Abstract
Recent evidence suggests that endothelial dysfunction and pathology of pulmonary vascular responses may serve as a precursor to smoking-associated emphysema. Although it is known that emphysematous destruction leads to vasculature changes, less is known about early regional vascular dysfunction which may contribute to and precede emphysematous changes. We sought to test the hypothesis, via multidetector row CT (MDCT) perfusion imaging, that smokers showing early signs of emphysema susceptibility have a greater heterogeneity in regional perfusion parameters than emphysema-free smokers and persons who had never smoked (NS). Assuming that all smokers have a consistent inflammatory response, increased perfusion heterogeneity in emphysema-susceptible smokers would be consistent with the notion that these subjects may have the inability to block hypoxic vasoconstriction in patchy, small regions of inflammation. Dynamic ECG-gated MDCT perfusion scans with a central bolus injection of contrast were acquired in 17 NS, 12 smokers with normal CT imaging studies (SNI), and 12 smokers with subtle CT findings of centrilobular emphysema (SCE). All subjects had normal spirometry. Quantitative image analysis determined regional perfusion parameters, pulmonary blood flow (PBF), and mean transit time (MTT). Mean and coefficient of variation were calculated, and statistical differences were assessed with one-way ANOVA. MDCT-based MTT and PBF measurements demonstrate globally increased heterogeneity in SCE subjects compared with NS and SNI subjects but demonstrate similarity between NS and SNI subjects. These findings demonstrate a functional lung-imaging measure that provides a more mechanistically oriented phenotype that differentiates smokers with and without evidence of emphysema susceptibility.
Keywords: pulmonary circulation, chronic obstructive pulmonary disease, pulmonary emphysema, computed tomography, hypoxic pulmonary vasoconstriction
Multidetector row CT (MDCT), along with the development of quantitative image analysis techniques, provides a comprehensive assessment of the lung (1–3). MDCT imaging has focused on evaluating the extent and distribution of emphysema, a subset of chronic obstructive pulmonary disease (COPD). In this paper, we focus specifically on smoking-associated emphysema. COPD is characterized by a progressive airflow limitation associated with an abnormal inflammatory response to noxious gases or particles and leads to irreversible damage in the airways with enlargement of the distal airspaces (4, 5). Although pulmonary hypertension is a well-known clinical feature of advanced COPD (6, 7), less is known about the vascular response and tone early in COPD pathogenesis. Recent evidence suggests that endothelial dysfunction and alterations in pulmonary vascular response occur early in COPD and may represent an important vascular pathway in the development of smoking-associated emphysema (8–10). In response to cigarette smoke, the lung reacts with the expansion of inflammatory cells. With inflammation, alveoli often are flooded, leading to regional hypoxia. The lung's normal response to alveolar hypoxia is hypoxic pulmonary vasoconstriction (HPV) resulting in the shunting of blood toward better-ventilated lung regions for improved oxygenation. In sheep (11) and humans (12, 13), imaging demonstrates that HPV is blocked in the presence of inflammation, and this block can be limited to the sites of inflammation (3). We therefore have hypothesized (3) that if inherent mechanisms fail to block the HPV response in the presence of inflammation, the inflammatory response may not be self-limited by the normal cascade of events and repair mechanisms, including delivery of bone marrow-derived progenitor cells (14, 15), thus leading to chronic inflammation, focal tissue destruction, and emphysema.
Mishima et al. (16) previously provided a hypothesis and quantitative, CT-based evidence suggesting that local mechanical weakening of the lung serves to promote progression of emphysema once the disease is established. In this paper, we hypothesize an underlying cause for the initial pathologic process and provide quantitative, functional MDCT-based evidence supporting the hypothesis. Dynamic 4D ECG-gated MDCT perfusion sequences are tailored to determine quantitatively regional perfusion param-eters, i.e., pulmonary blood flow (PBF) and mean transit time (MTT), through the detection of temporal changes in the density of the lung parenchyma resulting from a rapid, central i.v. bolus injection of iodinated contrast coupled with the application of indicator dilution theory and first-pass kinetics (17–20). We hypothesize that increased heterogeneity (larger regional variation) of PBF and MTT will exist in smokers with normal spirometry and MDCT-only findings of emphysema. This hypothesis is based on the notion that smokers susceptible to emphysema may fail to block HPV in the presence of inflammation, leading to increased heterogeneity of MTT, whereas smokers not susceptible to emphysema would block HPV in inflamed regions, maintaining a more homogenous PBF and MTT. In this study we formed a cohort of subjects to determine the normal range of MDCT perfusion parameters for subjects who had never smoked (NS) and then compared these results with findings in a smoking population split into two groups, those with (SCE) and without (SNI) subtle MDCT evidence of centrilobular emphysema as detected visually by an expert chest radiologist.
Results
Subject Characteristics.
