Abstract
A large body of literature suggests that motor sequence learning involves dopamine-modulated plastic processes in the basal ganglia. Sequence learning can occur both implicitly, without conscious awareness and intention to learn, and explicitly, i.e., under conscious control. Here, we investigated whether individual differences in implicit and explicit sequence learning of movement sequences in a group of 15 healthy participants are related to dopamine D2 receptor densities in functional subregions of the striatum. Sequence learning was assessed using the serial reaction time task, and measures of implicit and explicit knowledge were estimated using a process dissociation procedure. Correlation analyses were performed between these measures and D2 receptor densities, which had been measured previously with positron emission tomography. Striatal D2 densities were negatively related to measures of sequence learning. In the limbic subregion, D2 densities were specifically related to implicit but not explicit learning. These findings suggest that individual differences in striatal DA function underlie differences in sequence learning ability and support that implicit and explicit sequence learning depend on partly distinct neural circuitry. The findings are also in line with the general view that implicit learning systems are evolutionarily primitive and tend to rely more on phylogenetically old neural circuitry than does explicit learning and cognition.
Keywords: basal ganglia, PET, plasticity, procedural learning
A large body of literature has shown that movement sequence learning can be either explicit, that is under conscious control, or implicit, in which case the learning is nonintentional and results in knowledge that is inaccessible to consciousness (1). Dissociating these two forms of learning is difficult, because most learning measures can be influenced by both implicit and explicit knowledge to an unknown degree (2). One approach to this problem is to use a process dissociation procedure (PDP) (3), where performances in two generation tasks are compared: in one task, explicit and implicit knowledge both facilitate performance; in the other task, explicit knowledge facilitates performance, and implicit knowledge acts as a source of interference.
Many brain regions are activated during sequence learning (4–6). However, in studies of implicit sequence learning, basal ganglia activity is a highly consistent finding. PDP studies have specifically related striatal activity to the amount of implicit knowledge obtained (7). Basal ganglia mechanisms for motor learning, including sequence learning, appear to involve dopamine (DA)-modulated neuronal plasticity (8–11). For example nigrostriatal DA depletion impairs sequence learning in monkeys (12). Furthermore, sequence learning in humans is facilitated by the indirect DA agonist d-amphetamine and impaired by the DA antagonist haloperidol (13).
Recently, DA involvement in human sequence learning has been studied using PET. Badgaiyan et al. (14, 15) found a reduction of D2 receptor binding in the caudate nucleus and the putamen, during both implicit and explicit sequence learning tasks, indicating endogenous DA release. In contrast, Garraux et al. (16) found that lower pallidal DA release was related to faster sequence learning. It appears likely that a clearer picture of the roles of basal ganglia DA systems in implicit and explicit sequence learning could emerge if functional subregions of the striatum were studied separately. These have different connectivity (17), and it was recently shown (18) that correlations between striatal D2 receptor densities and cognitive abilities differ between functional subregions.
We studied implicit and explicit sequence learning using a serial reaction time task (SRTT) (19), followed by a PDP, in a participant group where D2 receptor densities had previously been measured with PET. The aim was to test whether functional subregions of the striatum are differently associated with measures of implicit and explicit sequence knowledge. For exploratory purposes we also investigated relations between sequence learning and D2 receptor densities in extrastriatal regions.
Results
Behavioral Data.
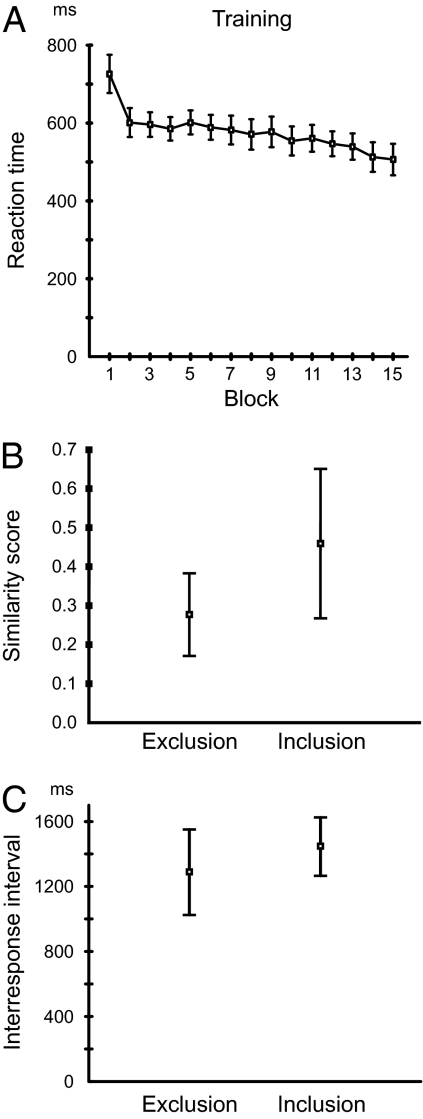
Mean performance in the SRTT for the whole group is shown in Fig. 1A. The learning curves show the mean RT of each training block, across all participants, as a function of block number (Block). In a repeated-measures ANOVA with mean RT as dependent variable and Block (1–15) as independent variable, a significant main effect was seen for Block [F(1, 14) = 13.56; P < 0.0001]. The mean number of errors per block was 2.17 ± 1.87 (SD).
