Abstract
In a previous study, we mapped spontaneous mitotic reciprocal crossovers (RCOs) in a 120-kb interval of chromosome V of Saccharomyces cerevisiae. About three-quarters of the crossovers were associated with gene conversion tracts. About 40% of these conversion tracts had the pattern expected as a consequence of repair of a double-stranded DNA break (DSB) of an unreplicated chromosome. We test this hypothesis by examining the crossovers and gene conversion events induced by gamma irradiation in G1- and G2-arrested diploid yeast cells. The gene conversion patterns of G1-irradiated cells (but not G2-irradiated cells) mimic conversion events associated with spontaneous RCOs, confirming our previous conclusion that many spontaneous crossovers are initiated by a DSB on an unreplicated chromosome.
Keywords: homologous recombination, DNA breaks, Saccharomyces cerevisae, loss of heterozygosity
Homologous mitotic recombination is an efficient method of repairing double-stranded breaks (DSBs) in the yeast Saccharomyces cerevisiae and other organisms (1). Although mitotic recombination was described in Drosophila more than 70 years ago (2), many of the basic properties of mitotic recombination are not understood. The chromosomes resulting from a mitotic recombination event are segregated into two daughter cells and one problem is that most analytic methods select for only one of the cells containing the recombinant chromosomes.
A method for selecting both daughter cells with the products expected from reciprocal crossing over (RCO) is shown in Fig. 1. A diploid yeast strain is constructed in which one copy of chromosome V has an ochre mutation in the CAN1 gene (can1-100); in the absence of an ochre suppressor, such strains are resistant to canavanine. On the other homologue, the CAN1 gene has been replaced by SUP4-o, a gene encoding an ochre-suppressing tRNA. The diploid is also homozygous for the ade2-1 ochre mutation. Yeast strains containing the ade2-1 allele without a nonsense suppressor are Ade− and form red colonies as a result of accumulation of red precursor to adenine (3). In the diploid depicted in Fig. 1, because of the presence of the SUP4-o gene, the cells are canavanine-sensitive, Ade+, and form white colonies.
Fig. 1.
Diploid strain used to select and map RCOs. The diploid PG311 has the can1-100 allele on one copy of chromosome V and a replacement of can1 sequences with the ochre-suppressor tRNA gene SUP4-o on the other (5). This strain is also homozygous for the ochre-suppressible ade2-1 mutation. The starting diploid is CanS and Ade+; strains with an unsuppressed ade2-1 mutation form red colonies (3). An RCO between the centromere and can1-100/SUP4-o can result in two CanR cells (rectangles at Bottom). Subsequent growth of these cells results in a red/white sectored colony. There are many single-nucleotide polymorphisms distinguishing each homologue (indicated by red and black circles) that can be used to map the position of the RCO.
A RCO between the centromere of chromosome V and the can1-100/SUP4-o markers will result in two CanR daughter cells that will subsequently grow to form a red/white sectored CanR colony (4). As a consequence of the RCO, polymorphisms distal to the crossover point become homozygous in the two sectors, whereas polymorphisms proximal to the exchange retain heterozygosity (Fig. 1). We mapped crossovers to a resolution of 4 kb in this 120-kb interval using a diploid constructed by mating haploids with diverged (0.1%) DNA sequences (5). Using PCR and restriction enzyme analysis (discussed below), we determined whether individual sectors were heterozygous or homozygous for the markers.
In meiotic tetrads in fungi, although heterozygous markers usually segregate 2:2 into the four spores (for example, 2A:2a), in some tetrads, there is a net loss of one allele and a net gain of the second (3A:1a or 1A:3a). Such events are called “gene conversions.” Meiotic gene conversions reflect DNA mismatch repair in the heteroduplex that initiates the crossover (6); mitotic gene conversions also occur (6). In our system, conversion events associated with RCOs are detectable as regions that are homozygous for one or more markers in one sector, but that remain heterozygous for the same markers in the other sector (dotted boxed region in Fig. 2A). In analogy with meiotic conversion events, this type of mitotic conversion is called “3:1.”
Fig. 2.
RCOs and gene conversion events associated with G2- and G1-induced DSBs. The homologues containing the SUP4-o and can1-100 alleles are shown in black and red, respectively, with circles indicating polymorphic sites. Dotted boxes mark gene conversion tracts and X's show the position of the RCO. (A) The 3:1 conversion tract. Recombination is initiated by a DSB on one of the black chromatids. The associated 3:1 gene conversion involves the broken chromatid receiving information from the red chromatid as observed in many studies of induced DSBs (1). Two linked markers are transferred as shown by the horizontal arrows. (B) The 4:0 conversion tract. Recombination is initiated by a DSB in an unreplicated black chromosome that is then replicated to yield two broken chromatids. The repair of one chromatid is associated with an RCO and conversion of two polymorphisms. The repair of the second DSB is not associated with an RCO but the same two sites are converted, yielding a 4:0 conversion event. (C) The 3:1/4:0 hybrid tract. The pattern of DSB formation and conversion is similar to that in Fig. 2B, except that the conversion tract associated with the second repair event is short, generating a hybrid 3:1/4:0 conversion.