Forty-eight subjects underwent perfusion MDCT imaging; seven subjects were excluded from the final cohort (two NS with bronchiectasis, one NS with emphysema on MDCT, one smoker with a large mediastinal mass, one smoker with respiratory alveolitis, and two subjects with contrast-injection errors). The remaining 41 subjects were categorized based on smoking status (NS or smoker), and current smokers were subcategorized further into two distinct groups, SCE and SNI, based on the presence (or absence) of emphysema on MDCT determined by a blind-read from an expert chest radiologist with 15 years’ experience. Baseline demographics, the diffusing capacity of the lung for carbon monoxide (DLCO), and spirometry measurements for the NS, SNI, and SCE groups are summarized in Table 1. None of the subjects had ground-glass opacities in the region of perfusion MDCT imaging. Of the 24 smokers imaged, 12 (50%) demonstrated emphysema on MDCT. Six of these 12 subjects had upper lobe emphysema only, two had upper and middle lobe emphysema, and four had emphysema present in all lobes. Two subjects had bullous changes. Compared with NS and SNI subjects, SCE subjects were collectively older and had a greater number of pack-years, and their prebronchodilator forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratios were in the lower range of normal (70–80% of predicted). However, there was overlap between the groups. No significant differences in spirometry measurements existed between groups. DLCO measurements for SCE subjects were significantly decreased compared with those for NS but not compared with those for SNI subjects (P = 0.02).
Table 1.
Characteristics of subjects
| Characteristic | NS | SNI | SCE |
| N | 17 | 12 | 12 |
| Gender (% male) | 35 | 58 | 58 |
| Age (years) | 31 ± 10 | 32 ± 14 | 46 ± 11 |
| BMI (kg/m2) | 24 ± 3 | 25 ± 3 | 25 ± 4 |
| Heart rate (bpm) | 68 ± 10 | 72 ± 11 | 70 ± 13 |
| Blood pressure (mm Hg) | |||
| Systolic | 116 ± 13 | 119 ± 15 | 128 ± 13 |
| Diastolic | 66 ± 11 | 65 ± 12 | 74 ± 7 |
| Creatinine (mg/dL) | 0.8 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.2 |
| Pack-years | — | 15 ± 14 | 31 ± 15 |
| Age started smoking (years) | — | 17 ± 3 | 16 ± 4 |
| BDI | 11.9 ± 0.2 | 11.3 ± 1.2 | 11.2 ± 1.3 |
| Daily cough in past 2 weeks (%) | 6 | 33 | 50 |
| Prebronchodilator FEV1 (%) | 98.3 ± 8.9 | 84.9 ± 16.7 | 98.2 ± 23.5 |
| Prebronchodilator FVC (%) | 100.7 ± 10.5 | 91.7 ± 11.5 | 103.9 ± 15.6 |
| Prebronchodilator FEV1/FV ()% | 81.4 ± 7.0 | 77.2 ± 11.6 | 74.5 ± 4.9 |
| DLCO (%) | 92.7 ± 16.0 | 79.4 ± 10.4 | 75.7 ± 19.6 |
| MLD (HU) | −849 ± 36 | −848 ± 18 | −846 ± 21 |
| EI −950 HU (%) | 6.3 ± 5.9 | 4.1 ± 4.3 | 4.3 ± 3.8 |
| EI −910 HU (%) | 34.8 ± 16.9 | 28.3 ± 14.9 | 26.0 ± 12.5 |
| α −950 HU | 1.7 ± 0.3 | 1.7 ± 0.2 | 1.6 ± 0.4 |
| α −910 HU | 0.9 ± 0.4 | 1.1 ± 0.4 | 1.0 ± 0.2 |
Results are expressed as mean ± SD unless noted. Spirometry and DLCO is reported as percent predicted. BDI, baseline dyspnea index; BMI, body mass index; DLCO, diffusing capacity of the lung for carbon monoxide; EI, emphysema index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; MLD, mean lung density; NS, persons who had never smoked; SCE smokers with centralobular emphysema present on MDCT; SNI, smokers with normal CT imaging studies.
Volumetric CT.
The CT-based emphysema index (EI, the proportion of lung volume below a threshold attenuation compared with the total volume of the lung), α [an index of hole-size distribution based on the measurements of Mishima et al. (16) and described in Methods], and mean lung density are summarized in Table 1. Although visual inspection served to divide the smokers into a group with apical, centrilobular emphysema and a group with normal imaging appearances, there were no significant differences between groups for whole-lung EI or α measurements obtained at thresholds of −950 Hounsfield units (HU) (EI: P = 0.4; α: P = 0.6) or −910 HU (EI: P = 0.3; α: P = 0.3). To determine the extent of emphysema specifically in the lung region imaged for perfusion, the lung was divided into thirds based on the distance from the apex to the costophrenic angle. EI analyses were performed on the middle third of the lung (covering the lung volume distal to the carina and ending near the diaphragm); this area corresponded to the region of perfusion imaging in all subjects. These EI measurements for the three groups did not demonstrate differences in the percent of emphysema-like lung with thresholds of −950 HU (P = 0.3) or −910 HU (P = 0.2). The mean percentages of emphysema-like volume for the middle third below −950 HU were 6.7%, 4.1%, and 4.0%, and those below −910 HU were 37.2%, 29.6%, and 26.5% for NS, SNI, and SCE subjects, respectively.