Fig. 1.
Behavioral data. Squares represent mean values, and whiskers represent the SEM for all participants (n = 15). (A) Performance during the serial reaction time task. Mean reaction time across all participants is shown as a function of training block number. (B) Performance in the generation tasks. Similarity scores for both the Inclusion and the Exclusion task are depicted. Inclusion scores were significantly higher than Exclusion scores (P < 0.0016). (C) Interresponse intervals in the generation tasks. No significant difference in mean interresponse interval was found between Inclusion and Exclusion.
Similarity scores (Materials and Methods) for the two generation tasks (Inclusion and Exclusion) are shown in Fig. 1B. Inclusion scores were significantly higher than Exclusion scores [t(14) = 3.89; P < 0.0016], i.e., sequences produced in Inclusion were more similar to the trained sequence that those produced in Exclusion. A single-sample t test was used to compare Inclusion and Exclusion scores with a baseline of 0.33, which would correspond to performance at chance level. Inclusion scores were significantly higher than baseline [t(14) = 2.59; P < 0.021], whereas a trend was found for Exclusion scores to be lower than baseline [t(14) = −1.92; P < 0.075]. Finally, we compared the interresponse intervals between responses in Inclusion and Exclusion (Fig. 1C). No significant difference in interrresponse interval was found [t(14) = 1.13; P = 0.28].
On the verbal question, 13 participants believed that the stimuli followed a sequence (answer 1), whereas two participants believed that the sequence was just sometimes following a pattern (answer 2). No one believed that the sequence was always random. These findings suggest that, on a group level, participants had acquired implicit as well as explicit sequence knowledge. Estimates of obtained implicit and explicit knowledge, i.e., I and E scores, were calculated from performance in the inclusion and exclusion tasks according to a standard procedure (Materials and Methods). These I and E scores were used in the subsequent correlation analyses.
Correlations Between Sequence Learning and Regional D2BP.
The D2 binding potential (D2BP) in striatal regions measured with [11C]raclopride and extrastriatal regions measured with [11C]FLB 457 showed both interregional and interindividual variability. Regional binding potentials for both ligands are summarized in Table 1.
Table 1.
D2 receptor binding potentials for all ROIs
| Measure | Mean | SD | Range |
| Limbic subregion | 2.00 | 0.19 | 1.80–2.42 |
| Associative subregion | 2.60 | 0.17 | 2.25–2.94 |
| Sensorimotor subregion | 3.01 | 0.28 | 2.52–3.69 |
| Thalamus | 2.58 | 0.47 | 1.86–3.28 |
| Insula | 1.14 | 0.22 | 0.82–1.54 |
| Anterior cingulate cortex | 0.66 | 0.23 | 0.28–1.13 |
| Frontal cortex | 0.39 | 0.14 | 0.21–0.64 |
| Dorsolateral prefrontal cortex (DLPFC) | 0.34 | 0.13 | 0.14–0.60 |
| Orbitofrontal cortex (OFC) | 0.42 | 0.19 | 0.21–0.75 |
| Medial frontal cortex (MFC) | 0.48 | 0.15 | 0.26–0.74 |
| Medial temporal lobe | 1.50 | 0.32 | 1.03–2.17 |
| Hippocampus | 0.92 | 0.25 | 0.57–1.41 |
| Amygdala | 2.71 | 0.79 | 1.47–4.09 |
The mean, SD, and range are given for each ROI.
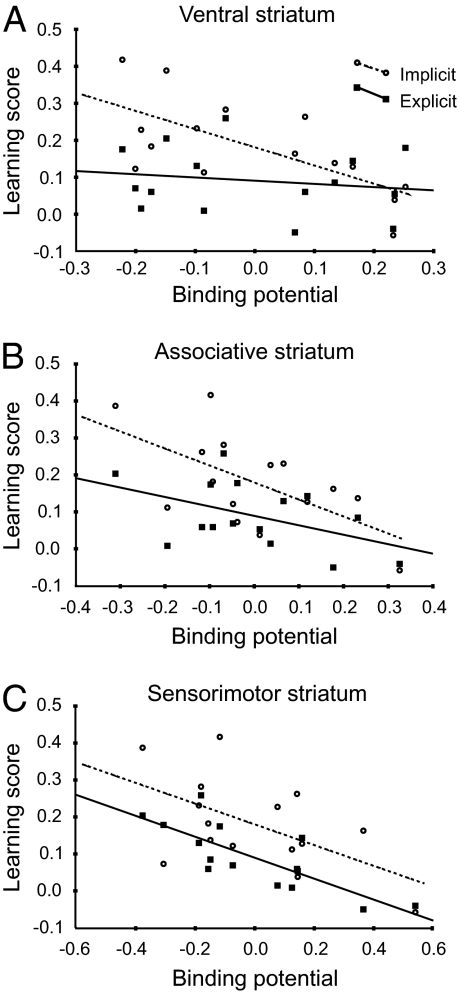
The striatum was divided into subregions (Materials and Methods) using functionally defined regions of interest (limbic, associative, and sensorimotor). In those functional subregions, different patterns of correlations with implicit and explicit learning were found (Fig. 2 A–C and Table 2). After Bonferroni correction D2BP in the limbic striatum showed a significant negative correlation with I (r = –0.68) but not with E (r = –0.18; Fig. 2A). D2BP in the associative striatum and the sensorimotor striatum showed negative correlations for both E and I, but only the correlation between D2BP and E in the sensorimotor striatum was statistically significant after Bonferroni correction (r = –0.82; Fig. 2C). For the correlations between D2BP in the sensorimotor striatum and I (r = –0.56), as well as for those between D2BP in the associative striatum and I and E (r = –0.61 and r = –0.49, respectively; Fig. 2B), a negative trend was observed (Table 2). A two-tailed Hotelling–Williams test was applied to compare the correlation coefficients of the E × D2BP and the I × D2BP correlations in the same striatal subregion. This analysis confirmed that for the limbic striatum, I had a significantly stronger relation to D2BP than had E [t(12) = 2.76, P = 0.009]. For the other subregions, no significant difference in r values was observed. No statistically significant correlations were observed between I or E scores and D2BP in extrastriatal regions (Table 3), or between error rate and D2BP in any region.
