Abstract
Global warming is impacting biodiversity by altering the distribution, abundance, and phenology of a wide range of animal and plant species. One of the best documented responses to recent climate change is alterations in the migratory behavior of birds, but the mechanisms underlying these phenotypic adjustments are largely unknown. This knowledge is still crucial to predict whether populations of migratory birds will adapt to a rapid increase in temperature. We monitored migratory behavior in a population of blackcaps (Sylvia atricapilla) to test for evolutionary responses to recent climate change. Using a common garden experiment in time and captive breeding we demonstrated a genetic reduction in migratory activity and evolutionary change in phenotypic plasticity of migration onset. An artificial selection experiment further revealed that residency will rapidly evolve in completely migratory bird populations if selection for shorter migration distance persists. Our findings suggest that current alterations of the environment are favoring birds wintering closer to the breeding grounds and that populations of migratory birds have strongly responded to these changes in selection. The reduction of migratory activity is probably an important evolutionary process in the adaptation of migratory birds to climate change, because it reduces migration costs and facilitates the rapid adjustment to the shifts in the timing of food availability during reproduction.
Keywords: adaptation, bird migration, climate change, genetic variation, natural selection
In the last three decades, mean surface temperatures have been increasing at an unprecedented rate, particularly at higher latitudes (1). This has had a profound impact on the distribution and ecology of many species and their survival (2, 3). Probably the biggest challenge for organisms living in seasonal habitats is to adapt to the changes in the timing of availability of resources, i.e., their phenology (4, 5). Whether a population will survive or even benefit from altered climatic conditions will be determined to a large extent by its capacity to adjust to rapidly changing environmental conditions (6–9). Phenotypic plasticity may be an important mechanism underlying recent phenotypic changes (10–13). However, the phenotypic response of the individuals of a population may become maladaptive if directional environmental changes exceed certain limits or if the environmental cues triggering phenotypic changes lose their predictive value (14). In such a situation, phenotypic plasticity will need to evolve and the capacity for evolutionary change will be pivotal for the long-term survival of populations (6, 9, 15, 16). To date there are only a few studies that have conclusively demonstrated adaptive evolution in response to the recent changes in climatic conditions (e.g., ref. 17; reviewed by refs. 4 and 15). It is unclear whether the limited number of studies is due to the difficulty of testing evolutionary response in the wild or because most recent phenotypic shifts have not involved evolutionary changes (11).
Due to their complex life cycle, migratory birds are sensitive models for studying the effects of climate change on animal populations, particularly the effects of mistiming (18–20). Migratory birds have responded strongly to the recent increase in temperature and the advancement of spring at their breeding sites by an advancement of spring migration (10, 15, 18–22) and, partly, by migrating shorter distances (23, 24). The strength of the response varies among populations and species (24–26). Differences in the ability to adjust to phenological changes at the breeding sites have been attributed, in part, to variation in migration distance and to the divergence of temperature changes at the breeding and wintering sites (27). Those populations that have not sufficiently adjusted to the altered conditions, or have not responded at all, have suffered declines (7, 8). Yet, the strength of the effects of mismatching may depend on the seasonality of food availability in the breeding areas, being largest in populations inhabiting habitats with a narrow food peak, such as forests (28). Among-population differences in the potential to adapt to environmental changes are also expected on theoretical grounds on the basis of differences in the extent to which populations are composed of both migrants and residents, i.e., in partial migration (29). In partially migratory populations, adaptive evolution of migration distance or the proportion of migrants is expected to be very fast as the response to selection on these traits is reinforced by high, favorable genetic correlations (29–31). It has been predicted that completely migratory populations may become partially migratory, which may increase their evolutionary response if mean migratory activity decreases, e.g., as a response to selection for shorter migration distance (29, 31). Although this process may constitute an important mechanism for migratory birds to adjust migration to rapid environmental changes, it has not yet been tested.
The blackcap (Sylvia atricapilla) is a small passerine bird breeding in the western Palaearctic and wintering in southern Europe and Africa that has strongly responded to recent increases in temperature and to concomitant environmental changes in central Europe. The advancement in spring arrival is the most consistent, and among the strongest, of all Passerine species’ responses studied in Europe (22, 32), and in some blackcap populations breeding in Central Europe there is a trend for later migration in autumn (33, 34, but see ref. 35). The blackcap is currently the model species for the study of the genetics and evolution of avian migration (31, 36, 37). It has been previously demonstrated that this species has a high potential for evolutionary changes in migratory behavior (36–38), which may facilitate the rapid establishment of new wintering areas in the wild (39, 40).
In view of the high evolutionary potential of the blackcap and recent phenological changes in its breeding, staging, and wintering areas in southern and central Europe (41), we expect this species to be a sensitive system to detect rapid adaptive changes in avian migration, and we expect to gain insight into the evolutionary processes involved. Here we test whether blackcaps have adapted migratory behavior to the recent increase in ambient temperature and to the resultant widening of the reproductive time window (42–44). Specifically, we predicted a shortening of migration distance and a delay in departure from the breeding grounds as a response to the global increase in temperature (45, 46).This study aims at unraveling the mechanisms underlying adaptation of migratory birds to climate change by continuously monitoring migratory behavior, and the effects causing its variation, using a combination of a long-term common garden experiment in time and selective breeding in captivity under a rigidly controlled environment. We demonstrate that there has been a strong evolutionary reduction in migratory activity and that this process will result in the rapid evolution of residency in an exclusively migratory bird population.