MDCT-Based Pulmonary Perfusion Parameters.
An example of perfusion data obtained from an SNI subject (Fig. 1) demonstrates time–attenuation curves obtained for the input arterial region of interest (ROI) and ROIs placed in the nondependent and dependent region of the lung parenchyma. PBF and MTT measurements for NS, SNI, and SCE subjects were determined for the whole imaged slab and are summarized in Table 2.
Fig. 1.
Dynamic time–attenuation curve data for an SNI subject. Graphs show corresponding time–attenuation curves demonstrating the dynamic change in HU as the bolus of contrast passes through the PA (Upper) and through the dependent and nondependent regions of the lung parenchyma (Lower). Regional PBF and MTT are obtained by applying indicator dilution theory to the data. (Inset) MDCT perfusion baseline image.
Table 2.
MDCT-based perfusion parameters
| Parameter | NS | SNI | SCE |
| PBF(mL/100 mL/min) | |||
| Mean ± SD | 221.5 ± 55.6 | 226.0 ± 47.5 | 210.3 ± 83.7 |
| Range (low–high) | 132.9–323.6 | 152.0–307.2 | 140.1–420.2 |
| CV | 0.43 ± 0.07 | 0.45 ± 0.11 | 0.58 ± 0.12 |
| MTT (seconds) | |||
| Mean ± SD | 4.68 ± 1.01 | 4.54 ± 0.70 | 4.08 ± 0.88 |
| Range (low–high) | 3.34–6.36 | 3.21–5.47 | 2.02–5.21 |
| CV | 0.45 ± 0.09 | 0.44 ± 0.08 | 0.58 ± 0.10 |
Results are expressed as mean ± SD unless noted. MTT, mean transit time; NS, persons who had never smoked; PBF, pulmonary blood flow; SCE, smokers with emphysema present on MDCT; SNI, smokers with normal MDCT imaging studies.
Whole lung.
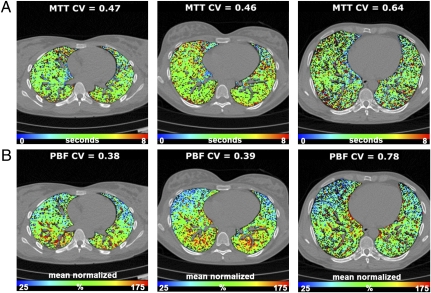
Absolute mean PBF (mL/100 mL/min) and MTT (seconds) were determined for each group, and no statistically significant differences were found among the three groups for either PBF (P = 0.8) or MTT measurements (P = 0.2). The one-way ANOVA demonstrated significant differences in coefficient of variation (CV) measurements for MTT between the groups (P = 0.0005), with post hoc analysis demonstrating significant differences in MTT CV measurements between the SCE group compared with the SNI and NS groups but no significant difference between the SNI and NS groups. Similarly, for PBF CV measurements the one-way ANOVA demonstrated significant differences in CV measurements between the groups (P = 0.0007) with post hoc analysis demonstrating a significant increase in CV in the SCE group compared with the SNI and NS groups but no significant difference between the SNI and NS groups. A representative PBF and MTT color map from a subject from each group demonstrates this difference in heterogeneity (Fig. 2). After accounting for differences in subject age, significant differences in CV in PBF (P = 0.02) and MTT (P = 0.02) still exist among groups. After accounting for subject age and pack-year smoking history specifically in the smoking population, the differences in CV between the SNI and SCE groups were diminished to a level of marginal nonsignificance for PBF (CV: P = 0.11) but were still significant for MTT (CV: P = 0.015). Because of concern that the use of different models of MDCT scanners might influence the observed differences in CV measurements, a subgroup of perfusion images from 10 NS and 10 SCE subjects, all performed on the 64-slice scanner, were compared, and significant CV differences for PBF and MTT were observed, similar to findings in the larger population.
Fig. 2.
Color-coded maps of perfusion parameters overlaid on an imaging slice for a subject in each group. (A) MTT maps for NS (Left), SNI (Center), and SCE (Right) subjects. Maps demonstrate significantly increased regional heterogeneity of MTT measurements in SCE subjects as compared with NS and SNI subjects. Range: 0–8 seconds. (B) PBF normalized to the mean PBF for NS (Left), SNI (Center), and SCE (Right) subjects. As in MTT findings, there is increased regional heterogeneity in mean normalized PBF measurements in SCE subjects compared with NS and SNI subjects. The range on the color scale for MTT is 0-8 seconds and the range for mean normalized PBF is 25–175%.
Nondependent and dependent comparison.