Fig. 2.
Explicit and implicit learning as a function of D2 receptor binding potential. Data are shown for the limbic (A), associative (B), and sensorimotor (C) subregion of the striatum. Correlation statistics are controlled for age and ROI volume.
Table 2.
Relationships of D2 binding potential in the striatum to implicit and explicit learning scores
| Region | Implicit learning score | Explicit learning score | ||
| Correlation r | P value | Correlation r | P value | |
| Limbic striatum | −0.68* | 0.007 | −0.18 | 0.55 |
| Associative striatum | −0.61 | 0.022 | −0.49 | 0.076 |
| Sensorimotor striatum | −0.56 | 0.038 | −0.82* | 0.000 |
*Correlations that were significant at a family alpha of 0.05, after Bonferroni correction (three tests, individual alpha 0.0167).
Table 3.
Relationships of D2 binding potential in extrastriatal regions to implicit and explicit learning scores
| Region | Implicit learning score | Explicit learning score | ||
| Correlation r | P value | Correlation r | P value | |
| Thalamus | 0.31 | 0.28 | −0.099 | 0.74 |
| Insula | −0.075 | 0.80 | −0.35 | 0.22 |
| Anterior cingulate cortex | −0.42 | 0.13 | −0.36 | 0.20 |
| Frontal cortex | 0.08 | 0.78 | −0.25 | 0.38 |
| Dorsolateral prefrontal cortex | 0.24 | 0.41 | −0.06 | 0.83 |
| Orbitofrontal cortex | −0.089 | 0.76 | −0.34 | 0.23 |
| Medial frontal cortex | −0.15 | 0.60 | −0.43 | 0.12 |
| Medial temporal lobe | −0.38 | 0.18 | −0.33 | 0.24 |
| Hippocampus | −0.12 | 0.68 | −0.35 | 0.22 |
| Amygdala | −0.43 | 0.13 | −0.18 | 0.54 |
No significant correlations were observed.
Discussion
We present correlations between D2BP and measures of sequence learning, estimated by the PDP. The validity of the PDP as a method to estimate sequence knowledge after SRTT training is supported by a number of studies showing that the PDP consistently detects differences in acquired explicit knowledge caused by the manipulation of various task conditions that affect sequence awareness (20, 21). We assume in the following that the correlational findings reveal regional differences in dopaminergic function that are related to differences in implicit and explicit sequence learning. However, our analysis pertains only to brain regions involved in individual differences in behavior. It is possible, of course, that dopaminergic systems in additional brain regions are implicated in sequence learning per se, but that these regions show little behaviorally relevant interindividual variability.
Striatal Subregions Have Specific Roles for Implicit and Explicit Sequence Learning.
The main finding of this study was that implicit and explicit sequential learning correlate differently with dopamine D2BP in functional subregions of the striatum. Specifically, D2BP in the limbic striatum showed a significant correlation only with implicit learning, and the magnitude of this correlation was significantly higher than the correlation between explicit learning and D2BP in the same region. This provides further support for the view that partly distinct processes in the human brain support implicit and explicit sequential learning.
Functional subregions of the striatum differ in their pattern of cortical projections. The sensorimotor striatum receives projections from the primary sensorimotor areas and the supplementary motor area (17). The associative striatum, defined as the precommissural putamen and the dorsal caudate nucleus, has afferents from frontal and parietal association areas (17). The limbic striatum has strong connections with orbitofrontal cortex and the amygdala (22). The present findings suggest that individual differences in dopaminergic mechanisms in the limbic subregion are relatively selectively related to differences in implicit sequence learning. Dopaminergic mechanisms in the sensorimotor and associative striatum, in contrast, may be related to both explicit and implicit sequence learning.
Reber (23, 24) has suggested that basic neural circuits for implicit learning developed earlier in evolution than did systems for explicit learning. On this view, implicit learning systems in general tend to involve phylogenetically old parts of the nervous system, to appear early in ontogeny, to be relatively robust in the face of psychological or neurological disorder, and to show weak, if any, relations to general intelligence and other cognitive abilities. Explicit learning systems, in contrast, develop late, typically show major impairments in neurological or psychiatric disease, and are substantially correlated with cognitive ability. Notably, the limbic subregion is the phylogenetically oldest subregion of the striatum, present even in primitive vertebrates such as the lamprey (25). The specific correlation found between the limbic striatum and implicit learning thus appears to fit a general evolutionary perspective on implicit and explicit systems: within the striatum, individual differences in the oldest subregions are apparently more related to differences in implicit than explicit sequence learning. However, both implicit and explicit sequence learning clearly also involve partly overlapping neocortical circuitry (see, e.g., 26, 27).