Results
Common Garden Experiment.
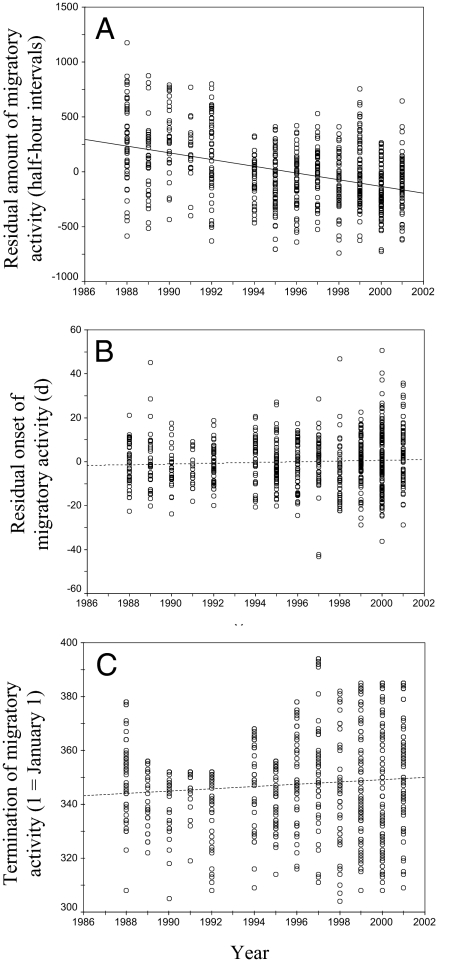
The measurement of nocturnal activity in birds collected in the wild (for details, see Materials and Methods) indicates that there was a significant reduction of migratory activity in southern German blackcaps between 1988 and 2001 (linear regression weighted by number of families: b = −30.2, F1,12 = 25.37, P < 0.001). Neither onset of migratory activity (b = 0.161, F1,12 = 0.99, P > 0.05) nor termination of activity (b = 0.402, F1,12 = 1.96, P > 0.05) changed significantly during this period (Fig. 1 and Table S1). The change in the amount of activity, however, did not change monotonously in the course of the 14-year period as indicated by the significant nonlinear components in the models with the best fit (Table S2). A chronological cluster analysis (47) identified two different groups of years 1988–1992 and 1994–2001 in our time series. Yet, the decrease in migratory activity was not significant in either of these two periods (regression on family means, 1988–1992: b = −24.0, F1,45 = 1.28, P > 0.05; 1994–2001: b = −9.6, F1,144 = 1.77, P > 0.05), nor did it differ significantly from each other [generalized linear interactive modeling (GLIM) period-by-year interaction: F1,12 = 1.07, P > 0.05], probably because of the small number of years studied. A comparison between trait means in the first (1988–1991) and the last 4 years of the study (1998–2001) revealed that migratory activity decreased by 326 half-hour intervals with activity, i.e., 45% or 1.1 standard deviations (SD) in this 10-year interval. Changes in the timing of migratory activity were much smaller and were significant only for the termination of activity. The difference was 1.3 days (0.09 SD) for the onset and 3.2 days (0.18 SD) for the termination of activity (Table S1).
Fig. 1.
Changes in migratory behavior in southern German blackcaps from 1988 to 2001. Amount (A), onset (B), and termination (C) of autumn migratory activity were measured in birds collected as nestlings in the wild and kept in the laboratory in environment-controlled chambers. Amount and onset of migratory activity are given as the residuals of a linear regression of these variables on hatching date. Lines represent the linear regression model fitted to the data. Solid lines indicate a significant change, broken lines indicate no change. Points give individual measurements.
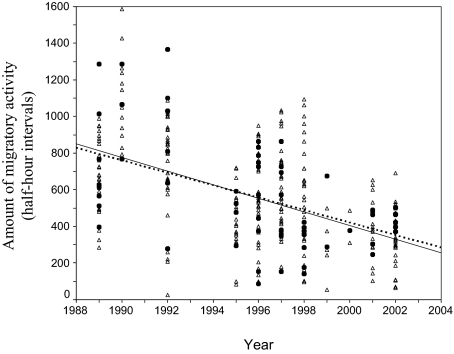
Changes in the amount of migratory activity were also found in birds that were kept under the same conditions but born in captivity, i.e., in individuals that hatched from eggs layed by birds raised under controlled environmental conditions (Fig. 2). The rate of change in the G1 generation (weighted linear regression: b = −34.03, F1,10 = 15.14, P < 0.01) did not differ from the rate of change in the parents of these birds (b = −38.09, F1,10 = 17.59, P < 0.01), which were sampled in the wild (GLIM generation-by-year interaction: F1,19 = 0.05, P > 0.05). This suggests that the changes observed were not induced by early environmental condition or maternal effects experienced by the individuals collected in the wild.
Fig. 2.
Changes in the amount of migratory activity in birds bred in aviaries (F1 generation; open triangles, broken line) and their parents collected in the wild (black dots, solid line). Lines represent the line of a linear regression fitted to the data. Parental data (black dots) are a subset of the data depicted in Fig. 1A.