We previously showed that mitotic conversion tracts associated with RCOs averaged 12 kb in length (5), considerably longer than meiotic conversion tracts (6). In addition, about 40% of the conversion tracts were not of the 3:1 type. For one type of exceptional tract (4:0 events), the same form of the polymorphism was homozygous in both sectors (dotted boxed region of Fig. 2B). We also detected conversion tracts with a 4:0 region immediately adjacent to a 3:1 region (Fig. 2C). Because the 4:0 and hybrid 3:1/4:0 tracts were much too frequent to represent two independent events, we suggested that both 4:0 and 3:1/4:0 hybrid tracts reflected repair of two DSBs, generated by replication of a broken chromosome to form two broken chromatids (Fig. 2 B and C). Repair of both DSBs associated with conversion tracts of the same size would generate the 4:0 tract and repair of the two DSBs with tracts of different sizes would produce the hybrid tracts.
Several other observations support the model shown in Fig. 2 B and C. First, X-rays and UV radiation stimulate mitotic recombination in G1-arrested yeast cells (7, 8). Second, Esposito (9) showed that some spontaneous heteroallelic conversions in yeast had the properties expected for events initiated in G1. Third, DSBs in G1 cells result in DNA ends that are inefficiently resected (10, 11), and these ends fail to recruit Rad52p (12). Fourth, cells irradiated in G2 have much more rapid repair than those irradiated in G1 (13). Thus, broken chromosomes generated in G1 are likely to replicate before DNA repair. In addition, G1-irradiated chicken cells have metaphase chromosomes in which both chromatids are broken at the same position, whereas G2-irradiated cells have metaphase chromosomes with a single broken chromatid (14).
To provide further evidence for this model, we examined RCOs and their associated conversion events induced by gamma irradiation of yeast cells synchronized in G1 or G2. We find that the conversion events associated with RCOs in cells irradiated in G1 are of the 3:1, 4:0, and 3:1/4:0 hybrid types. The conversions associated with RCOs induced by G2 irradiation are exclusively of the 3:1 type.
Results
Experimental Rationale.
The diploid used in the study (PG311) has markers allowing the selection of RCOs as canavanine-resistant red/white sectored colonies and is also heterozygous for the polymorphisms that allow mapping of the RCOs and associated conversion tracts (Fig. 1). Although most diploids have both MATa and MATα information, PG311 has a deletion of the MATα locus, allowing its synchronization in G1 using the α pheromone (15). We showed previously that the frequency and distribution of RCOs and conversion tracts were similar in PG311 and an isogenic diploid expressing both mating types (5). We synchronized PG311 in G2 using nocodazole (16).
Cell Viability and Frequency of RCOs in G1- and G2-Synchronized Cells.
PG311 cells synchronized in G1 or G2 were irradiated with 0, 50, or 100 Gy of gamma rays to induce DSBs. The average viabilities for the G1-arrested cells (±95% confidence limits) relative to 100% for unirradiated samples (five independent experiments) were: 100% (±10%) for cells treated with 50 Gy and 77% (±15%) for the samples treated with 100 Gy. The viabilities of the G2-irradiated samples at the 50 and 100 Gy doses were 110% (±25%) and 89% (±7%), respectively.
The average rates of sectored colonies per viable cell for the G1 cells were: 1.1 (±0.4) × 10−5, 0 Gy; 3.2 (±2.0) × 10−4, 50 Gy; and 2.6 (±1.1) × 10−4, 100 Gy. For the G2 cells, the comparable rates were: 9.9 (±7.8) × 10−6, 0 Gy; 2.4 (±0.9) × 10−4, 50 Gy; and 4.2 (±2.5) × 10−4, 100 Gy. Thus, in both G1- and G2-irradiated samples, the rates of RCOs were stimulated 20- to 40-fold.
Unexpectedly, the rate of RCOs in the G1-irradiated samples was not elevated by increasing the dose from 50 to 100 Gy. It is possible that cells have a limited capacity to repair G1-induced DSBs by the RCO pathway because of limiting amounts of one of the recombination proteins. Alternatively, because our estimates of the rates of RCOs have large confidence limits, it is possible that the similar rates of RCOs at the two radiation doses simply reflect the large confidence limits on the estimates.
Mapping of Gamma Ray-Induced Mitotic Crossovers and Associated Gene Conversions.
