Abstract
A phase II clinical trial with single-agent decitabine was conducted in older patients (≥60 years) with previously untreated acute myeloid leukemia (AML) who were not candidates for or who refused intensive chemotherapy. Subjects received low-dose decitabine at 20 mg/m2 i.v. over 1 h on days 1 to 10. Fifty-three subjects enrolled with a median age of 74 years (range, 60–85). Nineteen (36%) had antecedent hematologic disorder or therapy-related AML; 16 had complex karyotypes (≥3 abnormalities). The complete remission rate was 47% (n = 25), achieved after a median of three cycles of therapy. Nine additional subjects had no morphologic evidence of disease with incomplete count recovery, for an overall response rate of 64% (n = 34). Complete remission was achieved in 52% of subjects presenting with normal karyotype and in 50% of those with complex karyotypes. Median overall and disease-free survival durations were 55 and 46 weeks, respectively. Death within 30 days of initiation of treatment occurred in one subject (2%), death within 8 weeks in 15% of subjects. Given the DNA hypomethylating effect of decitabine, we examined the relationship of clinical response and pretreatment level of miR-29b, previously shown to target DNA methyltransferases. Higher levels of miR-29b were associated with clinical response (P = 0.02). In conclusion, this schedule of decitabine was highly active and well tolerated in this poor-risk cohort of older AML patients. Levels of miR-29b should be validated as a predictive factor for stratification of older AML patients to decitabine treatment.
Keywords: methylation, microRNA, azanucleoside
Acute myeloid leukemia (AML) is characterized by maturation arrest and proliferation of clonal myeloid precursors, leading to marrow failure and death within weeks to months if left untreated. The majority of newly diagnosed AML patients in the United States are aged ≥60 years, and the prognosis of older patients is dismal, with only ≈10% long-term survival with standard intensive chemotherapy (1). The poor outcome of older patients is because of an increased likelihood of comorbid illness leading to high rates of induction death, as well as increased frequency of several disease-specific factors associated with high risk of treatment failure, such as the presence of an antecedent hematologic disorder, complex karyotype, and expression of multidrug resistance mechanisms, among others (2). New therapies that target specific mechanisms of leukemogenesis and allow treatment of aged patients with comorbid illnesses are needed.
Epigenetic silencing of structurally normal genes by aberrant DNA methylation, mediated by DNA methyltransferase (DNMT) enzymes, has been shown to contribute to myeloid leukemogenesis by disrupting normal pathways of differentiation, proliferation, and apoptosis (3, 4). In contrast to structural changes such as mutation or deletion causing permanent loss of gene expression, epigenetic changes can be pharmacologically reversed, resulting in gene re-expression and restoration of normal cellular functions. Two azanucleoside DNMT inhibitors, azacitidine (5-azacytidine; Vidaza; Celgene, Inc.) and decitabine (5-aza-2'-deoxycytidine; Dacogen; Eisai, Inc.), are now approved in the United States for treatment of patients with myelodysplastic syndromes (MDS), a clonal myeloid disorder that often evolves to AML (5–7). Critical to successful therapy with both agents is the application of repetitive low-dose cycles of treatment at regular intervals (e.g., 4 weeks), allowing efficient incorporation of drug into the newly synthesized DNA of myeloid blasts undergoing mitosis during each treatment. In a randomized phase III study for higher risk MDS, azacitidine significantly improved overall survival (OS) compared with conventional care regimens (7). Notably, a subset of patients with low blast count AML (20–30% blasts) also had a survival benefit with azacitidine treatment (median survival, 24.5 months vs. 16.0 months for conventional care regimens) (8). Several schedules of decitabine have also shown promise for AML in early clinical trials (9–11). A low incidence of treatment-related toxicity has been reported for both of these agents, supporting their development for older AML patients, especially in those unable or unwilling to receive standard intensive chemotherapy.