All traits investigated had moderate to high heritabilities (h2), which may allow rapid evolutionary responses (Table 1). Maternal effects (m2) were significant only for the onset of migratory activity, where they accounted for about 25% of the phenotypic variance. However, maternal effects on the onset of migration did not change over time [weighted linear regression of mother best linear unbiased predictors (BLUPs) against time: b = 0.04, F1,12 = 0.69, P > 0.05]. Year effects (y2) were significant for all traits investigated, but they were particularly high for the “the amount of migratory activity” (Table 1).
Table 1.
Variance components for migratory traits in southern German blackcaps
| Trait | h2 | m2 | y2 |
| Amount of MA | 0.431 ± 0.065* | NS | 0.260 ± 0.083* |
| Onset of MA | 0.372 ± 0.073* | 0.248 ± 0.044* | 0.110 ± 0.047* |
| Termination of MA | 0.358 ± 0.059* | NS | 0.018 ± 0.013† |
Heritabilities (h2), maternal effects (m2), and year effects (y2) are given ±1 SE. MA, migratory activity. All variance components were standardized by the total phenotypic variance. Significance levels: NS P > 0.05,
*P < 0.05;
†P < 0.001.
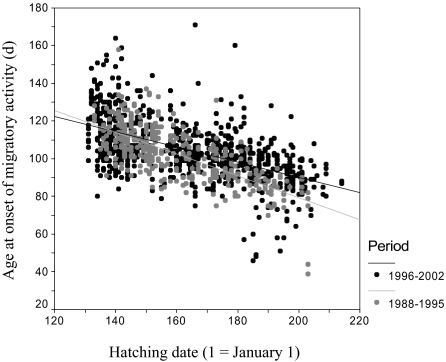
Observed changes in mean migratory activity were paralleled by changes in the relation between hatching date and the age at onset of migratory activity (Fig. 3; GLIM period-by-hatching-date interaction: F1,1237 = 21.78, P < 0.001). In the course of our study, individuals born late in the season significantly delayed age at onset of migratory activity, but there was no change in blackcaps hatching early. As a consequence, the population reaction norm became flatter during the course of the study (b1988–1995 = −0.580 ± 0.027, b1996–2002 = −0.405 ± 0.020).
Fig. 3.
Relationship between onset of migratory activity and hatching date in two study periods. Blackcaps hatched in the first half of the study (1988–1995) are represented by gray dots. Birds born in the second period (1996–2002) by black dots. The lines give the respective linear regression models fitted to the data in the two periods.
Selection Experiment.
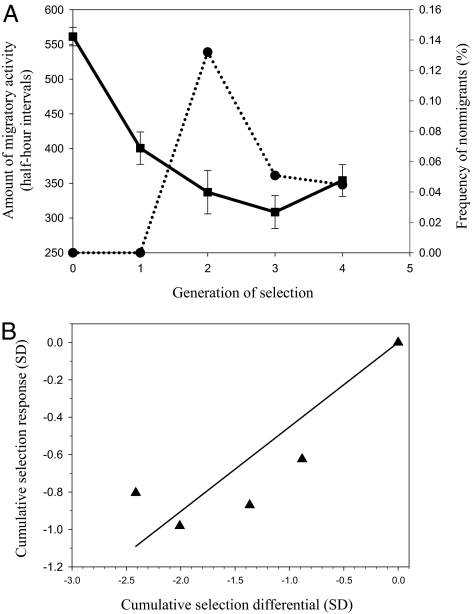
Southern German blackcaps showed a strong response to selection for lower amount of migratory activity in the artificial selection experiment (for details, see Materials and Methods). Both the mean amount of migratory activity (ANOVA: F5,645 = 29.58, P < 0.001) and the proportion of nonmigrants changed significantly (χ2 = 8.3, P < 001) after four generations of selection (see Fig. 4A). However, the response to selection was not uniform (Fig. 4B). The response of the amount of migratory activity was highest in the first generation of selection (realized h2 = 0.71) and decreased thereafter (realized h2 in G2 = 0.60; realized h2 in G3 = 0.21; realized h2 in G4 = 0.0). Differences in the amount of migratory activity were significant between the parental generation and all generations of selection (Tamahne's post hoc test T3: P < 0.001) and between G1 and G3 (Tamahne's post hoc test T3: P < 0.05), but not between all other generations of selection.
Fig. 4.
Response of amount of migratory activity and frequency of nonmigrants in southern German blackcaps to four generations of directional artificial selection for lower amount of migratory activity. (A) Changes in the mean amount of migratory activity (with standard errors, squares and continuous line) and in the frequency of nonmigratory individuals (circles, broken line). (B) Cumulative selection response as a function of the cumulative strength of selection for lower migratory activity. The linear regression model was fitted through the origin (b = 0.45, F1,4 = 50.6, P < 0.01).
As a correlated response to selection for lower migratory activity, the proportion of individuals with no migratory activity (i.e., nonmigrants) increased from zero in the parental population and in generation 1 (G1) to 14% in G2. In the G3 and G4 generations the frequency of nonmigrants was 5% and 4.5%, respectively. Differences in the proportions of nonmigrants were significant between generations 0 and 1 and all other generations (for comparison with G0: all P < 0.001; comparison G1 with G2: P < 0.001; comparison G1 with G3 or G1 with G4: P < 0.05). The frequency of nonmigrants did not significantly differ between generation 2 and later generations of selection (comparison G2 with G3: P = 0.136; G2 with G4: P = 0.090). The selection experiment in this naturally exclusively migratory population yielded a total of 14 nonmigratory individuals, i.e., individuals showing no migratory activity.