Crossovers and associated gene conversion events were mapped as described previously (5). DNA was isolated from both the red and white sectors of each sectored colony. For this analysis, we used 34 heterozygous polymorphisms in which one allele had a restriction enzyme recognition site that the other allele lacked. By PCR amplification of sequences containing the heterozygous alleles, followed by digestion with the appropriate restriction enzyme, we determined whether the cells of the sector were homozygous for one allele or the other, or were heterozygous.
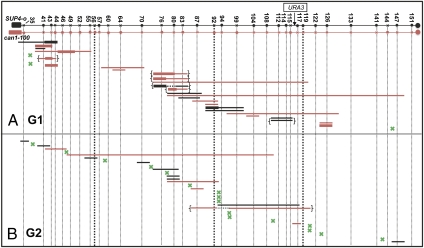
The mapping data for the G1- and G2-irradiated samples are shown in Fig. 3. We analyzed 29 sectors from the G1-irradiated cells (15 and 14 from the 50 and 100 Gy samples, respectively) and 31 from the G2-irradiated cells (7 and 24 from the 50 and 100 Gy samples, respectively). The patterns of crossovers and conversions were similar for the two doses of radiation and are summed for subsequent analysis. Green X's depict crossovers unassociated with gene conversion and the horizontal lines show conversion events associated with crossovers. The 3:1 tracts are shown as thin lines and 4:0 tracts as thick lines.
Fig. 3.
Mapping of RCOs and associated conversions in G1- and G2-irradiated cells. The markers are shown at the top with X's and O's indicating the absence and presence of diagnostic restriction sites, respectively. The thick vertical dotted lines mark the divisions that were used in the analysis of the distribution of recombination events (described in the text). Numbers at the top of the figure are the Saccharomyces Genome Database (SGD) coordinates of the markers. Conversion events are shown as horizontal lines with 3:1 events, 4:0 events, and 3:1/4:0 hybrid events indicated by thin lines, thick lines, and hybrid thin/thick lines, respectively. The color of the line shows which chromosome was the donor in the conversion event. Braces indicate complex conversion events and green X's represent RCOs that are not associated with an observable conversion tract. (A) Mapping of 29 RCO events in G1-irradiated cells. (B) Mapping of 31 RCO events in G2-irradiated cells.
In the G1-irradiated cells, we found 3:1, 4:0, and 3:1/4:0 hybrid tracts, whereas in the G2-irradiated sample, we observed only 3:1 tracts. Most (34 of 41) of the conversion events in G1- and G2-irradiated samples had three properties: (i) the conversion event involved a single donor chromosome, (ii) the conversion tracts were uninterrupted by markers that did not show conversion, and (iii) for 3:1 conversion tracts or the 3:1 portion of 3:1/4:0 hybrid tracts, the sectors that are homozygous or heterozygous for the markers had the patterns of segregation shown in Fig. 2 A and C in which the conversion event involves the chromatids that crossed over. Thus, if the conversion event involves donating information from the can1-100-containing chromosome, the sector that is homozygous for can1-100 is homozygous for the converted marker with the other sector retaining heterozygosity.
We observed two classes of exceptional conversion events (braced in Fig. 3). There were five sectored colonies in the G1-irradiated samples involving 3:1 conversion tracts in which the “wrong” sector (as defined in the previous paragraph) was homozygous for the marker (class 1). There were two sectored colonies in which the gene conversion tract was interrupted by a marker that did not undergo conversion (class 2). Mechanisms for these two classes will be presented in Discussion. Because we have a simple mechanism to explain the class 1 sectored colonies, we include this class, but not class 2, in the analyses described below.
Of the simple conversions observed in the G1-irradiated cells, we found fifteen 3:1, three 4:0, and seven 3:1/4:0 hybrid tracts. In the G2-irradiated cells, we observed fourteen 3:1 tracts and no 4:0 or hybrid tracts. These two distributions are significantly different (P = 0.02 by Fisher's exact test). In our previous study of conversion events associated with spontaneous RCOs in PSL100/101 (5), we found thirty-three 3:1, six 4:0, and fifteen 3:1/4:0 hybrid tracts. This distribution is not significantly different from that observed in the G1-irradiated cells (P = 1 by Fisher's exact test), but is significantly different from that observed in the G2-irradiated cells (P = 0.01 by Fisher's exact test). PSL100/101 are MATa/MATα diploids otherwise isogenic to the MATa/MATαΔ::NAT diploid PG311 (5). We also previously examined a small number of spontaneous events in PG311 and found five 3:1, three 4:0, and four 3:1/4:0 hybrid tracts. This distribution is not significantly different from that observed for the G1-induced events (P = 0.40) but is different from that observed for G2-induced events (P = 0.001). In summary, the types of gene conversion tracts resulting from G1-irradiated cells are similar to those associated with spontaneous mitotic crossovers and are different from those associated with G2-induced crossovers.