To date, several strategies have been employed to optimize or enhance the clinical activity of azanucleosides in clonal myeloid diseases. Foremost among these is the combination of DNMT inhibitors with histone deacetylase inhibitors, based on preclinical studies showing synergistic anticancer activity and re-expression of epigenetically silenced genes (12–14). Unfortunately, various combinations of these agents in AML and MDS have been associated with increased toxicity and, to date, no definitive clinical benefit over treatment with azacitidine or decitabine alone (10, 15, 16). In a recent study of a 10-d schedule of decitabine, we demonstrated promising clinical activity in previously untreated older AML patients who were not candidates for standard treatment, but we observed no clinical or biologic benefit of combining this agent with the histone deacetylase inhibitor valproic acid (10).
We now report results of a phase II study in this population using a previously untested schedule of decitabine as a single agent, beginning with 10 d of treatment per cycle with subsequent therapy modified to improve tolerability and response. The clinical results observed are comparable to those seen with more intensive chemotherapeutic regimens in this aged cohort of AML patients but with relatively low toxicity. We are also unique in reporting that higher pretreatment levels of miR-29b, a microRNA known to target DNMTs, were associated with subsequent clinical response to decitabine.
Results
Patient Characteristics and Treatment Groups.
Fifty-three patients with previously untreated AML by World Health Organization (WHO) criteria were enrolled on this single-center phase II study. Patient characteristics at diagnosis are summarized in Table 1. According to the validated Medical Research Council (MRC) prognostic risk score for clinical outcome in older AML patients (17), 49% of enrollees were poor risk, 32% standard risk, and 19% good risk. The median age was 74 years (range, 60–85); six subjects were aged 60 to 65 (11%) and eight subjects were aged 80 years or older (15%). Nineteen subjects (36%) had either AML arising from an antecedent hematologic disorder (n = 13) or therapy-related AML (n = 6). Twenty-one subjects (40%) had normal karyotype, 16 subjects (30%) complex karyotype (≥3 abnormalities; 12 subjects with ≥5 abnormalities), one t(8;21), and 13 other cytogenetic abnormalities. Forty-eight subjects (91%) had at least two of the following: age ≥70, antecedent hematologic disorder, unfavorable karyotype, or WHO performance status ≥2. To provide descriptive data on the risk of toxic death with treatment, we used the hematopoietic stem cell transplantation index (18); 26 subjects (49%) had comorbidity scores of ≥3.
Table 1.
Patient characteristics
| Patient characteristic | Result |
| Median age (years) | 74 (range, 60–85) |
| 6 patients, 60–65 years | |
| 35 patients, 65–79 years | |
| 8 patients, ≥80 years | |
| Male/Female (n) | 34/19 |
| AML rising from AHD (n) | 13 |
| t-AML (N) | 6 |
| Median presenting WBC count, ×103/μL | 2.7 (range, 0.4–150.0) |
| Median BM blasts,% | 52 (range, 20–92%) |
| Cytogenetics (n) | |
| CBF | 1, patient had t(8;21)(q22;q22),,−7 |
| Normal karyotype | 21 |
| Complex karyotype | 16 (12 with ≥5 abnormalities) |
| Other | 13 |
| MRC* prognostic risk score, percent of patients | |
| Good | 19 |
| Standard | 32 |
| Poor | 49 |
AHD, antecedent hematologic disorder; BM, bone marrow; CBF, core binding factor; n, number of patients; t-AML, therapy-related AML.
*See ref. 17.
Clinical Responses.
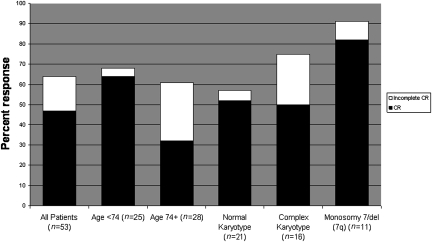
Complete remission (CR) was defined according to International Working Group (IWG) published criteria (19). For simplicity, in this manuscript additional IWG response categories of CR with incomplete count recovery and morphologic leukemia-free state (without count recovery) are referred to collectively as “incomplete CR.” Of 53 subjects, 25 achieved CR [47%; 95% confidence interval (CI): 33–61%] (Fig. 1). An additional nine subjects achieved incomplete CR for an overall response rate of 64% (34 of 53; 95% CI: 50–77%). Responses were seen in all age groups, in subjects with both low and high presenting WBC count, in both de novo and secondary AML, and in all cytogenetic subsets. The overall response rate for subjects below the median age of 74 years was 68% (64% CR); the overall response rate in subjects aged ≥74 years was 61% (32% CR). The CR rate in subjects with presenting WBC counts ≥15,000/μL (range, 15,000–150,000/μL) was 57% (8 of 14 subjects), including 50% (4 of 8) for those subjects presenting with WBC count ≥ 50,000/μL. Of the 19 subjects with secondary or t-AML, 9 achieved CR (47%), and 5 more achieved incomplete CR. Thus, the overall response rate for this subset was 74% (95% CI: 49–91%).