Discussion
In many bird populations migratory behavior has recently changed in response to profound ongoing climatic shifts (24, 25, 48). This study on the blackcap provides strong evidence for microevolution as a viable mechanism for these rapid adaptive changes in migration. Within a period of 14 years, we observed a marked reduction in migratory activity and a change in the response of the age at onset of migration activity to hatching date. These changes are in accord with the expected response of migratory birds to the observed increase in ambient temperature (18) and are, to our knowledge, a unique demonstration of evolutionary change of migratory behavior in response to climate change. The results of our selection experiment in which we simulated current selection in the wild favoring shorter migration distances further suggest that this process will lead to the evolution of partial migration. Our experiments demonstrate that selection for lower migratory activity will result in the evolution of residency in exclusively migratory populations of birds without the need for an introduction of “residency genes” by mutation or gene flow. This result is hitherto the strongest support for the threshold model of migration (29), which predicts a correlated selection response between migratory activity and the frequency of migrants (or nonmigrants).
Several lines of evidence support our conclusion that observed phenotypic changes in migratory behavior reflect genetic shifts in the population. First, as indicated by the “animal model” analyses, all monitored traits have moderate to high heritabilities, and, in accord with this finding, we demonstrate in the artificial selection experiment that migratory activity strongly responds to artificial selection. Therefore, it is likely that migratory behavior has responded to changes in selection regimes in the wild. Recent reports of significant shortening of migration distances and the increase of residency in a number of short- and middle-distance migrants associated with climatic changes in the last 30 years in Europe suggest that such changes in selection and phenotypic shifts are taking place (23, 24, 29). Moreover, the advancement of spring in southern Germany (49) and the advancement and lengthening of the breeding season in this blackcap population (44) indicate that selection on the timing of migration has changed in this population. An increase of observations of blackcaps wintering in this area (50) is in accord with our results, which suggest that residency is increasing in this population. Secondly, our “common garden experiment” was designed to minimize environmental variation. Throughout the experiment, birds were held in captivity, i.e., in an environment where temperature, humidity, and food availability were held constant. It is therefore likely that significant year effects reflect genetic changes rather than changes induced by shifts in environmental conditions. The “animal model” analyses further revealed that neither among-year variation in early environmental effects nor maternal effects could explain the observed phenotypic changes in migratory behavior. The observed reduction in the amount of migratory activity was, therefore, not confounded by shifts in maternal or early environmental effects. This conclusion is further supported by the fact that the reduction of migratory activity in birds born in captivity—i.e., from mothers that were kept under controlled laboratory conditions for almost a year—did not differ from the rate of change in their parents (Fig. 2), which were collected in the wild.
One interesting finding of our study was that the rate of evolutionary change in migratory activity had not been constant over the study period. We actually observed a strong reduction between 1988 and 1994, and a very small, nonsignificant change between 1994 and 2001. One possible explanation is that in the course of the study the strength of selection has been fluctuating, and that it has been particularly strong in the early years. Although we currently do not have any evidence supporting this hypothesis, it seems a likely explanation given that fluctuations in the strength and direction of selection are a common phenomenon in natural populations in the wild (see refs. 17, 51) and in their adjustment to anthropogenic changes of the environment (12).
Alternatively, or in addition, the rapid reduction in migratory activity between 1988 and 1994 could be due to gene flow. Immigration from southern blackcap populations, which are known to migrate later and have shorter migration distances (15), is not unlikely as many European bird species, including the blackcap, are expanding their breeding ranges to the north (52, 53). Moreover, populations of blackcaps breeding in central Europe and wintering in the United Kingdom and Ireland have been rapidly growing in the last decades (39, 40). Both gene flow from southern populations and from birds migrating to Great Britain and Ireland would result in a shortening of migration distance and a shift in migration times. A combination of selection and gene flow from populations that are less migratory could result in very high rates of evolutionary change in response to the large-scale increase in temperature, as found in this study. Unfortunately, we lack information on immigration to our population. Stable isotope studies may help in identifying the composition and dynamics of bird populations that are currently altering migratory behavior (31, 39).
As a consequence of the recent advancements in the availability of food during the reproductive season, the timing of reproduction has advanced in a large number of animal species, particularly in birds (2, 3, 42, 43). However, in some populations of migratory birds, particularly in long-distance migrants, the adjustment of the timing of breeding has been constrained by the lack of phenotypic change in the timing of spring arrival (54), causing population declines (7, 8, 28). Earlier arrival is thus a prerequisite for earlier breeding. Selection for shorter migration distance may be driven by the reduction of the costs of migration, which in some populations may be considerable (55), or by changes in food availability on the wintering sites, as in blackcaps wintering in Great Britain, which increased survival due to the setting up of feeding tables (40). Yet, more importantly, a shortening of migration distance may be advantageous because it facilitates earlier migration and breeding, as a consequence of the physiological response to the exposure to an altered photoperiod (18, 56).