The limbic striatum has a central role in the processing of reward (28, 29). In the present paradigm there was no extrinsic reward present. Yet, it appears possible that in our human participants, the instruction to perform as fast and accurately as possible created sufficient incentive motivation to involve DA mechanisms in the limbic striatum. It could also be speculated that gradual improvements in performance, e.g., decreases in reaction time, served as an implicit positive feedback signal that made the task intrinsically rewarding. Because the limbic striatum communicates mainly with orbitofrontal rather than sensorimotor cortical regions (22), it appears likely that this subregion is more directly involved in the processing of motivational signals than in the learning of sequential associations between stimuli or responses. However, the limbic corticostriatal loop can exert a powerful influence on sensorimotor and associative corticostriatal loops through striatonigral and thalamocortical projections (28, 29).
In our study, both implicit and explicit learning correlated negatively with D2BP in the associative and sensorimotor striatum. All correlations were relatively high even though only the correlation between explicit learning and D2BP in the sensorimotor striatum survived Bonferroni correction. However, for neither of these two striatal subregions was there a significant difference between the strengths of the correlations with implicit and explicit learning. This suggests that both the associative and the sensorimotor striatum are involved in implicit as well as explicit learning. There is ample evidence from both human and animal studies (10, 29–31) that early and advanced stages of motor sequence learning depend on independent circuits in the basal ganglia. Early learning stages are thought to involve mainly the associative striatum whereas later stages are dependent on the sensorimotor striatum. To investigate this, an experimental paradigm measuring differences in D2BP during different stages of a sequence learning task may be informative.
Why Did D2BP and Sequence Learning Show a Negative Correlation?
The majority of earlier studies have found positive relations between D2BP and various cognitive abilities (18, 32). It may appear counterintuitive, therefore, that all correlations found in the present study were negative, i.e., more sequence knowledge was related to lower D2BP. We would like to make two comments in relation to this point.
First, because D2BP is a function of both receptor density and apparent affinity, it impossible to dissociate these parameters based on a single PET measurement (33). For [11C]raclopride, the binding has been shown to be sensitive to endogenous dopamine levels (34). Thus, low binding potential here could in principle reflect both the density of D2 receptors and the baseline concentration of DA. Thus, it cannot be excluded that individuals having low D2BP and high performance in motor learning have high concentration of endogenous DA in the striatum. The use of DA-release paradigms, in particular with agonist radioligands sensitive to high- and low-affinity states of DA receptors (35), could aid in disentangling the effect of endogenous DA on sequence learning.
Second, DA is likely to play different roles for implicit motor learning and cognitive functions. As suggested previously, implicit learning systems tend to rely less on systems involved in higher cognitive functions than do explicit learning systems. Accordingly, the correlations between implicit learning and cognitive abilities (23, 36–38) are very weak. Explicit learning, in contrast, is substantially correlated with general intelligence (36–38). In relation to the present findings, as well as the negative relationship between striatal DA release and sequence learning found by Garraux et al. (16), it should also be emphasized that there is no simple relation between activity in the DA system and motor learning. Rather, recent findings show that plastic processes such as long-term potentiation and long-term depression in the basal ganglia can be both facilitated and inhibited in a complex and subtle way by DA, acting through D1 as well as D2 receptors (39).
Materials and Methods
Participants.
The participants recruited for the present study were control subjects in a previous study on Restless Leg Syndrome (40). Fifteen subjects (7 male, 8 female) agreed to participate. The age range was 41–65 years (56 ± 8; mean ± SD). Mean years of education were 15 ± 4 years. Subjects had no history of significant psychiatric or somatic illness. None of the subjects were nicotine users, and they were required to abstain from products containing caffeine or alcohol during the days of PET examinations. All subjects gave verbal and written informed consent and the study was approved by the Ethics and Radiation Safety committees of Karolinska Institutet (Dnr. 2007/704–31/4, 02–431).
MR and PET Experimental Procedure.
MR and head fixation system.
MR images were acquired using a 1.5T GE Signa system with T1- and T2-weighted protocols. T2-weighted images were examined for structural pathology at subject inclusion. To allow for the same head position in all measurements and to minimize head movement, a plaster helmet was made for each subject individually and used during both MRI and PET examinations (41). T1-weighted images were reconstructed using a 256 × 256 × 156 matrix with an original resolution of 1.02 × 1.02 × 1 mm3 and were used for the subsequent data analysis. For three subjects the original z-axis resolution was 1.2 mm and in one case 1.5 mm, due to temporary problems with the MRI scanner.
PET examinations.
PET studies were performed on an ECAT Exact HR system (CTI Siemens) run in 3D mode (42). The transaxial resolution of this system is 3.8 mm full width at half maximum (FWHM) at the center of the field of view, 4.5 mm FWHM tangentially, and 7.4 mm radially at a distance of 20 cm from the center. The axial resolution is 4 mm FWHM at the center and 6.8 mm, 20 cm from the center. Before each emission scan, a transmission scan of 10 min was performed using three rotating 68Ge–68Ga sources. Data from the transmission scan was used for attenuation correction.