Fig. 1.
Response rates for all patients and for selected subsets. Percent response is noted by each bar graph. CR is noted in black, and additional patients with incomplete CR are noted in white. Shown are response rates for all patients, patients below or above the median age of 74 years, and selected cytogenetic subsets, respectively.
CR occurred in all cytogenetic subsets. For subjects with a normal karyotype, 52% (11 of 21) achieved CR, and one more achieved incomplete CR. For subjects with complex karyotypes (defined as ≥3 abnormalities), 50% (8 of 16) achieved CR, and 4 more had incomplete CR, for an overall response rate of 75%. For evaluable subjects with monosomy 7 or del(7q) [occurring as a sole abnormality (n = 1), in complex karyotypes (n = 9), or with t(8;21) (n = 1)], 91% responded (10 of 11) with nine CRs. Fourteen of the 25 CR subjects (56%) had abnormal karyotypes at diagnosis, and 71% (10 of 14) achieved cytogenetic CR.
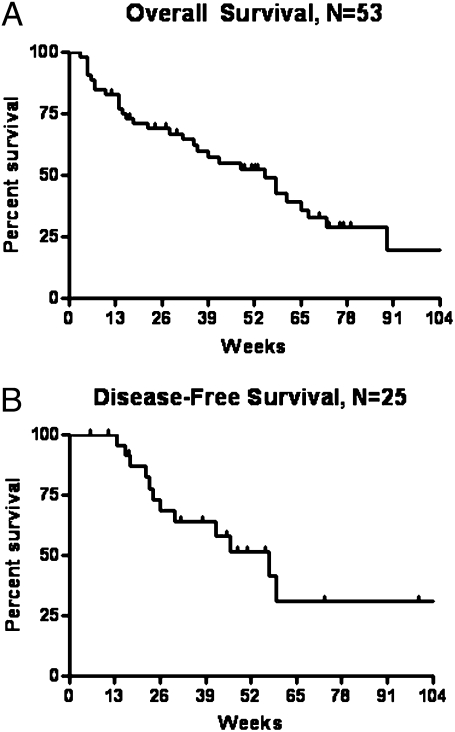
Median OS for all subjects was 55 weeks (95% CI: 36–72 weeks) (Fig. 2A). Median disease-free survival (DFS) for CR subjects was 46 weeks (95% CI: 30 weeks—not yet reached) (Fig. 2B). Cause of death in nonsurviving subjects was refractory/relapsed disease (56%), infection (19%), other cancers (4%), and unknown in one case. Four subjects received nonmyeloablative allogeneic stem cell transplants after achievement of CR.
Fig. 2.
Survival results. (A) Overall survival and (B) disease-free survival. Vertical marks reflect last follow-up times for censored observations.
Treatment Received.
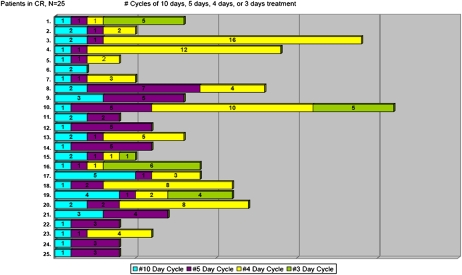
Overall, 300 cycles of decitabine were administered. The median number of cycles given for all subjects was four (range, 1–21+). For subjects who achieved CR, the median number of cycles received was eight (range, 2–21+), with a median of three cycles given before achievement of CR (range, 1–6). A typical pattern of response with treatment emerged: responding subjects often received one to two cycles of decitabine for 10 d before achieving at least incomplete CR (no disease but without full blood count recovery), often with robust platelet count recovery but continued neutropenia. Following one to two additional abbreviated cycles (4–5 d of treatment per cycle), neutrophil response also occurred, and full CR was confirmed. A detailed graphic of cycles administered for each CR patient is depicted in Fig. 3. Currently, 11 subjects continue to receive decitabine, 10 as maintenance and 1 with no response following two cycles.