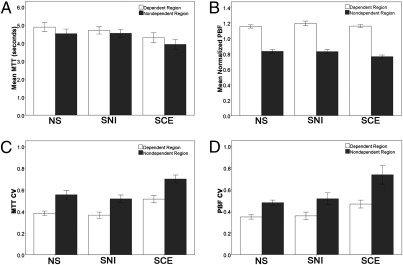
Regional data are graphed in Fig. 3. MTT measurements did not vary significantly through the lung volume sampled. No significant differences were observed in mean MTT in nondependent, dependent, and whole-lung MTT measurements. PBF measurements demonstrate an increasing flow gradient from nondependent to dependent regions of the lung in all three groups. CV measurements of PBF and MTT exhibit significantly higher CV in the nondependent region than in the dependent region for NS subjects (PBF: P = 0.0001; MTT: P = 0.00001), SNI subjects (PBF: P = 0.003; MTT: P = 0.001) and SCE subjects (PBF: P = 0.001; MTT: P = 0.007).
Fig. 3.
MTT and PBF measurements (mean ± SEM) for dependent and nondependent regions of the lung. (A) Mean MTT did not differ between the nondependent and dependent regions for any of the groups. (B) In all three groups, mean normalized PBF measurements demonstrate increased flow in the dependent region and decreased flow in the nondependent region of the lung. (C) In all three groups the CV of MTT is increased in the nondependent region compared with the dependent region. (D) In all three groups the CV of mean normalized PBF is greater in the nondependent region than in the dependent region.
Discussion
The data from this study suggest that heterogeneity of flow may serve as a functional “phenotype” of smoking-related centrilobular emphysema. We assume that all smokers have the same inflammatory events, but in this study the SCE group, who are hypothesized to have an HPV response associated with inflammation, have significantly patchier perfusion parameters, and the SNI group, who are hypothesized to block HPV in inflamed regions, do not show an increased CV of perfusion parameters relative to the NS group, who do not have the smoking-related inflammatory events. Interestingly, although DLCO was within a lower range of normal for both smoking groups, the SCE group showed a significant difference in DLCO compared with the NS group, but NS vs. SNI and SNI vs. SCE DLCO values were not significantly different from each other. This finding is not surprising, because both groups were likely to have inflamed lung regions. The perfusion findings support our hypothesis that failure to block HPV with local inflammation may serve as a precursor to destruction in a smoking population susceptible to emphysema (and may be genetically based). It is known that, in response to noxious particles and gases in cigarette smoke, the lung reacts by recruiting inflammatory cells. Pulmonary vascular changes characterized by a thickening of the vessel wall have been characterized early in the history of COPD (21, 22). More recently it has been observed that, in the presence of inflammation, there is an enhanced delivery of progenitor cells to the lung (14, 15). Remy-Jardin et al. (23) observed an increased propensity for the lung to develop emphysema in regions characterized by ill-defined ground-glass opacities and micronodules characteristic of inflammation. There are also suggestions in the literature that, in response to inflammatory processes, the HPV response normally is blocked (12, 13). In a sheep model, Easley et al. (11) used MDCT to demonstrate the regional failure of HPV in a lung region flooded with endotoxin but not with saline, and Hoffman et al. (3) showed the coexistence of an intact HPV in normal lung regions and a failure of HPV in inflamed lung regions. If HPV shuts down blood flow in an emphysema-susceptible smoking population (increased CV of perfusion parameters), this response would tend to shut down the cascade of events serving to resolve an inflammatory event, including the blockade of progenitor cells that would serve to repair the damage caused by the inflammatory response. Furthermore, if increased vascular tone in an inflamed region is a critical event in the development of emphysema, this increased vascular tone would have greater consequences in the lung apex where blood flow is further compromised in the upright body posture because of gravitational effects, explaining the predominance of apical pathology in smoking-related emphysema. Barr and colleagues (24, 25) recently have demonstrated a correlation between impaired left ventricular (LV) filling and percent emphysema in groups of nonsmokers, ex-smokers, and current smokers. The correlation between impaired LV filling with emphysema was greater in current smokers than in former smokers, consistent with the notion that increased vascular resistance in inflamed lung regions of smokers susceptible to emphysema may contribute to the LV-filling defect.
Another possible explanation of the current study's findings is that the increased PBF and MTT heterogeneity result from microvascular destruction caused by microemphysema (including very peripheral destruction of vascular and airway structures) undetectable with the resolution of MDCT or, as has been suggested recently, early destruction of peripheral airways (26). If such microvascular or peripheral airways destruction was present in our population, neither the α measure nor the density mask index of emphysema served as an index of this destruction. Loss of peripheral pulmonary capillary beds because of parenchymal destruction is a known feature of advanced disease (21) but has not been demonstrated in transformational regions. If our findings were the result of very peripheral disease that was undetectable by purely anatomy-based metrics on the MDCT image, these findings would indicate that functional imaging provides a differentiating phenotype, whereas anatomical imaging with MDCT provides no differences including quantitative measures of emphysema-like lung at a threshold of either −950 or −910 HU. Regardless of the underlying mechanism (failure to block HPV in the presence of inflammation or microdestruction with loss of peripheral vascular and airway structures), the increased CV of MTT and PBF measurements in this population identifies a mechanistic phenotype differentiating our NS and SNI subjects from our SCE subjects. Currently, both density and airways changes are considered to be the primary imaging-based phenotypes serving to differentiate COPD groups (27–29). We describe a functionally based, image-derived phenotype (CV of PBF and MTT) within regions of lung with no density-based changes, and this phenotype may link more closely to specific disease etiology.