[11C]Raclopride and [11C]FLB 457 were prepared from [11C]methyltriflate as described previously (43, 44). The radioligands were administered i.v. as a rapid bolus and the cannula was flushed with saline. Radioactivity in the brain was measured during 51 min for [11C]raclopride and 87 min for [11C]FLB 457 by a consecutive sequence of frames (3 × 1, 4 × 3, 6 × 6 and 3 × 1, 4 × 3, 12 × 6 min, respectively). The images were reconstructed using a Hann filter (2 mm FWHM).
Image processing and analysis.
The MR images were realigned to the AC–PC plane using the SPM2 software (Wellcome Department of Imaging Neuroscience, Institute of Neurology, London) and resliced to a resolution of 2 × 2 × 2 mm3. For each subject, both PET images were coregistered to the resliced MR image using the normalized mutual information method implemented in SPM2 (45). During the process of reslicing, the PET images were resampled to have the same resolution as the MR images, to minimize loss of information. For determination of regional radioligand binding, regions of interest (ROIs) were manually delineated on each individual MR image using Human Brain Atlas software (46).
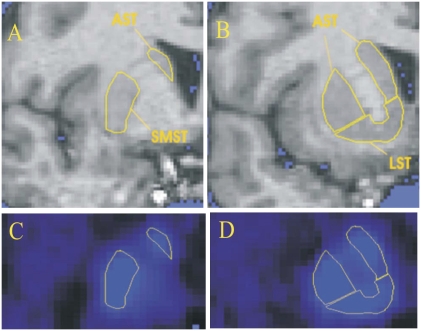
For [11C]raclopride examinations, striatal ROIs were defined as functional subregions according to a method described previously (47, 48) in which striatum is divided into limbic, associative, and sensorimotor subregions based on differential connectivity (17). This approach has previously been used to study D2-receptor binding in relation to cognitive performance (18). [11C]FLB 457 provides a signal for a series of cortical and subcortical extrastriatal regions. In the present study, the selection of ROIs for [11C]FLB 457 examinations was guided by earlier studies that identified extrastriatal regions as being activated in sequence learning paradigms: thalamus (26, 49, 50), insula (7, 26), anterior cingulate cortex (7, 26, 51), medial temporal lobe (50, 52), dorsolateral prefrontal cortex (26, 50, 51), and medial frontal cortex (7, 27). For exploratory purposes, the orbitofrontal cortex, as well as separate ROIs for the hippocampus and the amygdala within the medial temporal lobe, were also investigated. ROIs for the thalamus were defined using a modified version of a procedure described previously (53–55). Finally, a ROI for cerebellum was drawn below the petrosal bone using five slices, corresponding to a thickness of 10 mm. For an example of ROI delineation, see Fig. 3.
Fig. 3.
Regions of interest. (A and B) Coronal MRI sections depicting manually drawn ROIs in the right striatum in one subject, posterior and anterior to the anterior commisure, respectively. The lateral direction is to the left in the images. (C and D) Corresponding PET sections showing [11C]raclopride binding in the same subject, with the ROIs superimposed. LST, limbic striatum; AST, associative striatum; SMST, sensorimotor striatum.
The ROIs were transferred to the series of PET images to generate time-activity curves (TACs). This was done for each subregional ROI individually, on both the right and the left side. Spatially weighted averages of the original curves were then calculated to create TACs for larger regions. D2 receptor binding potential (D2BP) values were calculated using the simplified reference tissue model with the cerebellar TAC as input function (56). This approach has previously been found suitable for both [11C]raclopride and [11C]FLB 457 (56–58). D2BP in this context represents the ratio of receptor density (Bmax) and apparent affinity (Kd) (33).
Behavioral Measurements.
Stimulus presentation and data collection were controlled by a script written in the E-Prime software package (Psychological Software Tools, Inc.). All stimuli were presented on a computer monitor and designed using CorelDraw 11 (Corel, Inc.). Responses were collected from the numerical keypad of the PC keyboard. Processing of behavioral data was performed in MatLab 7 (MathWorks, Inc.), and all statistical analyses were made in Statistica 7.1 (StatSoft, Inc.), except for the Hotelling–Williams test, which was implemented in MatLab 7. During the experiment, the participants were seated in front of the computer, at a distance of around 60 cm from the screen. The following sequence was used in the training: 3-4-2-3-1-2-1-4-3-2-4-1. This sequence is a 12-element second-order conditional sequence, i.e., each element (1, 2, 3, or 4) is uniquely predicted by the two preceding elements, but in no case by the immediately preceding element alone. There are no repetitions of elements, each element occurs three times, and all possible first-order transitions occur once (for a discussion of sequence properties, see ref. 59).
Experimental procedure.
Behavioral testing was performed approximately 1 year after the PET scan. The experiment consisted of a serial reaction time task (SRTT) (19) followed by two tests of explicit knowledge: first, a verbal question on stimulus regularities and, second, two generation tasks that were analyzed using a process dissociation procedure (PDP).