Fig. 3.
Cycle intensity and number of cycles administered for patients achieving CR. Numerated in the column on the left is each individual CR patient (n = 25). Each color-coded bar graph shows the number of treatment courses received. In blue are cycles of 10 d of decitabine therapy, in purple 5-d cycles, in yellow 4-d cycles, and in green 3-d cycles.
Toxicities.
Infection and febrile neutropenia were common before neutrophil response. During the first two cycles of therapy, grade 3 or higher infection occurred in 31 subjects (58%). Fever without documented infection occurred in an additional 5 subjects for a total of 36 subjects with febrile neutropenia (68%) during this interval. Only one subject died within 30 days. Death within 8 weeks from the start of treatment occurred in eight subjects (15%) and was because of infection in each case; seven had active leukemia at the time of death. Following achievement of CR, however, infectious complications were rare. Myelosuppression during 129 cycles of maintenance decitabine given after CR was minimal; none of these cycles resulted in admission for myelosuppression or infectious complications that were related to drug. Other grade 3 or higher nonhematologic toxicities during the first two cycles of therapy were relatively uncommon and are summarized in Table 2.
Table 2.
Nonhematologic toxicities, grade ≥3 experienced during the first two cycles of treatment
| Toxicity | Patients with grade ≥ 3 in cycles 1 and 2 (n) |
| Infection | |
| Documented infection | 31 |
| Febrile neutropenia | 5 |
| Constitutional | |
| Fatigue | 3 |
| Fever | 2 |
| Gastrointestinal | |
| Anorexia | 1 |
| Dysgeusia | 1 |
| Mucositis/gingivitis | 3 |
| Pulmonary | |
| Dyspnea | 3 |
| Hypoxia | 8 |
| Cardiac | |
| Prolonged QTc | 2 |
| Decreased left ventricular ejection fraction | 1 |
| Arrhythmia | 3 |
| Hypertension | 2 |
| Skin | |
| Decubitus ulcer | 1 |
| Rash | 2 |
| Neurologic | |
| Confusion | 1 |
| Syncope | 3 |
| Abnormal gait | 2 |
| Other | |
| Pain | 7 |
| Thrombosis | 3 |
| Hemorrhage/hematoma | 5 |
miR-29b Expression Associated with Clinical Response to Decitabine.
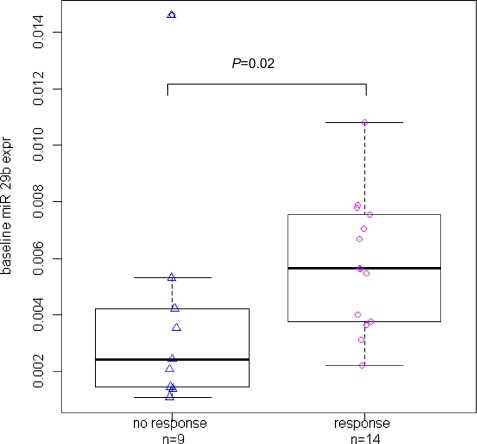
Twenty-three subjects had pretreatment samples available for testing; 11 achieved CR, 3 incomplete CR, and 9 had no response. From unselected diagnostic bone marrow samples, we measured pretreatment RNA expression levels of DNMT1, DNMT3a, and DNMT3b. We also measured expression of estrogen receptor (ESR1), a gene that is commonly hypermethylated in AML (10). As we previously reported that miR-29b targets DNMTs, we also assessed pretreatment levels of this microRNA (20, 21). Subjects who responded to decitabine treatment had a higher pretreatment level of miR-29b than nonresponders (P = 0.02) (Fig. 4). Notably, responders also had a trend for lower pretreatment levels of the miR-29b target DNMT3a compared with nonresponders (P = 0.06). No differences were observed with regard to expression levels of DNMT1, DNMT3b, or ESR1.