Our cohort consisted of individuals with normal spirometry and with no clear differences in MDCT densitometric-based emphysema measurements between groups, indicating a population of smokers with preclinical emphysema. The SCE group had significantly lower DLCO, and the SNI group trended toward lower DLCO as compared with the NS group, suggesting that inflammatory processes were present in the smokers. The presence of emphysema, mainly in the apical lobes, did not typically correspond to the region of lung sampled for perfusion imaging. Therefore, the increased heterogeneity demonstrated in smokers susceptible to emphysema is not simply the result of gross parenchymal destruction.
We also explored the relationship of perfusion in the nondependent and dependent regions of the lung, demonstrating an increasing blood flow gradient in the supine position consistent with gravitational forces fighting perfusion, most likely in zones 2 and 3 (30). This gradient is present in all groups and therefore did not relate to smoking status. The gradient is consistent with reports in the literature from CT (18), MR (31–34) and PET (35, 36) imaging modalities. The high spatial resolution perfusion maps obtained with MDCT perfusion imaging demonstrated regional heterogeneity in the lung parenchyma. When quantified by CV measurements, an increased heterogeneity was demonstrated in the nondependent region compared with the dependent region for both MTT and PBF measurements in all three groups. Smoking status did not change this regional difference in heterogeneity during supine imaging. We hypothesize that in the upright body posture, the interplay between HPV in inflamed regions and gravitational-based reduction of perfusion parameters may make the lung apices more susceptible to events leading to the development of emphysema (in the current investigation, to smoking-related centrilobular emphysema).
This study is limited by its cross-sectional study design. A subpopulation of the SNI subjects might be susceptible to emphysema not revealed by current imaging evidence. The SNI group tended to be younger than the SCE subjects. Although there was a significant difference in our SCE population versus the NS and SNI groups, there was overlap among the populations in regards to MTT and PBF measures. SNI subjects with higher CV measurements represent a group of potential interest regarding emphysema susceptibility. Following the SNI smokers longitudinally to assess if there is a correlation between higher CV measurements and the development and progression of centrilobular emphysema would facilitate such an evaluation.
The functional CT method used in this study captures 4D data (3D volume plus time) at baseline and during a sharp bolus injection of contrast, allowing the collection of time–intensity data necessary for first-pass kinetics. This protocol is not the standard clinical protocol used to evaluate regional perfused blood volume (slow prolonged bolus of contrast injected to cause a prolonged enhancement of blood) but rather is tailored to provide quantitative data regarding regional PBF (in mL/100 mL/min) and MTT (in seconds). The perfusion scans were performed using axial scanning to image a multislice slab of lung just below the carina and cephalad to the diaphragm dome. This region was selected for several reasons: (i) Large branches of the pulmonary arteries were available for assessing the arterial input function used in the perfusion calculations, (ii) this region of lung was not visually or quantitatively affected by emphysema, and (iii) we hypothesize that the enhanced heterogeneity of perfusion will be present everywhere in the lung, whereas apical emphysema is caused by hypoxic pulmonary vasoconstriction confounded by the effects of gravity over time. The MDCT perfusion technique assesses a region of lung corresponding to the z-axis coverage of the multidetector scanner array. Various altered scanner designs may, in the future, provide access to perfusion parameters over the whole lung. However, the partial lung coverage presented here is adequate for phenotypic differentiation among smoking populations. Derivatives of such imaging may find clinical utility if subphenotype selection becomes important for treatment selection or outcomes assessment; initially, such imaging will aid in elucidating disease etiology and drug and device discovery. Because there is a strong relationship between regional vascular resistance and compliance (37), one would expect there to be correlations between MDCT measures of regional perfused pulmonary blood volume and pulmonary perfusion parameters. Thus, simpler means of measuring perfused pulmonary blood volume, requiring only a single volume scan using dual-energy MDCT (38), may serve as a surrogate for the imaging methods outlined in this study. Furthermore, MR mea-sures of regional perfusion (39) might provide similar measures of perfusion heterogeneity.
In summary, perfusion MDCT imaging provides a minimally invasive means of obtaining functional information about the pulmonary vasculature and provides valuable information as we continue to characterize a vascular phenotype of emphysema. Our data show a blood flow pattern of significantly increased heterogeneity, indicative of increased regional variability in PBF and MTT in a subset of smokers with minimal visual MDCT-based evidence of centrilobular emphysema and normal spirometry. These findings do not prove but are consistent with our hypothesis that inflammation-based HPV occurs in smokers susceptible to centrilobular emphysema but is blocked successfully in smokers without signs of emphysema susceptibility. An alternative explanation is that microdestruction that is undetectable by visual or quantitative CT measures occurs in the region of lung in which blood flow measures were made. In either case, our functional MDCT measures provide a functional phenotype that may be mechanistic in nature and of importance in the differentiation of patient populations as part of a search for genotypes associated with COPD and other potentially genetic-based lung disease.
Methods
Study Population.