Conversion Tract Sizes in G1- and G2-Irradiated Cells.
Because the lengths of the conversion tracts do not have a symmetrical distribution, we examined the median length of the tracts (95% confidence limits shown in parentheses). We measured the lengths of individual conversion tracts by averaging the minimum length (the distance between markers included within the tract) and the maximum length (the distance between the closest markers not included in the tract). Because all homologous recombination events are thought to require heteroduplex formation (1), it is likely that the RCOs with no detectable associated conversion events reflect short regions of heteroduplex that do not include any markers. Consequently, we estimated the lengths of conversion tracts for the RCO events with no detectable tract by assigning them a length that was the average of the maximum length (the distance between the flanking markers in the interval with the RCO) and the minimum length (1 bp).
The median length of conversion tracts associated with spontaneous RCOs in PSL100/101 was 6.5 kb (95% confidence limits of 5.1–8.6 kb; ref. 5), considerably longer than meiotic conversion tracts [1–5 kb in different studies (5, 17–19)]. The median conversion tract size associated with the G1-irradiated samples was 7.3 kb (5.4–11.4 kb), similar to the size associated with spontaneous crossovers. The median tract size for G2-irradiated cells was 2.7 kb (1.8–3.6 kb), significantly smaller than that associated with spontaneous events or G1-irradiated cells. If mitotic conversion tracts induced in G2 are shorter than those induced in G1, then one would expect that a smaller fraction of RCOs would be associated with conversion in G2-irradiated cells. A total of 26 of the 29 RCOs in G1-irradiated cells were associated with conversion events whereas only 15 of the 31 RCOs in G2-irradiated cells were conversion associated, a significant difference (P < 0.001 by Fisher's exact test).
By the nonparametric Mann-Whitney test, the tracts in the G1 samples were also significantly longer than those of the G2 samples (P < 0.001), although there was no significant difference in the lengths of the G1 and spontaneous tracts (P = 0.17). In contrast, by the same test, the G2 tracts were significantly shorter than the spontaneous tracts (P < 0.001). In summary, the G1-associated conversion tracts were longer than the G2-associated tracts, and G1-associated tracts were similar in size to those associated with spontaneous RCOs. In these comparisons, we included 4:0 and 3:1/4:0 hybrid tracts as single events. We also did the Mann-Whitney test, comparing G1 and G2 conversions, in which each 4:0 and hybrid tracts was counted as two events; the G1 tracts were still significantly longer than the G2 tracts (P < 0.001).
Distribution of Gamma-Radiation-Induced Crossovers and Conversion Events.
We previously showed that the distribution of spontaneous conversion events was nonrandom in the region between CAN1 and CEN5 (5). When this interval was divided into four quandrants (shown as thick dotted lines in Fig. 3), the quadrant near can1-100/SUP-o had significantly more events and the quadrant near CEN5 had significantly fewer events than expected for a random distribution. A similar analysis (chi-square analysis) of the G1- and G2-associated conversion events indicated no significant deviation from a random distribution. This result is not surprising because the distribution of gamma-radiation-induced DNA damage is likely to be random.
Discussion
Our main conclusion is that DNA damage induced by gamma radiation in G1-arrested yeast cells results in crossovers and gene conversion events that closely mimic the recombination events that occur spontaneously. Recombination events induced by irradiating G2-arrested cells are significantly different from spontaneous and G1-induced events. These results support our previous conclusion that many spontaneous mitotic recombination events reflect DNA damage occurring in unreplicated chromosomes.
Effect of Gamma Irradiation on Cell Viability and the Frequency of RCOs in Diploid Cells Irradiated in G1 and G2.
We found that 50 and 100 Gray (Gy) of gamma radiation had no significant effect on the viability of G2-arrested cells and only a small effect on the viability of G1-arrested cells. In most previous studies, the viability of G2-arrested cells is unaffected by doses of radiation of 100 Gy or less; the viability of G1-arrested cells is usually reduced about 2-fold by doses of 100 Gy (20–22). In agreement with these previous studies, gamma rays induced mitotic recombination in both G1- and G2-arrested cells. The level of induction in our study was about 30-fold at the same dose of radiation for both G1- and G2-arrested cells. In analyzing mitotic gene conversion between homologues (heteroallelic recombination), Fabre et al. (23) and Kadyk and Hartwell (21) observed very strong stimulation of conversion in G1-irradiated cells (about 100-fold) and only about 10-fold stimulation of conversion in G2-irradiated cells. These results were interpreted as indicating that sister chromatids were the preferred substrate for repair in G2 cells, reducing the frequency of heteroallelic conversion.