Fig. 4.
Difference in baseline miR-29b expression levels in decitabine between responders vs. nonresponders. Individual patient data are shown as the points in the figure, with the overlaid boxplots showing differences in the distribution of these data. In the boxplots, the thick middle line in the box represents the median baseline miR-29b expression value for that group; the top and bottom edges of the box itself represent the 75th and 25th percentiles, respectively; and the length of the box represents the interquartile range (difference between the 75th and 25th percentile as a measure of spread of the distribution). The P value is from a comparison of the continuous baseline miR-29b expression levels between responders and nonresponders using the nonparametric Wilcoxon rank-sum test.
Discussion
This study targeted AML patients over age 60 who were “unfit” for standard intensive cytarabine/anthracycline induction chemotherapy (“7+3”). The MRC prognostic risk-score profile in the current study is similar to that in the nonintensively treated cohort from the MRC AML11 trial (17), which served as the basis for the validated prognostic risk-score model that was developed, suggesting that the target population was studied. In the MRC AML11 trial, poor-risk patients treated nonintensively had 1-year survival of only 10%; poor-risk patients treated intensively had slightly better 1-year survival of 30%. Clearly, new therapies are needed to improve outcomes for older AML patients.
In the current single-agent decitabine trial, clinical responses were observed in all age groups, types of disease (de novo or secondary), and cytogenetic subsets including patients with complex karyotype who typically fare quite poorly with intensive therapy (1). Median OS was slightly more than 1 year. Interesting results were seen in patients harboring monosomy 7 or del(7q). Patients with this abnormality, most in the setting of complex karyotypes, had a response rate of 91%. This finding supports observations in MDS trials suggesting a favorable response to azanucleoside treatment for patients harboring aberrations of chromosome 7 (22, 23).
Although unintended patient-selection bias cannot be entirely overcome without randomization, examination of objective characteristics and prognostic risk scores provides a reasonable framework for comparing these single-center results with other clinical trials. Despite the high frequency of factors predicting poor outcome, clinical outcomes from our trial compare favorably with results achieved with conventional regimens in “fit” patient populations treated intensively (1, 17, 24, 25). Recently, Lowenberg, et al. reported results of an international, multicenter, randomized phase III study of high-dose vs. standard-dose daunorubicin in previously untreated older AML patients (24). The median age was 67 years. Of 813 patients enrolled, 21% had secondary AML, 22% unfavorable cytogenetic risk, and 88% WHO performance status of 0 or 1. In the present study, the median age was 74 years; 36% of patients had secondary AML, 34% unfavorable cytogenetic risk (using the same classification schema), and 70% performance status of 0 or 1. As the negative influences of increasing age, secondary AML, adverse risk cytogenetics, and poor performance status on outcome for older AML patients are well established (1, 2), the cohort of subjects treated in the present trial would be expected to have had a significantly inferior outcome, irrespective of the treatment given. Yet, 1-year survival estimates appear similar between the two trials.
Likewise, the decitabine results compare favorably with other treatment approaches in unfit patients (11, 17, 26–28). The current standard for this group is low-dose cytarabine, based on positive survival results from a randomized trial of cytarabine vs. hydroxyurea and best supportive care. With low-dose cytarabine in unfit older AML patients, the CR rate was 17%, with a median survival of only 3 to 4 months (26). More recently, Cashen et al. reported results of a three-institution trial of single-agent decitabine, targeting a patient population similar to that in the present study (median age in both studies was 74 years) (29). For 55 AML patients of age ≥ 60 who were not candidates for intensive therapy, the investigators administered 5 d of decitabine treatment per cycle, an approach identical to that previously published for MDS (30, 31). With the 5-d approach, the CR rate was 24% and the overall response rate 25%. Median overall survival was 7.7 months. Interestingly, in that study only one patient with a peripheral blast count ≥1,000/μL achieved CR (1 out of 14 patients). In contrast, the 10-d treatment approach in our study was associated with responses in patients with proliferative disease, as the CR rate in those with a presenting WBC count ≥15,000/μL (range, 15,000–150,000/μL) was 57% (8 of 14 patients), including four of eight patients with a presenting WBC count > 50,000/μL. Induction death rates were similar between the two studies; thus, increasing the decitabine treatment duration to 10 d did not appear to increase early death. Although definitive comparison would require further study, these results support the likelihood that the 10-d decitabine schedule, with subsequent cycles abbreviated based on response and toxicity as described, is more effective than a 5-d induction/consolidation approach in previously untreated older AML patients. Clofarabine is another agent in ongoing studies for older AML patients. Clinical results from our trial are similar to those reported with clofarabine (28, 32), and head-to-head comparison of these relatively low-intensity drug regimens would be informative in selection of the optimal approach for this age and risk group of patients.