The SRTT was composed of 15 training blocks during which participants were exposed to consecutive four-choice reaction time (RT) trials. Participants were told to respond to the stimuli as quickly and accurately as possible. No mention was made to the participant of the sequential structure of the stimuli. Each block consisted of 96 trials, each including eight repetitions of the 12-item target sequence, giving a total of 1,440 total trials or 120 sequence repetitions. Throughout a training block, four squares—corresponding to sequence elements 1, 2, 3, and 4—were presented in a horizontal arrangement along the middle of the computer monitor. On each trial, a stimulus in the form of a small drawing of a mouse appeared in one of the squares and remained until the participant pressed the correct key. Four different response buttons—the F1 to F4 keys of the keyboard—were used, corresponding to the four stimulus locations. As soon as the participant gave the correct response, the next stimulus appeared; thus the response to stimulus interval was 0 ms. Erroneous responses were signaled to the participant by means of a tone. Procedural learning in the SRTT is commonly examined by including a final training block, where the stimulus order either follows a nontrained control sequence or is random (see, e.g., ref. 60). Because we used a PDP to estimate implicit and explicit sequence knowledge, and because exposing participants to a different sequence before the PDP might interfere with obtained sequence knowledge, we chose not to include a final control block in the SRTT.
After completion of the SRTT, a multiple-choice question on the perceived regularity of the stimuli was presented on the computer screen. The participant was asked to choose which of the three following alternatives best characterized the pattern of stimuli: 1, “the pattern was always predictable”; 2, “the pattern was sometimes predictable”; and 3, “the pattern was always random.”
Thereafter, the participant was informed that the stimuli had followed a repeating sequence. Finally, the participant was instructed to perform the two generation tasks. In the Inclusion task, the instruction was to generate sequences that were similar to the pattern used in SRTT. In the Exclusion task, the participant was asked to generate sequences, but to try not to include the pattern used in the SRTT. Participants were instructed to generate different sequences and not to use repetitions. Each generation task was interrupted after 96 trials, i.e., eight sequences of 12 elements. The order of the two generation tasks was randomized.
Data analysis.
The mean reaction time in each training block was used as a measure of procedural learning. Procedural learning was investigated by applying a repeated-measures ANOVA with RT as the dependent variable and Block as the independent variable.
Estimates of implicit and explicit sequence knowledge were calculated as in Destrebecqz and Cleeremans (20). First, the number of generated triplets (chunks of three consecutive elements) that were part of the SRTT sequence was counted for the Inclusion and Exclusion performances. Because the generated sequences were 96 elements long, the maximum number of correct triplets that could be generated in each task was 94. Hence, we divided the number of correct triplets by 94 to obtain a target sequence similarity measure. Expected similarity at random chance level is 0.33. An assumption of the study (see the introduction) is that both implicit and explicit knowledge act additively to increase similarity scores in Inclusion, whereas similarity scores in Exclusion are decreased by explicit knowledge and increased by implicit knowledge, which acts as interference in this task, i.e.:
Inclusion = I + E + Baseline, and
Exclusion = I – E + Baseline,
where Inclusion and Exclusion are similarity scores in the corresponding generation tasks, Baseline is expected similarity score during random performance (i.e., 0.33), and I and E are implicit and explicit learning scores, respectively. Note that good performers have a low similarity score in Exclusion, because the task is to avoid producing the target sequence. I and E scores were estimated from these equations.
Within-participant differences in similarity scores and interresponse intervals on the Inclusion and Exclusion tasks were investigated with t tests for dependent samples. To test whether similarity scores were higher than baseline (0.33), a single-sample t test was employed. Mean time intervals between responses (interresponse intervals) were also compared (t test for dependent samples) between Exclusion and Inclusion, because a significant difference in interresponse interval could indicate a difference in task difficulty. Relations between sequence learning and D2 receptor densities were examined by computing partial product–moment correlations between I and E scores and radioligand binding for each ROI, controlling for age and the ROI volume. Age was controlled for as D2-receptor availability has been shown to decline with age (61). To have a significance level of 0.05 (family alpha) after multiple comparisons, all correlations were Bonferroni corrected, i.e., an alpha of 0.0167 was used for the individual tests (n = 3). To statistically test whether the correlation between implicit learning and D2BP in a striatal subregion differed from the correlation between explicit learning and D2BP in the same subregion, we employed the Hotelling–Williams test for dependent overlapping correlations (62).