Selection for rapid development in birds born late in the season—the so-called calendar effect—is expected to be relaxed as the start of autumn and winter is delayed, and the probability of encountering unfavorable conditions in early autumn decreases (46). Moreover, if hatching is advanced, this norm of reaction may become maladaptive for juvenile birds, because they would start migration unseasonably early (56). The observed trend for a reduction of the age at migration onset in response to hatching date (Fig. 3) is probably a result of these seasonal shifts. If the delay of autumn and the advancement of spring continue, we expect this calendar effect to disappear.
Both the shortening of migration distance and the attenuation of the response of migration onset to hatching date are changes that will modify the photoperiodic control of seasonal life-history events, like molt, migration, and reproduction. This is in line with the prediction that evolutionary responses to climate change will primarily involve changes in photoperiodism (4). A latitudinal shift of wintering areas to the north, as a consequence of a shortening of migration distance, will advance spring migration and gonadal growth (56). For migratory birds, this acceleration of the annual cycle seems an effective mechanism of adjusting the timing of reproduction to the rapid advancement of spring on the breeding grounds. However, this mechanism of rapid adaptation to the effects of climate change, which may be a common response of bird populations that migrate short to moderate distances (30), like the blackcap population studied, may not work for most long-distance migrants. In populations where large areas of unsuitable habitat are located between current breeding and wintering areas a gradual shortening of migration distance may not be possible (29, 57). Habitat loss in current winter ranges of many long-distance migrants, which is predicted to increase with global warming, will worsen this situation, because it will cause an increase in migration distance (45, 58) and prevent adaptation. Moreover, high rates of evolutionary change, as found here for the amount of migratory activity in the blackcap, increase the risk of extinction and may not be sustained for a longer period (59). This will particularly affect small populations. Low rates of phenotypic change in long-distance migratory birds (10, 15), and recent population declines (7, 8, 60, 61), suggest that in these populations the potential for evolutionary change may be limited.
Materials and Methods
Subjects.
We studied autumn migratory activity in a blackcap population breeding in woods near Radolfzell (47°46′N, 9°E), southwestern Germany. Between 1988 and 2001, we collected a total of 757 nestlings at an age of 5–7 days from 199 nests in the wild (Table S1) and transferred them to the laboratory, where they were hand-raised. We selected 102 of these birds for breeding experiments that we conducted between 1989 and 2002. A total of 97 successful clutches were produced by these pairs, which yielded 247 G1 offspring. In the selection experiment, we further bred another 250 birds in different lines for up to five generations (generations G2–G5). At about 30 days of age, all birds were transferred to individual cages equipped with movable perches, which allowed the continuous recording of locomotory activity (see below). As of this age, blackcaps were kept in climate-controlled chambers with ad libitum food and water and at a constant temperature of 20 °C ± 1.5 °C and a relative humidity of 75–80%. Light and dark cycles simulated the natural photoperiod experienced by birds from this population (for details, see ref. 62).
Control of Experimental Conditions.
Common garden experiments allow us to draw inferences about genetic changes in wild populations if experimental conditions are strictly controlled and do not change over time (15, 63). To achieve this, we collected nestlings at an early age, hand-raised them, and studied birds in climate-controlled chambers in captivity, which allowed us to follow a highly standardized protocol for rearing, keeping, and measuring birds throughout the complete study from 1988 through 2002. Moreover, the implementation and control of the experimental protocol was done by the same group of people (i.e., Peter Berthold, Ulrich Querner, and Gabriele Mohr). Although sample sizes differed among years, the samples collected are likely to be random samples from the wild population with regard to the birds' migratory behavior, as we did not know the behavior of individuals or their parents at the time they were sampled. Moreover, all birds were collected in the same woodland plots.
Measurement of Migratory Activity.
We monitored migratory behavior in SW-German blackcaps by measuring migratory restlessness. Migratory restlessness is the fraction of locomotory activity that nocturnal migrants display in captivity at night and that mirrors the time course and extent of migration in the wild, particularly in hand-raised birds in their first year (36, 64). We recorded migratory activity of each bird during its first “migratory journey,” i.e., in the autumn (September 1–December 17) of the year the bird was born. We measured three parameters of autumn migratory activity: (i) The onset of activity, which was defined as the first night on which a birds was active at night during at least 5 half-hour intervals; (ii) the termination of activity, i.e., the last day with a minimum of 5 half-hour intervals with night activity; and (iii) the total amount of migratory activity measured in number of half-hour intervals of night activity between the onset and termination of activity. In all analyses, we corrected onset and amount of migratory activity for the effect of hatching date. All variables were normally distributed and variances were independent of means. For the amount of activity, this was the case after log transformation.
Estimation of Variance Components.