Finally, we are unique in identifying that higher pretreatment levels of miR-29b were associated with achievement of clinical response to decitabine, recognizing that these subset analyses were done in a limited number of subjects. We and others have previously shown that miR-29b is involved in the regulation of DNA methylation and is down-regulated in AML (20, 21). Previous studies showed that restoration of normal miR-29b expression levels with a synthetic miR-29b oligonucleotide in AML cell lines and primary blasts reduced DNA hypermethylation by directly downregulating DNMT3a and 3b and indirectly targeting DNMT1 through Sp1. This led to re-expression of aberrantly hypermethylated and silenced genes (ESR1 and p15) (21). A unifying interpretation of our findings is that higher baseline miR-29b expression in myeloid blasts leads to lower DNMT levels and increased sensitivity to the hypomethylating effects of decitabine. Consistent with this hypothesis, we observed a trend for lower DNMT3a expression in responders compared to nonresponders. The findings warrant further exploration of these markers and support the putative disease targets described.
In conclusion, this single-center phase II study of a previously untested schedule of decitabine shows promising remission and survival results. Decitabine as a single agent with this schedule provides a framework upon which to build future combination studies to improve outcomes for older AML patients. Multicenter studies with the regimen should be performed. Expression of miR-29b and/or DNMT3a should be validated as a stratification tool in selection of older AML patients for decitabine-based treatments.
Materials and Methods
Eligibility Criteria and Study Design.
This study enrolled 53 subjects aged ≥60 years with previously untreated AML who were not candidates for or who refused to receive intensive cytarabine/anthracycline-based induction therapy. Subjects were required to have a stable WBC count (WBC <40,000/μL for 1 week) before initiation of therapy, total bilirubin ≤1.5 mg/dL, creatinine ≤2.0 mg/dL, ALT/AST ≤2 × upper limit of normal, and WHO performance status ≤2. Informed written consent approved by The Ohio State University Human Studies Committee was obtained on all subjects before study entry. All of the experiments involving human subjects were conducted according to the principles expressed in the Declaration of Helsinki. Prior cytokine or immunomodulatory therapy for MDS was permitted, but prior azacitidine or decitabine was not allowed.
Hydroxyurea was permitted before enrollment and during cycle 1 of treatment, if necessary, to maintain a WBC count <40,000/μL. Decitabine was initially administered at 20 mg/m2 i.v. over 1 h on days 1 to 10, every 4 weeks. Treatment during subsequent cycles was individually customized based on response and toxicity as follows: (i) subjects with evidence of persistent AML (≥5% blasts) received repeated cycles of the 10-d course of decitabine; (ii) subjects with no morphologic evidence of AML (<5% blasts) received a 5-d course of decitabine; (iii) following a 5-d course of treatment, subjects without evidence of AML but who had grade 4 neutropenia (<500/μL) of at least 14-d duration received dose reduced therapy (from 5 to 4 d per cycle) for the next cycle. A second dose reduction was permitted if the neutropenia occurred again following a 4-d course of treatment (from 4 to 3 d per cycle). However, dose reduction below 5 d per cycle was not done for subjects with evidence of minimal residual disease detected by flow cytometry or cytogenetics; these subjects continued to receive 5 d of treatment per cycle. Maintenance therapy for responding subjects continued indefinitely in the absence of relapse or unacceptable toxicity. Before removal from study because of treatment failure, subjects received two to four cycles of treatment.