From November 2004 to April 2008, 48 subjects were recruited as part of an effort to establish a normative lung atlas. The University of Iowa institutional review board approved this study, and written informed consent was obtained from all subjects before they entered the study. Study inclusion criteria were “never smokers” (with a total smoking history of less than 1 pack-year, NS) and smokers currently smoking one pack per day. Exclusion criteria were known heart disease, kidney disease, diabetes, presence of metal in the lung field, pregnancy, an X-ray/CT scan in the past 12 months, contrast allergies, a glomerular filtration rate less than 60 mL/min, and a body mass index over 32. NS with clinically important pathology detected on MDCT were excluded, as were smokers with significant lung disease other than emphysema. The baseline dyspnea index was determined (40). Prebronchodilator spirometry including DLCO measurements were performed via a V6200 Body Box (Sensor Medics) or an OWL body plethysmograph (Ferraris Respiratory), verified for equivalency. Spirometry quality followed the American Thoracic Society and European Respiratory Society guidelines (41).
MDCT Imaging.
Heart rate, ECG, arterial pressure, and pulse oximetry oxygen saturation (SpO2) were monitored continuously (Philips IntelliVue patient monitoring system, M8010A,) during imaging. MDCT imaging consisted of spiral noncontrast volumetric scans of the lung obtained during breath-hold at two fixed, spirometry-controlled lung volumes, functional residual capacity (FRC: 20% vital capacity) and total lung capacity (TLC: 100% of vital capacity), and a dynamic ECG-gated perfusion scan performed at an FRC breath-hold. MDCT scanners were upgraded throughout the study with each scanner's perfusion protocol selected to maximize the similarity of spatial resolution. Breath-holds were obtained with the aid of a laboratory-developed lung volume controller system that allowed the instantaneous measurement of airflow and airway pressures and held lung volumes appropriately with the aid of a computer-controlled balloon valve on the expiratory port. Volumetric MDCT scan times were under 10 seconds with a z-coverage of 22–30 cm, adjusted to capture the whole lung. Voxels were near isotropic at 0.47 mm × 0.47 mm × 0.5 mm. The following imaging protocol was used with our 64-slice MDCT scanner: 100 milliampere-seconds (mAs), 120 kV, 1 mm pitch, 512 × 512 matrix, and B31f reconstruction kernel. ECG-gated axial dynamic MDCT perfusion imaging acquires 4D information on a volume of lung during the administration of a sharp, central bolus (0.5 mL/kg, up to a total volume of 40 mL) of iodinated contrast agent (Visipaque 350;GE Healthcare) delivered over 2 seconds by a power injector (Mark V ProVis; MedRad) through a 5F percutaneously introduced central line in the superior vena cava. Central line placement was performed by a chest or interventional radiologist with the aid of fluoroscopy at the MDCT scanner. Perfusion is assessed with subjects in the supine position during a breath-hold at FRC. We believe it is important to image at FRC because it is well recognized that at higher lung volumes blood flow is reduced in the nondependent lung regions. Images collected during a dynamic sequence consisted of 11 (4-slice scanner) or 14 (16-slice or 64-slice scanners) time points including a baseline followed by the bolus contrast injection as it flowed through the lung fields. Beta-blockers were not necessary to control heart rate. Scanner-based perfusion imaging parameters are summarized in Table S1. The maximum effective radiation dose (conservatively calculated) is 10.6 mSv for the volume scans and 3.2 mSv for the perfusion scan.
Image Analysis.
Mean lung density and an EI for thresholds of −950 and −910 HU were obtained from TLC images using Pulmonary Workstation software (PW; VIDA Diagnostics) (42). Because images were obtained with three different scanners over the course of 4 years, EI thresholds were modified to correct for interscan variation using the attenuation of air outside the body and blood from the aorta. α, defined as the negative slope of the cumulative cluster–size distribution of areas with attenuation lower than a specified threshold, was determined for thresholds of −950 HU and −910 by methods described by Mishima et al. (16). PBF and MTT were determined using indicator dilution theory with Pulmonary Analysis Software Suite (PASS), a laboratory-developed software program (2, 43). Segmented lung parenchymal voxels were binned in 3 × 3 ROIs (1.4 mm × 1.4 mm × 1.2 mm) resulting in regional data for 2.4 mm3. Regional PBF and MTT measurements were determined based on time–attenuation curves for lung parenchyma ROIs, assuming a bolus injection, residue detection model (17, 19, 20). In this model, PBF is defined as the ratio of change in HU to the area under the input time–attenuation arterial curve, and MTT is assessed as the area under the residue curve divided by the maximum height. Data were filtered to remove major airways and vessels (ensuring ROIs were predominately lung parenchyma) using the following criteria: fractional air content: 0.4–0.9; fractional blood content: 0.02–0.5; and χ2 curve fit: 0–200,000. To compare regional differences across groups without intersubject variability from differing cardiac outputs, PBF was normalized to the mean PBF.
Statistical Analysis.