To determine the potential for evolutionary change and the importance of nongenetic effects, we estimated heritabilities, maternal effects, and year effects using an “animal model” approach (65). We computed variance components using the multivariate restricted maximum likelihood (REML) procedure implemented in the software ASREML 2.0 (66). We used the complete pedigree information to fit individual animal models that allowed us to decompose phenotypic variance into causal variance components (65). The complete pedigree comprised 1,254 individuals that we actually studied, and 398 dummy individuals (our base population) for which we did not have any data. These individuals were the unidentified parents of birds collected in the wild. Dummy parents were assumed to be the same for each clutch and different among broods, assuming that within one clutch individuals were full sibs and all unknown parents bred only once. In the model for the estimation of variance components, we treated “hatching date” as a linear covariate, “sex” as fixed effect, and “individual,” “mother,” and “birth year” as random effects. Mother's identity fitted as a random effect yields general maternal effects (i.e., genetic and environmental). The significance of the random effects was tested using a log-likelihood ratio test. Random effects were considered in the final model if the deviance of the model including the effect was significantly lower than the deviance of the model excluding it. A significant birth-year effect may identify among-year environmental and genetic effects (67). Given the rigidly controlled environmental conditions in our experimental setup, significant year effects are likely to indicate genetic changes in the composition of the population.
Selection Experiment.
Between 1996 and 2002 we conducted an artificial selection experiment in which we bred a total of 305 blackcaps using the birds collected in the wild between 1995 and 2001 (see above). We selected for lower migratory activity in a selection line, which we ran up to five generations. To simulate selection processes in the wild, and to prevent inbreeding, we chose to crossbreed ≈50% of the birds produced in captivity with birds collected in the wild (see ref. 68 for details of the selection regime followed). As a consequence, we did not have “pure” generations of selection, particularly for the later generations (G3–G5). In the analyses of data, we therefore used the parent with the longest selection history as reference to determine the generation of selection (e.g., a backcross of a G1 with an individual of the parental generation is considered here as a G2). Because sample size in generation 5 was too low, we only considered four generations of selection (G1–G4) in the final analyses.
Supplementary Material
Acknowledgments
We thank all the people from the Vogelwarte Radolfzell that helped us in breeding, rearing, and keeping blackcaps, particularly Gabriele Mohr and Ulrich Querner. Timothy Coppack, Loeske Kruuk, Erik Postma, Stein Sæether, Arie van Noordwijk, and Marcel Visser commented on previous versions of the manuscript. This study was supported by the Max Planck Society and the Deutsche Forschungs Gemeinschaft. Part of the analyses were done during a “short visit” of F.P. to the University of Edinburgh, which was financially supported by the Royal Society of London.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910361107/DCSupplemental.
References
- 1.Solomon SD, et al. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- 2.Parmesan C. Ecological and evolutionary response to recent climate change. Annu Rev Ecol Evol Syst. 2006;37:637–669. [Google Scholar]
- 3.Walther G-R, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 4.Bradshaw WE, Holzapfel CM. Genetic response to rapid climate change: It's seasonal timing that matters. Mol Ecol. 2008;17:157–166. doi: 10.1111/j.1365-294X.2007.03509.x. [DOI] [PubMed] [Google Scholar]
- 5.Visser ME, Both C. Shifts in phenology due to global climate change: The need for a yardstick. Proc R Soc Lond B Biol Sci. 2005;272:2561–2569. doi: 10.1098/rspb.2005.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berteaux D, Réale D, McAdam AG, Boutin S. Keeping pace with fast climate change: Can arctic life count on evolution? Integr Comp Biol. 2004;44:140–151. doi: 10.1093/icb/44.2.140. [DOI] [PubMed] [Google Scholar]
- 7.Both C, Bouwhuis S, Lessells CM, Visser ME. Climate change and population declines in a long-distance migratory bird. Nature. 2006;441:81–83. doi: 10.1038/nature04539. [DOI] [PubMed] [Google Scholar]
- 8.Møller AP, Rubolini D, Lehikoinen E. Populations of migratory bird species that did not show a phenological response to climate change are declining. Proc Natl Acad Sci USA. 2008;105:16195–16200. doi: 10.1073/pnas.0803825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Visser ME. Keeping up with a warming world: Assessing the rate of adaptation to climate change. Proc R Soc Lond B Biol Sci. 2008;275:649–659. doi: 10.1098/rspb.2007.0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gienapp P, Leimu R, Merilä J. Responses to climate change in avian migration time—Microevolution or phenotypic plasticity? Clim Res. 2007;35:25–35. [Google Scholar]
- 11.Gienapp P, Teplitsky C, Alho JS, Mills JA, Merilä J. Climate change and evolution: Disentangling environmental and genetic responses. Mol Ecol. 2008;17:167–178. doi: 10.1111/j.1365-294X.2007.03413.x. [DOI] [PubMed] [Google Scholar]