Our conclusion that DSBs generated in G1 and G2 result in RCOs with similar efficiencies is not necessarily in conflict with the previous studies (21, 23). In mitosis, less than 10% of gene conversion events are associated with crossing over (24). Conversion events unassociated with crossovers are not detected in our system. One interpretation of our results is that the repair of DSBs leading to RCOs is not preferentially between sister chromatids, unlike the repair of DSBs leading to conversions unassociated with RCOs. Thus, in mitosis, as in meiosis (25, 26), conversions unassociated with crossovers may involve a pathway different from conversions associated with crossovers.
Only a small fraction of DSBs is associated with RCOs. Because a dose of 800 Gy of radiation results in 250 DSBs in a G2-arrested diploid (16), we calculate that about 30% of the G2-arrested diploids treated with 100 Gy would have a DSB in the can1–CEN5 interval. Because the observed frequency of RCOs in the diploids irradiated in G2 at this dose was only about 4 × 10−4, we conclude that most (at least 99%) of the DSBs are repaired by a mechanism that does not lead to an RCO (for example, gene conversion without an associated RCO or sister chromatid crossovers). Although we cannot rule out a low frequency of nonhomologous end-joining (NHEJ) repair events, NHEJ is a minor pathway for the repair of radiation-induced DNA damage compared to homologous recombination (27).
Patterns of Gene Conversion Associated with RCOs.
The conversion events in the G1-irradiated cells resemble those previously observed associated with spontaneous RCOs. The 4:0 and 3:1/4:0 events (about 40% of the total) can be simply explained by repair of two DSBs resulting from the replication of a broken chromosome (Fig. 2 B and C). The 3:1 events in the G1-irradiated cells could be formed in two different ways. First, the 3:1 conversion event could reflect repair of a G1-associated DSB, if the repair of one DSB was associated with conversion of an adjacent marker, but the repair of the second DSB was not. Alternatively, because gamma rays induce DSBs:single-strand nicks:base damage, in a ratio of 1:20:40 (28), the 3:1 conversions could reflect a DSB generated by replicating a nicked DNA molecule. Single-stranded nicks are recombinogenic in yeast (29). Replication of nicked DNA molecules can give rise to a recombinogenic DSB (22, 30). In addition, nicked or gapped DNA molecules can stimulate conversion directly, without being converted to a DSB (31–33).
All of the simple gene conversions induced by irradiating G2-synchronized cells were 3:1 events. The most straightforward explanation of this result is that irradiation resulted in recombinogenic DSBs in only one of two sister chromatids. Repair of this DSB then resulted in a 3:1 conversion associated with the RCO (Fig. 2A).
Complex Conversion Events.
As described in Results, we found two classes of complex conversions (braced in Fig. 3 and Fig. S1). The class 1 events represent conversion in which the “wrong” sector is homozygous for the marker or in which there is a crossover within the conversion tract. As shown in Fig. 2A, because the conversion event is associated with a crossover, in a 3:1 conversion event in which the “red” alleles are donated, the red sector should be homozygous for the red allele and the white sector heterozygous. In the class 1 event depicted in Fig. 4, for the 3:1 portion of the hybrid tract, the red sector is heterozygous and the white sector is homozygous. This pattern of segregation is explicable as a consequence of the independent repair of two DSBs (Fig. 4). All of the class 1 sectored colonies can be explained by similar mechanisms (5).
Fig. 4.

Mechanism for generating a class 1 complex conversion event by a double repair event. As shown in Fig. 2A, if the event initiates on the SUP4-o-containing chromosome, the sector that is homozygous for SUP4-o will be heterozygous for the marker and the sector that is homozygous for can1-100 will be homozygous. In class 1 complex events, this expectation is violated, because the sector that is homozygous for SUP4-o is homozygous for the marker in the 3:1 portion of the hybrid tract. This pattern is readily explained by a double repair event of a G1-associated DSB. Repair of the first DSB is associated with the RCO and conversion of two markers. Repair of the second DSB is unassociated with a crossover but involves conversion of three markers, two on one side of the DSB and one on the other.
In class 2 events, the continuity of the gene conversion tract was interrupted by one marker that was not converted. Such a conversion tract could result from a heteroduplex including multiple markers (and, therefore, multiple mismatches) in which one mismatch was repaired in the opposite direction of the other mismatches. Such “patchy” events have been observed previously in studies of mitotic (34) and meiotic gene conversion (35).
Conversion Tract Sizes Associated with RCOs in Synchronized G1 and G2 Cells.
The conversion tracts in G1-irradiated cells were approximately two times longer than those in G2-irradiated cells. One explanation of this difference is that it reflects the kinetics of processing and repair of DSBs induced in G1 and G2. More specifically, because mitotic recombination in G1 cells is inefficient, the broken ends generated in G1 are likely to undergo extensive processing as cells enter S before recombination is initiated. In contrast, in G2 cells, the time interval between producing the DSB and initiating the recombination event would likely be short, leading to shorter gene conversion tracts.