SPSS (Windows 15.0, SPSS, Inc) was used for statistical comparisons. Data are expressed as mean ± SD for the whole-lung volume and the nondependent and dependent thirds of the lung. Nondependent and dependent regions correspond to the upper and lower thirds of the supine lung. Heterogeneity of PBF and MTT is assessed by its CV. One-way ANOVA followed by post hoc analysis using the Bonferroni method was used to determined statistical differences, with a P value less than 0.05 considered significant. Additional analyses accounting for the subject's age were performed to compare CV between groups. Paired t tests assessed differences in the dependent vs. nondependent regions.
Supplementary Material
Acknowledgments
The authors thank Heather Baumhauer, Joanie Wilson, and Angie Delsing for help with patient recruitment; Jered Sieren, Lisa Hudson, and John Morgan for assistance during imaging; Dr. Shilang Sun, Dr. Jafar Golzarian, and Dr. Steve Burke for their assistance in central venous line placement; and Junfeng Guo for software assistance. This work was funded by National Institutes of Health Grant R01 HL-064368 (toE.A.H.).
Footnotes
Conflict of interest statement: E.A.H. and G. M. are founders and shareholders of VIDA Diagnostics, a company commercializing lung-imaging software derived from laboratory research.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913880107/DCSupplemental.
References
- 1.Hoffman EA, Chon D. Computed tomography studies of lung ventilation and perfusion. Proc Am Thorac Soc. 2005;2:492–498, 506. doi: 10.1513/pats.200509-099DS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman EA, et al. Characterization of the interstitial lung diseases via density-based and texture-based analysis of computed tomography images of lung structure and function. Acad Radiol. 2003;10:1104–1118. doi: 10.1016/s1076-6332(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman EA, Simon BA, McLennan G. State of the art. A structural and functional assessment of the lung via multidetector-row computed tomography: Phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:519–532. doi: 10.1513/pats.200603-086MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 5.Rabe KF, et al. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 6.Barberà JA, Peinado VI, Santos S. Pulmonary hypertension in chronic obstructive pulmonary disease. Eur Respir J. 2003;21:892–905. doi: 10.1183/09031936.03.00115402. [DOI] [PubMed] [Google Scholar]
- 7.Thabut G, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127:1531–1536. doi: 10.1378/chest.127.5.1531. [DOI] [PubMed] [Google Scholar]
- 8.Barr RG, et al. Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: The Emphysema and Cancer Action Project (EMCAP) Study. Am J Respir Crit Care Med. 2007;176:1200–1207. doi: 10.1164/rccm.200707-980OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanazawa H, Asai K, Hirata K, Yoshikawa J. Possible effects of vascular endothelial growth factor in the pathogenesis of chronic obstructive pulmonary disease. Am J Med. 2003;114:354–358. doi: 10.1016/s0002-9343(02)01562-0. [DOI] [PubMed] [Google Scholar]
- 10.McAllister DA, et al. Arterial stiffness is independently associated with emphysema severity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:1208–1214. doi: 10.1164/rccm.200707-1080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Easley RB, Fuld MK, Fernandez-Bustamante A, Hoffman EA, Simon BA. Mechanism of hypoxemia in acute lung injury evaluated by multidetector-row CT. Acad Radiol. 2006;13:916–921. doi: 10.1016/j.acra.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 12.Gust R, Kozlowski J, Stephenson AH, Schuster DP. Synergistic hemodynamic effects of low-dose endotoxin and acute lung injury. Am J Respir Crit Care Med. 1998;157:1919–1926. doi: 10.1164/ajrccm.157.6.9704110. [DOI] [PubMed] [Google Scholar]
- 13.Schuster DP, Marklin GF. The effect of regional lung injury or alveolar hypoxia on pulmonary blood flow and lung water measured by positron emission tomography. Am Rev Respir Dis. 1986;133:1037–1042. doi: 10.1164/arrd.1986.133.6.1037. [DOI] [PubMed] [Google Scholar]
- 14.Peinado VI, Ramírez J, Roca J, Rodriguez-Roisin R, Barberà JA. Identification of vascular progenitor cells in pulmonary arteries of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2006;34:257–263. doi: 10.1165/rcmb.2005-0255OC. [DOI] [PubMed] [Google Scholar]
- 15.Ishizawa K, et al. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett. 2004;556:249–252. doi: 10.1016/s0014-5793(03)01399-1. [DOI] [PubMed] [Google Scholar]
- 16.Mishima M, et al. Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proc Natl Acad Sci USA. 1999;96:8829–8834. doi: 10.1073/pnas.96.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chon D, Beck KC, Larsen RL, Shikata H, Hoffman EA. Regional pulmonary blood flow in dogs by 4D-X-ray CT. J Appl Physiol. 2006;101:1451–1465. doi: 10.1152/japplphysiol.01131.2005. [DOI] [PubMed] [Google Scholar]