- 12.Hendry AP, Farrugia TJ, Kinnison MT. Human influences on rates of phenotypic change in wild animal populations. Mol Ecol. 2008;17:20–29. doi: 10.1111/j.1365-294X.2007.03428.x. [DOI] [PubMed] [Google Scholar]
- 13.Teplitsky C, Mills JA, Alho JS, Yarrall JW, Merilä J. Bergmann's rule and climate change revisited: Disentangling environmental and genetic responses in a wild bird population. Proc Natl Acad Sci USA. 2008;105:13492–13496. doi: 10.1073/pnas.0800999105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlaepfer MA, Runge MC, Sherman PW. Ecological and evolutionary traps. Trends Ecol Evol. 2002;17:474–480. [Google Scholar]
- 15.Pulido F, Berthold P. Microevolutionary response to climate change. Adv Ecol Res. 2004;35:151–183. [Google Scholar]
- 16.Stockwell CA, Hendry AP, Kinnison MT. Contemporary evolution meets conservation biology. Trends Ecol Evol. 2003;18:94–101. [Google Scholar]
- 17.Grant PR, Grant BR. Unpredictable evolution in a 30-year study of Darwin's finches. Science. 2002;296:707–711. doi: 10.1126/science.1070315. [DOI] [PubMed] [Google Scholar]
- 18.Coppack T, Both C. Predicting life-cycle adaptation of migratory birds to global climate change. Ardea. 2002;90:369–378. [Google Scholar]
- 19.Møller AP, Fiedler W, Berthold P, editors. Birds and Climate Change. Oxford: Elsevier; 2004. [Google Scholar]
- 20.Robinson RA, et al. Climate Change and Migratory Species. UK: British Trust for Ornithology, The Nunnery, Thetford; 2005. [Google Scholar]
- 21.Gordo O. Why are bird migration dates shifting? A review of weather and climate effects on avian migratory phenology. Clim Res. 2007;35:5–23. [Google Scholar]
- 22.Lehikoinen E, Sparks TH, Zalakevicius M. Arrival and departure dates. Adv Ecol Res. 2004;35:1–31. [Google Scholar]
- 23.La Sorte FA, Thompson FR., 3rd Poleward shifts in winter ranges of North American birds. Ecology. 2007;88:1803–1812. doi: 10.1890/06-1072.1. [DOI] [PubMed] [Google Scholar]
- 24.Visser ME, Perdeck AC, van Balen JH, Both C. Climate change leads to decreasing bird migration distances. Glob Chang Biol. 2009;15:1859–1865. [Google Scholar]
- 25.Rubolini D, Møller AP, Rainio K, Lehikoinen E. Intraspecific consistency and geographic variability in temporal trends of spring migration phenology among European bird species. Clim Res. 2007;35:135–146. [Google Scholar]
- 26.Végvári Z, Bókony V, Barta Z, Kovács G. Life history predicts advancement of avian spring migration in response to climate change. Glob Chang Biol. 2010;16:1–11. [Google Scholar]
- 27.Jones T, Cresswell W. The phenology mismatch hypothesis: Are declines of migrant birds linked to uneven global climate change? J Anim Ecol. 2010;79:98–108. doi: 10.1111/j.1365-2656.2009.01610.x. [DOI] [PubMed] [Google Scholar]
- 28.Both C, et al. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proc R Soc Lond B Biol Sci. 2010;277:1259–1266. doi: 10.1098/rspb.2009.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulido F, Berthold P, van Noordwijk AJ. Frequency of migrants and migratory activity are genetically correlated in a bird population: Evolutionary implications. Proc Natl Acad Sci USA. 1996;93:14642–14647. doi: 10.1073/pnas.93.25.14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berthold P. Towards a comprehensive theory for the evolution, control and adaptability of avian migration. Ostrich. 1999;70:1–11. [Google Scholar]
- 31.Pulido F. The genetics and evolution of avian migration. Bioscience. 2007;57:165–174. [Google Scholar]
- 32.Rainio K, Tøttrup AP, Lehikoinen E, Coppack T. Effects of climate change on the degree of protandry in migratory songbirds. Clim Res. 2007;35:107–114. [Google Scholar]
- 33.Bezzel E, Jetz W. Delay of the autumn migration period in the blackap (Sylvia atricapilla) 1966-1993—A response to “global warming”? J Ornithol. 1995;136:83–87. [Google Scholar]
- 34.Nowakowski JK. Terms of autumn migration of the genus Sylvia in central Poland. Ring. 1999;21:3–13. [Google Scholar]
- 35.Hüppop K, Hüppop O. An atlas of bird ringing on the island of Helgoland. Part 3: Changes of spring and autumn migration times from 1960-2001 (Translated from German) Vogelwarte. 2005;43:217–248. [Google Scholar]
- 36.Berthold P. Control of Bird Migration. London: Chapman & Hall; 1996. [Google Scholar]
- 37.Berthold P. Bird Migration. 2nd Ed. Oxford: Oxford Univ Press; 2001. [Google Scholar]
- 38.Pulido F, Berthold P. Quantitative genetic analysis of migratory behavior. In: Berthold P, Gwinner E, Sonnenschein E, editors. Avian Migration. Heidelberg: Springer; 2003. pp. 53–77. [Google Scholar]
- 39.Bearhop S, et al. Assortative mating as a mechanism for rapid evolution of a migratory divide. Science. 2005;310:502–504. doi: 10.1126/science.1115661. [DOI] [PubMed] [Google Scholar]
- 40.Berthold P, Helbig AJ, Mohr G, Querner U. Rapid microevolution of migratory behaviour in a wild bird species. Nature. 1992;360:668–670. [Google Scholar]
- 41.Menzel A, et al. European phenological response to climate change matches the warming pattern. Glob Chang Biol. 2006;12:1969–1976. [Google Scholar]
- 42.Crick HQP, Dudley C, Glue DE, Thomson DL. UK birds are laying eggs earlier. Nature. 1997;388:526. [Google Scholar]
- 43.Crick HQP, Sparks TH. Climate change related to egg-laying trends. Nature. 1999;399:423–424. [Google Scholar]
- 44.Schaefer T. Adaptation and nest predation in the blackcap (Translated from German). PhD thesis (Göttingen University) 2002. Available at http://deposit.ddb.de/cgi-bin/dokserv?idn=965459160. Accessed on March 25, 2010.