From previous studies, it is unclear whether the length of conversion tracts has a simple relationship with the amount of resection. In a study of transformed linearized plasmids, mutations resulting in less resection had smaller conversion tracts (36), whereas, in experiments examining HO-induced recombination, mutations that increased resection did not result in longer conversion tracts (37). It should also be noted that 5′ to 3′ resection of a DNA molecule broken in G1, followed by replication, would result in two chromatids with asymmetric double-stranded gaps. By this model, the conversion of a G1-associated DSB (gap repair?) might be mechanistically different from conversion events of a G2-associated DSB (mismatch repair in a heteroduplex).
Mechanism of Spontaneous Mitotic Reciprocal Crossovers.
Our analysis supports the model for spontaneous mitotic RCOs shown in Fig. 2 B and C. We suggest that about half of spontaneous mitotic RCOs initiate by formation of a DSB on an unreplicated chromosome. This estimate is based on the observation that 40% of conversion tracts are 4:0 or 3:1/4:0 hybrid tracts. Because 3:1 conversion tracts can also be generated by a DSB formed in G1, this estimate is a minimum. The DSB could be formed in G1 (as shown) or in an unreplicated portion of a chromosome that has already initiated DNA synthesis. This broken chromosome is then replicated to yield two chromatids broken at the same position.
We suggest that the resulting broken ends are processed independently by either degradation of both DNA strands, producing a gap, or by processing of one strand 5′ to 3′. Although, in studies of HO-induced DSBs in yeast, nuclease degradation of only one of the two strands is observed (24, 38), degradation of both strands producing a gap has been recently observed (39). Depending on the extent of processing the four broken ends, the subsequent repair events can result in 3:1, 4:0, or 3:1/4:0 hybrid conversion tracts associated with the RCO. Alternatively, some fraction of spontaneous RCOs can be generated by a DSB formed in S (possibly as a consequence of a stalled DNA replication fork) and repaired in G2. The conversion events associated with this type of DSB would be expected to be exclusively of the 3:1 type (Fig. 2A). As argued above, the DNA ends of a G2-associated DSB would be quickly engaged in recombination, subjected to limited nuclease processing, and thus be associated with shorter conversion tracts than for G1-associated events.
There are several points to be mentioned. First, our conclusions are based on only a subset of mitotic recombination events, those that give rise to RCOs. Gene conversion events that are not associated with RCOs, sister chromatid exchanges, and nonhomologous end-joining events are not detected by our system. Second, we do not know the source of the G1-associated DSBs. These DSBs could reflect unrepaired DNA lesions generated by the action of topoisomerases, excision of closely spaced damaged bases located on different strands, or other types of DNA damage repair gone awry. Alternatively, a G1-initiated DSB could be mimicked by a chromosome broken in G2 that escaped repair and was segregated into a daughter cell. Because no 4:0 or hybrid conversion tracts are observed in G2-irradiated cells, however, this pathway is probably a minor (or nonexistent) one. Finally, since the experiments of Stern in 1936, most geneticists have assumed that mitotic crossovers occur in G2. The model shown in Fig. 2 B and C is completely consistent with Stern's observations, although not Stern's interpretation of his observations.
Materials and Methods
Description of the Diploid Yeast Strain PG311.
The construction of the diploid PG311 has been described previously (5). The genotype of PG311 is MATa/MATαΔ::NAT ade2-1/ade2-1 trp1-1/TRP1 ura3-1/URA3 can1-100/can1-Δ::SUP4-o gal2/GAL2 ho/ho::hisG. In addition, PG311 is heterozygous for an insertion of the gene encoding hygromycin resistance centromere distal to the can1-100 allele (V9229::HYG) and heterozygous for an insertion of LEU2 on the right arm of chromosome V (V261553::LEU2).
Media.
Rich growth medium [YPD (yeast extract, peptone, dextrose)] and omission media were made according to standard recipes (40), except that the omission medium contained only 10 μg/mL of adenine. The solid medium used to select for mitotic crossovers lacked arginine (SD −arg) and contained 120 μg/mL canavanine. The solid medium used as the control nonselective medium (SD −arg) contained no canavanine.
Synchronization and Irradiation of Cells in G1 and G2.
The PG311 strain was synchronized in G1 using alpha factor and in G2 using nocodazole. Cells were irradiated with a Shepherd Mark 1 Cesium-137 irradiator. The details of the synchronization and irradiation procedures are in SI Materials and Methods.
Genetic and Physical Analysis of Irradiated Synchronized Cells.