Correlative Studies.
Correlative studies were performed with unselected bone marrow mononuclear cells collected before the first cycle of treatment, if material was available. DNMT1-, DNMT3a-, DNMT3b-, ESR1-, and miR-29b-expression studies were prioritized and conducted using quantitative RT-PCR, as previously described (21). DNMT1, DNMT3a, DNMT3b, and ESR1 levels were normalized to the internal control ABL and miR-29 to RNA U44. Three subjects with available material were excluded from these analyses, because they were not evaluable for correlation of response (two because of early death and one because of withdrawal of consent after one cycle). These subjects are included as nonresponders in the clinical analyses.
Statistical Analysis.
Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 3.0. IWG responses were assigned by the experimental therapeutics group at our institution. OS and DFS were estimated according to the method of Kaplan and Meier. DFS was calculated for CR patients from date of initial response (CR or incomplete CR) until relapse or death. Simon's two-stage optimal design was used. The primary endpoint was the rate of CR. The statistical design followed the following parameters: α = 0.1, β = 0.1, response probability of poor drug (p0) = 0.15, response probability of good drug (p1) = 0.35. After completing the second-stage target accrual of 33 subjects, the study was expanded and 22 additional subjects were treated to better assess tolerability of the regimen as well as correlative endpoints of interest. All patients who received any decitabine are included in the analyses, except for two patients accrued after November 1, 2009 who both remain on therapy. Four patients received nonmyeloablative allogeneic stem cell transplants after achieving CR, and these patients were censored for DFS at the time of transplantation. Descriptive statistics to include means, standard deviations, and frequencies were computed for DNMT1, -3a, -3b, ESR1, and miR-29b. Expression levels in pretreatment marrow samples from responding or nonresponding patients were compared using Wilcoxon rank sum tests; multiple comparison corrections were not used given the exploratory nature of the analyses.
Acknowledgments
We thank the patients and their families for participation in this clinical trial. This study was supported in part by National Institutes of Health and National Cancer Institute Grants K23CA120708 (to W.B.); R01 CA102031 (to G.M.), N01-CM-62207 (to M.V.-C.), and K12CA133250 (to J.C.B. and A.W.). A.W. is a Paul Calabresi Clinical Scholar. This work was also supported in part by the Leukemia and Lymphoma Society (J.C.B.), the Coleman Leukemia Research Foundation (C.D.B.), the D. Warren Brown Foundation (J.C.B.), and the Sidney Kimmel Foundation for Cancer Research (R.G.). This study has been registered on the National Cancer Institute Clinical Trials Network (NCT00492401).
Footnotes
The authors declare no conflict of interest.
References
- 1.Farag SS, et al. Cancer and Leukemia Group B 8461. Pretreatment cytogenetics add to other prognostic factors predicting complete remission and long-term outcome in patients 60 years of age or older with acute myeloid leukemia: results from Cancer and Leukemia Group B 8461. Blood. 2006;108:63–73. doi: 10.1182/blood-2005-11-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelbaum FR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 4.Baylin SB, et al. Hypermethylation of the 5′ region of the calcitonin gene is a property of human lymphoid and acute myeloid malignancies. Blood. 1987;70:412–417. [PubMed] [Google Scholar]
- 5.Silverman LR, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 7.Fenaux P, et al. International Vidaza High-Risk MDS Survival Study Group. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenaux P, et al. Azacitidine prolongs overall survival compared with con-ventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:521–523. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 9.Issa JP, et al. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2′-deoxycytidine (decitabine) in hematopoietic malig-nancies. Blood. 2004;103:1635–1640. doi: 10.1182/blood-2003-03-0687. [DOI] [PubMed] [Google Scholar]
- 10.Blum W, et al. Phase I study of decitabine alone or in combination with valproic acid in acute myeloid leukemia. J Clin Oncol. 2007;25:3884–3891. doi: 10.1200/JCO.2006.09.4169. [DOI] [PubMed] [Google Scholar]