- 45.Doswald N, et al. Potential impacts of climatic change on the breeding and non-breeding ranges and migration distance of European Sylvia warblers. J Biogeogr. 2009;36:1194–1208. [Google Scholar]
- 46.Pulido F, Coppack T, Berthold P. Genetic variation and phenotypic plasticity may explain adaptive changes in the timing of autumn migration. Ring. 2001b;23:149–158. [Google Scholar]
- 47.Legendre P, Legendre L. Numerical Ecology. 2nd Ed. Amsterdam: Elsevier; 1998. [Google Scholar]
- 48.Fiedler W. Recent changes in migratory behavior of birds: A compilation of field observations and ringing data. In: Berthold P, Gwinner E, Sonnenschein E, editors. Avian Migration. Heidelberg: Springer; 2003. pp. 21–38. [Google Scholar]
- 49.Menzel A, Estrella N, Fabian P. Spatial and temporal variability of the phenological seasons in Germany from 1951 to 1996. Glob Chang Biol. 2001;7:657–666. [Google Scholar]
- 50.Ornithologischen Arbeitsgemeinschaft Bodensee. (1989-2002) Circular of the Lake Constance Ornithological Working Group (OAB). Available at http://www.bodensee-ornis.de/rundbrief/archiv/. Accessed on March 25, 2010.
- 51.Siepielski AM, DiBattista JD, Carlson SM. It's about time: The temporal dynamics of phenotypic selection in the wild. Ecol Lett. 2009;12:1261–1276. doi: 10.1111/j.1461-0248.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 52.Böhning-Gaese K, Lemoine N. Importance of climate change for the ranges, communities and conservation of birds. Adv Ecol Res. 2004;35:211–236. [Google Scholar]
- 53.Thomas CD, Lennon JJ. Birds extend their ranges northwards. Nature. 1999;399:213. [Google Scholar]
- 54.Both C, Visser ME. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature. 2001;411:296–298. doi: 10.1038/35077063. [DOI] [PubMed] [Google Scholar]
- 55.Sillett S, Holmes RT. Variation in survivorship of a migratory songbird throughout its annual cycle. J Anim Ecol. 2002;71:296–308. [Google Scholar]
- 56.Coppack T, Pulido F. Photoperiodic response and the adaptability of avian life-cycles to climate change. Adv Ecol Res. 2004;35:131–150. [Google Scholar]
- 57.Coppack T, et al. Can long-distance migratory birds adjust to the advancement of spring by shortening migration distance? The response of the pied flycatcher to latitudinal photoperiodic variation. Glob Chang Biol. 2008;14:2516–2522. [Google Scholar]
- 58.Barbet-Massin M, Walther BA, Thuiller W, Rahbek C, Jiguet F. Potential impacts of climate change on the winter distribution of Afro-Palaearctic migrant passerines. Biol Lett. 2009;5:248–251. doi: 10.1098/rsbl.2008.0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lynch M, Lande R. Evolution and extinction in response to environmental change. In: Kareiva PM, Kingsolver JG, Huey RB, editors. Biotic Interactions and Global Change. Sunderland, MA: Sinauer; 1993. pp. 234–250. [Google Scholar]
- 60.Berthold P, Fiedler W, Schlenker R, Querner U. 25-year study of the population development of Central European songbirds: A general decline, most evident in long-distance migrants. Naturwissenschaften. 1998;85:350–353. [Google Scholar]
- 61.Sanderson FJ, Donald PF, Pain DJ, Burfield IJ, van Bommel FPJ. Long-term population declines in Afro-Palearctic migrant birds. Biol Conserv. 2006;131:93–105. [Google Scholar]
- 62.Pulido F, Coppack T. Correlation between timing of juvenile molt and onset of migration in the blackcap Sylvia atricapilla. Anim Behav. 2004;68:167–173. [Google Scholar]
- 63.van Noordwijk AJ, et al. A framework for the study of genetic variation in migratory behavior. J Ornithol. 2006;147:221–233. [Google Scholar]
- 64.Coppack T, Becker SF, Becker PJJ. Circadian flight schedules in night-migrating birds caught on migration. Biol Lett. 2008a;4:619–622. doi: 10.1098/rsbl.2008.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kruuk LEB. Estimating genetic parameters in natural populations using the “animal model”. Philos Trans R Soc Lond B Biol Sci. 2004;359:873–890. doi: 10.1098/rstb.2003.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gilmour AR, Gogel BJ, Cullis BR, Thompson R. ASReml User Guide Release 2.0. UK: VSN International, Hemel Hempstead; 2006. [Google Scholar]
- 67.Postma E. Implications of the difference between true and predicted breeding values for the study of natural selection and micro-evolution. J Evol Biol. 2006;19:309–320. doi: 10.1111/j.1420-9101.2005.01007.x. [DOI] [PubMed] [Google Scholar]
- 68.Berthold P, Mohr G, Querner U. Control and evolutionary potential of obligate partial migration: Results of a two-way selective breeding experiment with the Blackcap (Sylvia atricapilla) J Ornithol. 1990;131:33–45. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.