We monitored the rate of RCOs by measuring the frequency of CanR red/white sectored colonies (5). We purified cells from the red and white sectors and determined whether they were homozygous or heterozygous for the 34 restriction fragment-length polymorphisms used to map crossovers and conversions. This analysis involved generating a PCR fragment containing the polymorphism and treating the fragment with the relevant restriction enzyme. Details of the procedure are in SI Materials and Methods and Lee et al. (5).
Statistical Analyses.
Depending on the nature of the comparison, we used Fisher's exact test, the goodness of fit χ2 test, or the Mann-Whitney test on the VassarStats Website (http://faculty.vassar.edu/lowry/VassarStats.html). Calculations of confidence intervals of the median were made using table B11 of Altman (41).
Supplementary Material
Acknowledgments
We thank all members of the Petes laboratory, S. Jinks-Robertson, L. Symington, J. Westmoreland, and D. Gordenin for useful discussions and/or comments about the manuscript. The research was supported by National Institutes of Health Grants GM24110 and GM52319.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1001940107/DCSupplemental.
References
- 1.Aguilera A, Rothstein R. Molecular Genetics of Recombination. Berlin: Springer; 2007. [Google Scholar]
- 2.Stern C. Somatic crossing over and segregation in Drosophila melanogaster. Genetics. 1936;21:625–730. doi: 10.1093/genetics/21.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones EW, Fink GR. In: The Molecular Biology of the Yeast Saccharomyces: Metabolism and Gene Expression. Strathern JN, Jones EW, Broach JR, editors. Plainview, NY: Cold Spring Harbor Lab Press; 1982. pp. 181–299. [Google Scholar]
- 4.Barbera MA, Petes TD. Selection and analysis of spontaneous reciprocal mitotic cross-overs in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2006;103:12819–12824. doi: 10.1073/pnas.0605778103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee P, et al. A fine-structure map of spontaneous mitotic crossovers in the yeast Saccharomyces cerevisiae. PLoS Genet. 2009;5:e1000410. doi: 10.1371/journal.pgen.1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petes TD, Malone RE, Symington LS. In: The Molecular and Cellular Biology of the Yeast Saccharomyces. Broach JR, Jones EW, Pringle JR, editors. Plainview, NY: Cold Spring Harbor Lab Press; 1991. pp. 407–521. [Google Scholar]
- 7.Wildenberg J. The relation of mitotic recombination to DNA replication in yeast pedigrees. Genetics. 1970;66:291–304. doi: 10.1093/genetics/66.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabre F. Induced intragenic recombination in yeast can occur during the G1 mitotic phase. Nature. 1978;272:795–798. doi: 10.1038/272795a0. [DOI] [PubMed] [Google Scholar]
- 9.Esposito MS. Evidence that spontaneous mitotic recombination occurs at the two-strand stage. Proc Natl Acad Sci USA. 1978;75:4436–4440. doi: 10.1073/pnas.75.9.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aylon Y, Liefshitz B, Kupiec M. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J. 2004;23:4868–4875. doi: 10.1038/sj.emboj.7600469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ira G, et al. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature. 2004;431:1011–1017. doi: 10.1038/nature02964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lisby M, Rothstein R, Mortensen UH. Rad52 forms DNA repair and recombination centers during S phase. Proc Natl Acad Sci USA. 2001;98:8276–8282. doi: 10.1073/pnas.121006298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunborg G, Resnick MA, Williamson DH. Cell-cycle-specific repair of DNA double strand breaks in Saccharomyces cerevisiae. Radiat Res. 1980;82:547–558. [PubMed] [Google Scholar]
- 14.Takata M, et al. Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J. 1998;17:5497–5508. doi: 10.1093/emboj/17.18.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bucking-Throm E, Duntze W, Hartwell LH, Manney TR. Reversible arrest of haploid yeast cells in the initiation of DNA synthesis by a diffusible sex factor. Exp Cell Res. 1973;76:99–110. doi: 10.1016/0014-4827(73)90424-2. [DOI] [PubMed] [Google Scholar]
- 16.Argueso JL, et al. Double-strand breaks associated with repetitive DNA can reshape the genome. Proc Natl Acad Sci USA. 2008;105:11845–11850. doi: 10.1073/pnas.0804529105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Judd SR, Petes TD. Physical lengths of meiotic and mitotic gene conversion tracts in Saccharomyces cerevisiae. Genetics. 1988;118:401–410. doi: 10.1093/genetics/118.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borts RH, Haber JE. Length and distribution of meiotic gene conversion tracts and crossovers in Saccharomyces cerevisiae. Genetics. 1989;123:69–80. doi: 10.1093/genetics/123.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mancera E, Bourgon R, Brozzi A, Huber W, Steinmetz LM. High-resolution mapping of meiotic crossovers and non-crossovers in yeast. Nature. 2008;454:479–485. doi: 10.1038/nature07135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bronborg G, Williamson DH. The relevance of the nuclear division cycle to radiosensitivity in yeast. Mol Gen Genet. 1978;162:277–286. doi: 10.1007/BF00268853. [DOI] [PubMed] [Google Scholar]