Acknowledgments
This work was supported by the Swedish Research Council, the Swedish Governmental Agency for Innovation Systems (Vinnova), and the Karolinska Institute/National Institutes of Health Graduate Partnership Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Stadler MA, Frensch PA, editors. Handbook of Implicit Learning. New York: Sage; 1997. [Google Scholar]
- 2.Shanks DR. Implicit learning. In: Lamberts K, Goldstone R, editors. Handbook of Cognition. Thousand Oaks, CA: Sage; 2005. pp. 202–220. [Google Scholar]
- 3.Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. J Mem Lang. 1991;30:513–541. [Google Scholar]
- 4.Hallett M. Motor learning. In: Freund H-J, Jeannerod M, Hallett M, Leiguarda R, editors. Higher-Order Motor Disorders: From Neuroanatomy and Neurobiology to Clinical Neurology. Oxford: Oxford Univ Press; 2005. pp. 123–140. [Google Scholar]
- 5.Ullén F. Neuropsychology of movement sequence learning. In: Eliasson A-C, Burtner PA, editors. Improving Hand Function in Children with Cerebral Palsy: Theory, Evidence and Intervention. London: Mac Keith; 2008. pp. 43–60. [Google Scholar]
- 6.Ashe J, Lungu OV, Basford AT, Lu X. Cortical control of motor sequences. Curr Opin Neurobiol. 2006;16:213–221. doi: 10.1016/j.conb.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Destrebecqz A, et al. The neural correlates of implicit and explicit sequence learning: Interacting networks revealed by the process dissociation procedure. Learn Mem. 2005;12:480–490. doi: 10.1101/lm.95605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graybiel AM. Building action repertoires: Memory and learning functions of the basal ganglia. Curr Opin Neurobiol. 1995;5:733–741. doi: 10.1016/0959-4388(95)80100-6. [DOI] [PubMed] [Google Scholar]
- 9.Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- 10.Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Curr Opin Neurobiol. 2002;12:217–222. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- 11.Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto N, Hanakawa T, Maki S, Graybiel AM, Kimura M. Role of nigrostriatal dopamine system in learning to perform sequential motor tasks in a predictive manner. J Neurophysiol. 1999;82:978–998. doi: 10.1152/jn.1999.82.2.978. [DOI] [PubMed] [Google Scholar]
- 13.Kumari V, et al. Effects of acute administration of d-amphetamine and haloperidol on procedural learning in man. Psychopharmacoyl. 1997;129:271–276. doi: 10.1007/s002130050190. [DOI] [PubMed] [Google Scholar]
- 14.Badgaiyan RD, Fischman AJ, Alpert NM. Striatal dopamine release in sequential learning. Neuroimage. 2007;38:549–556. doi: 10.1016/j.neuroimage.2007.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badgaiyan RD, Fischman AJ, Alpert NM. Explicit motor memory activates the striatal dopamine system. Neuroreport. 2008;19:409–412. doi: 10.1097/WNR.0b013e3282f6435f. [DOI] [PubMed] [Google Scholar]
- 16.Garraux G, Peigneux P, Carson RE, Hallett M. Task-related interaction between basal ganglia and cortical dopamine release. J Neurosci. 2007;27:14434–14441. doi: 10.1523/JNEUROSCI.1595-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: An analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451–474. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- 18.Cervenka S, Bäckman L, Cselényi Z, Halldin C, Farde L. Associations between dopamine D2-receptor binding and cognitive performance indicate functional compartmentalization of the human striatum. Neuroimage. 2008;40:1287–1295. doi: 10.1016/j.neuroimage.2007.12.063. [DOI] [PubMed] [Google Scholar]
- 19.Nissen MJ, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cognit Psychol. 1987;19:1–32. [Google Scholar]
- 20.Destrebecqz A, Cleeremans A. Can sequence learning be implicit? New evidence with the process dissociation procedure. Psychon Bull Rev. 2001;8:343–350. doi: 10.3758/bf03196171. [DOI] [PubMed] [Google Scholar]
- 21.Fu QF, Fu XL, Dienes Z. Implicit sequence learning and conscious awareness. Conscious Cogn. 2008;17:185–202. doi: 10.1016/j.concog.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Reber AS, Walkenfeld FF, Hernstadt R. Implicit and explicit learning: Individual differences and IQ. J Exp Psychol Learn Mem Cogn. 1991;17:888–896. doi: 10.1037//0278-7393.17.5.888. [DOI] [PubMed] [Google Scholar]
- 24.Reber AS. The cognitive unconscious: An evolutionary perspective. Conscious Cogn. 1992;1:93–133. [Google Scholar]
- 25.Pombal MA, El Manira A, Grillner S. Organization of the lamprey striatum—transmitters and projections. Brain Res. 1997;766:249–254. doi: 10.1016/s0006-8993(97)00701-4. [DOI] [PubMed] [Google Scholar]
- 26.Aizenstein HJ, et al. Regional brain activation during concurrent implicit and explicit sequence learning. Cereb Cortex. 2004;14:199–208. doi: 10.1093/cercor/bhg119. [DOI] [PubMed] [Google Scholar]
- 27.Willingham DB, Salidis J, Gabrieli JDE. Direct comparison of neural systems mediating conscious and unconscious skill learning. J Neurophysiol. 2002;88:1451–1460. doi: 10.1152/jn.2002.88.3.1451. [DOI] [PubMed] [Google Scholar]
- 28.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 29.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 30.Lehéricy S, et al. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci USA. 2005;102:12566–12571. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyachi S, Hikosaka O, Miyashita K, Kárádi Z, Rand MK. Differential roles of monkey striatum in learning of sequential hand movement. Exp Brain Res. 1997;115:1–5. doi: 10.1007/pl00005669. [DOI] [PubMed] [Google Scholar]