- 11.Cashen AF, et al. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28:556–561. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 12.Cameron EE, Bachman KE, Myöhänen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 13.Klisovic MI, et al. Depsipeptide (FR 901228) promotes histone acetylation, gene transcription, apoptosis and its activity is enhanced by DNA methyltransferase inhibitors in AML1/ETO-positive leukemic cells. Leukemia. 2003;17:350–358. doi: 10.1038/sj.leu.2402776. [DOI] [PubMed] [Google Scholar]
- 14.Liu S, et al. Interplay of RUNX1/MTG8 and DNA methyltransferase 1 in acute myeloid leukemia. Cancer Res. 2005;65:1277–1284. doi: 10.1158/0008-5472.CAN-04-4532. [DOI] [PubMed] [Google Scholar]
- 15.Gore SD, et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–6369. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Manero G, et al. Phase 1/2 study of the combination of 5-aza-2′-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108:3271–3279. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheatley K, et al. United Kingdom National Cancer Research Institute Haematological Oncology Clinical Studies Group and Acute Myeloid Leukaemia Subgroup. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol. 2009;145:598–605. doi: 10.1111/j.1365-2141.2009.07663.x. [DOI] [PubMed] [Google Scholar]
- 18.Giles FJ, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007;136:624–627. doi: 10.1111/j.1365-2141.2006.06476.x. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, et al. International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Fabbri M, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci USA. 2007;104:15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garzon R, et al. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood. 2009;113:6411–6418. doi: 10.1182/blood-2008-07-170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rüter B, Wijermans P, Claus R, Kunzmann R, Lübbert M. Preferential cytogenetic response to continuous intravenous low-dose decitabine (DAC) administration in myelodysplastic syndrome with monosomy 7. Blood. 2007;110:1080–1082, author reply 1083. doi: 10.1182/blood-2007-03-080630. [DOI] [PubMed] [Google Scholar]
- 23.Furlan I, et al. Intriguing response to azacitidine in a patient with juvenile myelomonocytic leukemia and monosomy 7. Blood. 2009;113:2867–2868. doi: 10.1182/blood-2008-12-195693. [DOI] [PubMed] [Google Scholar]
- 24.Löwenberg B, et al. Dutch-Belgian Cooperative Trial Group for Hemato-Oncology (HOVON); German AML Study Group (AMLSG); Swiss Group for Clinical Cancer Research (SAKK) Collaborative Group. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361:1235–1248. doi: 10.1056/NEJMoa0901409. [DOI] [PubMed] [Google Scholar]
- 25.Chauncey TR, et al. Sequential phase II Southwest Oncology Group studies (S0112 and S0301) of daunorubicin and cytarabine by continuous infusion, without and with ciclosporin, in older patients with previously untreated acute myeloid leukaemia. Br J Haematol. 2010;148:48–58. doi: 10.1111/j.1365-2141.2009.07919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burnett AK, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109:1114–1124. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- 27.Harousseau JL, et al. FIGHT-AML-301 Investigators. A randomized phase 3 study of tipifarnib compared with best supportive care, including hydroxyurea, in the treatment of newly diagnosed acute myeloid leukemia in patients 70 years or older. Blood. 2009;114:1166–1173. doi: 10.1182/blood-2009-01-198093. [DOI] [PubMed] [Google Scholar]
- 28.Kantarjian HM, et al. Phase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. J Clin Oncol. 2010;28:549–555. doi: 10.1200/JCO.2009.23.3130. [DOI] [PubMed] [Google Scholar]
- 29.Cashen AF, Schiller GJ, O'Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28:556–561. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 30.Kantarjian H, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–57. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 31.Steensma DP, et al. Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: the alternative dosing for outpatient treatment (ADOPT) trial. J Clin Oncol. 2009;27:3842–3848. doi: 10.1200/JCO.2008.19.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faderl S, et al. A randomized study of clofarabine versus clofarabine plus low-dose cytarabine as front-line therapy for patients aged 60 years and older with acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2008;112:1638–1645. doi: 10.1182/blood-2007-11-124602. [DOI] [PMC free article] [PubMed] [Google Scholar]