- 21.Kadyk LC, Hartwell LH. Sister-chromatids are preferred over homologs as substrates for recombinational repair in Saccharomyces cerevisae. Genetics. 1992;132:387–402. doi: 10.1093/genetics/132.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galli A, Schiesl RH. Cell division transforms mutagenic lesions into deletion-recombinogenic lesions in yeast cells. Mutat Res. 1999;429:13–26. doi: 10.1016/s0027-5107(99)00097-4. [DOI] [PubMed] [Google Scholar]
- 23.Fabre F, Boulet A, Roman H. Gene conversion at different points in the mitotic cycle of Saccharomyces cerevisiae. Mol Gen Genet. 1984;195:139–143. doi: 10.1007/BF00332736. [DOI] [PubMed] [Google Scholar]
- 24.Paques F, Haber JE. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allers T, Lichten M. Differential timing and control of noncrossover and crossover recombination in meiosis. Cell. 2001;106:47–57. doi: 10.1016/s0092-8674(01)00416-0. [DOI] [PubMed] [Google Scholar]
- 26.Borner GV, Kleckner N, Hunter N. Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition transition in meiosis. Cell. 2004;117:29–45. doi: 10.1016/s0092-8674(04)00292-2. [DOI] [PubMed] [Google Scholar]
- 27.Siede W, Friedl AA, Dianova I, Eckardt-Schupp F, Friedberg EC. The Saccharomyces cerevisiae Ku autoantigen homologue affects radiosensitivity only in the absence of homologous recombination. Genetics. 1996;142:91–102. doi: 10.1093/genetics/142.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powell S, McMillan TJ. DNA damage and repair following treatment with ionizing radiation. Radiother Oncol. 1990;19:95–108. doi: 10.1016/0167-8140(90)90123-e. [DOI] [PubMed] [Google Scholar]
- 29.Strathern JN, Weinstock KG, Higgins DR, McGill CB. A novel recombinator in yeast based on gene II protein from bacteriophage f1. Genetics. 1991;127:61–73. doi: 10.1093/genetics/127.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cortés-Ledesma F, Aguilera A. Double-strand breaks arising by replication through a nick are repaired by cohesin-dependent sister-chromatid exchange. EMBO Rep. 2006;7:919–926. doi: 10.1038/sj.embor.7400774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fabre F, Chan A, Heyer WD, Gangloff S. Alternative pathways involving Sgs1/Top3, Mus81/Mms4, and Srs2 prevent formation of toxic recombination intermediates from single-stranded gaps created by DNA replication. Proc Natl Acad Sci USA. 2002;99:16887–16892. doi: 10.1073/pnas.252652399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lettier G, et al. The role of DNA double-strand breaks in spontaneous homologous recombination in S. cerevisiae. PLoS Genet. 2006;2:1–14. doi: 10.1371/journal.pgen.0020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mozlin AM, Fung CW, Symington LS. Role of the Saccharomyces cerevisiae Rad51 paralogs in sister chromatid recombination. Genetics. 2008;178:113–126. doi: 10.1534/genetics.107.082677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nickoloff JA, Sweetser DB, Clikeman JA, Khalsa GJ, Wheeler SL. Multiple heterologies increase mitotic double-strand break-induced allelic gene conversion tracts in yeast. Genetics. 1999;153:665–679. doi: 10.1093/genetics/153.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Symington LS, Petes TD. Expansions and contractions of the genetic map relative to the physical map of yeast chromosome III. Mol Cell Biol. 1988;8:595–604. doi: 10.1128/mcb.8.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Symington LS, Kang LE, Moreau S. Alteration of gene conversion tract length and associated crossing over during plasmid gap repair in nuclease-deficient strains of Saccharomyces cerevisiae. Nucleic Acids Res. 2000;28:4649–4656. doi: 10.1093/nar/28.23.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishna S, et al. Mre11 and Ku regulation of double-strand break repair by gene conversion and break-induced replication. DNA Repair (Amst) 2007;6:797–808. doi: 10.1016/j.dnarep.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mimitou EP, Symington LS. DNA end resection: Many nucleases make light work. DNA Repair (Amst) 2009;8:983–995. doi: 10.1016/j.dnarep.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zierhut C, Diffley JFX. Break dosage, cell cycle stage and DNA replication influence DNA double strand break response. EMBO J. 2008;27:1875–1885. doi: 10.1038/emboj.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guthrie C, Fink GR. Guide to Yeast Genetics and Molecular Biology. San Diego: Academic; 1991. [Google Scholar]
- 41.Altman DG. Practical Statistics for Medical Research. New York: CRC; 1990. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.