- 18.Dakin JH, Evans TW, Hansell DM, Hoffman EA. Regional pulmonary blood flow in humans and dogs by 4D computed tomography. Acad Radiol. 2008;15:844–852. doi: 10.1016/j.acra.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman EA, Tajik JK, Kugelmass SD. Matching pulmonary structure and perfusion via combined dynamic multislice CT and thin-slice high-resolution CT. Comput Med Imaging Graph. 1995;19:101–112. doi: 10.1016/0895-6111(94)00035-2. [DOI] [PubMed] [Google Scholar]
- 20.Won C, et al. CT-based assessment of regional pulmonary microvascular blood flow parameters. J Appl Physiol. 2003;94:2483–2493. doi: 10.1152/japplphysiol.00688.2002. [DOI] [PubMed] [Google Scholar]
- 21.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 22.Wright JL, Churg A. Advances in the pathology of COPD. Histopathology. 2006;49:1–9. doi: 10.1111/j.1365-2559.2006.02395.x. [DOI] [PubMed] [Google Scholar]
- 23.Remy-Jardin M, et al. Longitudinal follow-up study of smoker's lung with thin-section CT in correlation with pulmonary function tests. Radiology. 2002;222:261–270. doi: 10.1148/radiol.2221001154. [DOI] [PubMed] [Google Scholar]
- 24.Barr RG, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vonk-Noordegraaf A. The shrinking heart in chronic obstructive pulmonary disease. N Engl J Med. 2010;362:267–268. doi: 10.1056/NEJMe0906251. [DOI] [PubMed] [Google Scholar]
- 26.Hogg JC, McDonough JE, Gosselink JV, Hayashi S. What drives the peripheral lung-remodeling process in chronic obstructive pulmonary disease? Proc Am Thorac Soc. 2009;6:668–672. doi: 10.1513/pats.200907-079DP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coxson HO. Quantitative computed tomography assessment of airway wall dimensions: Current status and potential applications for phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:940–945. doi: 10.1513/pats.200806-057QC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coxson HO, Rogers RM. Quantitative computed tomography of chronic obstructive pulmonary disease. Acad Radiol. 2005;12:1457–1463. doi: 10.1016/j.acra.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Nakano Y, et al. Quantitative assessment of airway remodeling using high-resolution CT. Chest. 2002;122(6, Suppl):271S–275S. [PubMed] [Google Scholar]
- 30.West JB, Dollery CT, Naimark A. Distribution of blood flow in isolated lung; Relation to vascular and alveolar pressures. J Appl Physiol. 1964;19:713–724. doi: 10.1152/jappl.1964.19.4.713. [DOI] [PubMed] [Google Scholar]
- 31.Ley S, et al. Quantitative 3D pulmonary MR-perfusion in patients with pulmonary arterial hypertension: Correlation with invasive pressure measurements. Eur J Radiol. 2007;61:251–255. doi: 10.1016/j.ejrad.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 32.Prisk GK, et al. Pulmonary perfusion in the prone and supine postures in the normal human lung. J Appl Physiol. 2007;103:883–894. doi: 10.1152/japplphysiol.00292.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hopkins SR, et al. Vertical gradients in regional lung density and perfusion in the supine human lung: The Slinky effect. J Appl Physiol. 2007;103:240–248. doi: 10.1152/japplphysiol.01289.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohno Y, et al. Quantitative assessment of regional pulmonary perfusion in the entire lung using three-dimensional ultrafast dynamic contrast-enhanced magnetic resonance imaging: Preliminary experience in 40 subjects. J Magn Reson Imaging. 2004;20:353–365. doi: 10.1002/jmri.20137. [DOI] [PubMed] [Google Scholar]
- 35.Schuster DP, Kaplan JD, Gauvain K, Welch MJ, Markham J. Measurement of regional pulmonary blood flow with PET. J Nucl Med. 1995;36:371–377. [PubMed] [Google Scholar]
- 36.Musch G, et al. Topographical distribution of pulmonary perfusion and ventilation, assessed by PET in supine and prone humans. J Appl Physiol. 2002;93:1841–1851. doi: 10.1152/japplphysiol.00223.2002. [DOI] [PubMed] [Google Scholar]
- 37.Lankhaar JW, et al. Quantification of right ventricular afterload in patients with and without pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2006;291:H1731–H1737. doi: 10.1152/ajpheart.00336.2006. [DOI] [PubMed] [Google Scholar]
- 38.Pontana F, et al. Lung perfusion with dual-energy multidetector-row CT (MDCT): Feasibility for the evaluation of acute pulmonary embolism in 117 consecutive patients. Acad Radiol. 2008;15:1494–1504. doi: 10.1016/j.acra.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 39.Ohno Y, et al. Assessment of bolus injection protocol with appropriate concentration for quantitative assessment of pulmonary perfusion by dynamic contrast-enhanced MR imaging. J Magn Reson Imaging. 2007;25:55–65. doi: 10.1002/jmri.20790. [DOI] [PubMed] [Google Scholar]
- 40.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85:751–758. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- 41.Miller MR, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 42.Müller NL, Staples CA, Miller RR, Abboud RT. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest. 1988;94:782–787. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]
- 43.Guo J, Fuld MK, Alford SK, Reinhard JM, Hoffman EA. Pulmonary Analysis Software Suite 9.0: Integrating quantitative measures of function with structural analyses. In: Brown M, editor. First International Workshop on Pulmonary Image Analysis. 2008. pp. 283–292. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.