- 32.Cropley VL, Fujita M, Innis RB, Nathan PJ. Molecular imaging of the dopaminergic system and its association with human cognitive function. Biol Psychiatry. 2006;59:898–907. doi: 10.1016/j.biopsych.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 1984;15:217–227. doi: 10.1002/ana.410150302. [DOI] [PubMed] [Google Scholar]
- 34.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: A critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Seneca N, et al. Effect of amphetamine on dopamine D2 receptor binding in nonhuman primate brain: A comparison of the agonist radioligand [11C]MNPA and antagonist [11C]raclopride. Synapse. 2006;59:260–269. doi: 10.1002/syn.20238. [DOI] [PubMed] [Google Scholar]
- 36.Feldman J, Kerr B, Streissguth AP. Correlational analyses of procedural and declarative learning performance. Intelligence. 1995;20:87–114. [Google Scholar]
- 37.Waber DP, et al. Motor sequence learning and reading ability: Is poor reading associated with sequencing deficits? J Exp Child Psychol. 2003;84:338–354. doi: 10.1016/s0022-0965(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 38.Unsworth N, Engle RW. Individual differences in working memory capacity and learning: Evidence from the serial reaction time task. Mem Cognit. 2005;33:213–220. doi: 10.3758/bf03195310. [DOI] [PubMed] [Google Scholar]
- 39.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cervenka S, et al. Support for dopaminergic hypoactivity in restless legs syndrome: A PET study on D2-receptor binding. Brain. 2006;129:2017–2028. doi: 10.1093/brain/awl163. [DOI] [PubMed] [Google Scholar]
- 41.Bergström M, et al. Head fixation device for reproducible position alignment in transmission CT and positron emission tomography. J Comput Assist Tomogr. 1981;5:136–141. doi: 10.1097/00004728-198102000-00027. [DOI] [PubMed] [Google Scholar]
- 42.Wienhard K, et al. The ECAT EXACT HR: Performance of a new high resolution positron scanner. J Comput Assist Tomogr. 1994;18:110–118. [PubMed] [Google Scholar]
- 43.Langer O, et al. Precursor synthesis and radiolabelling of the dopamine D2 receptor ligand. J Labelled Comp Radiopharm. 1999;42:1183–1193. [Google Scholar]
- 44.Sandell J, et al. Improved specific radioactivity of the PET radioligand [C-11]FLB 457 by use of the GE Medical Systems PETtrace MeI MicroLab. J Labelled Comp Radiopharm. 2000;43:331–338. [Google Scholar]
- 45.Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging. 1997;16:187–198. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- 46.Roland PE, et al. Human brain atlas: For high-resolution functional and anatomical mapping. Hum Brain Mapp. 1993;1:173–184. doi: 10.1002/hbm.460010303. [DOI] [PubMed] [Google Scholar]
- 47.Mawlawi O, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Martinez D, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: Amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 49.Hazeltine E, Grafton ST, Ivry R. Attention and stimulus characteristics determine the locus of motor-sequence encoding. A PET study. Brain. 1997;120:123–140. doi: 10.1093/brain/120.1.123. [DOI] [PubMed] [Google Scholar]
- 50.Fletcher PC, et al. On the benefits of not trying: Brain activity and connectivity reflecting the interactions of explicit and implicit sequence learning. Cereb Cortex. 2005;15:1002–1015. doi: 10.1093/cercor/bhh201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berns GS, Cohen JD, Mintun MA. Brain regions responsive to novelty in the absence of awareness. Science. 1997;276:1272–1275. doi: 10.1126/science.276.5316.1272. [DOI] [PubMed] [Google Scholar]
- 52.Schendan HE, Searl MM, Melrose RJ, Stern CE. An FMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37:1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- 53.Buchsbaum MS, et al. PET and MRI of the thalamus in never-medicated patients with schizophrenia. Am J Psychiatry. 1996;153:191–199. doi: 10.1176/ajp.153.2.191. [DOI] [PubMed] [Google Scholar]
- 54.Gilbert AR, et al. Thalamic volumes in patients with first-episode schizophrenia. Am J Psychiatry. 2001;158:618–624. doi: 10.1176/appi.ajp.158.4.618. [DOI] [PubMed] [Google Scholar]
- 55.Yasuno F, et al. Low dopamine d(2) receptor binding in subregions of the thalamus in schizophrenia. Am J Psychiatry. 2004;161:1016–1022. doi: 10.1176/appi.ajp.161.6.1016. [DOI] [PubMed] [Google Scholar]
- 56.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 57.Olsson H, Halldin C, Farde L. Differentiation of extrastriatal dopamine D2 receptor density and affinity in the human brain using PET. Neuroimage. 2004;22:794–803. doi: 10.1016/j.neuroimage.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 58.Olsson H, Halldin C, Swahn CG, Farde L. Quantification of [11C]FLB 457 binding to extrastriatal dopamine receptors in the human brain. J Cereb Blood Flow Metab. 1999;19:1164–1173. doi: 10.1097/00004647-199910000-00013. [DOI] [PubMed] [Google Scholar]
- 59.Reed J, Johnson P. Assessing implicit learning with indirect tests: Determining what is learned about sequence structure. J Exp Psychol Learn Mem Cogn. 1994;20:585–594. [Google Scholar]
- 60.Wilkinson L, Shanks DR. Intentional control and implicit sequence learning. J Exp Psychol Learn Mem Cogn. 2004;30:354–369. doi: 10.1037/0278-7393.30.2.354. [DOI] [PubMed] [Google Scholar]
- 61.Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neurosci Biobehav Rev. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 62.Williams EJ. The comparison of regression variables. J R Stat Soc B. 1959;21:396–399. [Google Scholar]