Abstract
Deletion of acetyl CoA carboxylase-2 (Acc2) reportedly causes leanness in the setting of hyperphagia. To determine the cellular basis for these effects, we generated a mouse model in which Acc2 can be selectively deleted by the action of Cre recombinase. Deletion of Acc2 from skeletal muscle, the predominant site of Acc2 expression, had no effect on body weight, food intake, or body composition. When Acc2 was inactivated in the germline, Acc2 knockout (Acc2KO) mice displayed no differences in body weight, food intake, body composition, or glucose homeostasis as compared to controls on chow or high fat diet. Total malonyl CoA content and fatty acid oxidation rates in skeletal muscle of Acc2KO mice were unchanged, suggesting metabolic compensation in response to the loss of Acc2. The limited impact of Acc2 deletion on energy balance raises the possibility that selective pharmacological inhibition of Acc2 for the treatment of obesity may be ineffective.
Keywords: acetyl CoA carboxylase, energy balance, malonyl CoA, obesity
Increased rates of fatty acid oxidation have been associated with increased energy expenditure and leanness (1–7). The rate-limiting step in fatty acid oxidation is the delivery of long chain fatty acids into the mitochondria by the action of carnitine palmitoyl transferase-1 (CPT-1) (8, 9). CPT-1 transfers carnitine to acetylated fatty acids allowing them to be shuttled across the outer mitochondrial membrane for oxidation inside the mitochondrion. CPT-1 activity is tightly regulated by malonyl CoA, the product of carboxylation of acetyl CoA (10). Malonyl CoA is generated through the action of acetyl CoA carboxylase of which there are two major isoforms. Acc-1 is localized in the cytosol and is the predominant isoform expressed in lipogenic tissues including white fat and liver. Malonyl CoA produced by Acc-1 is thought to be used selectively as a substrate for de novo lipogenesis (11). Acc2 is localized to the mitochondria via its unique N-terminal domain and is found predominantly in oxidative tssues such as skeletal muscle and heart (12). Malonyl CoA generated by Acc2 at the surface of the mitochondria has been proposed to act directly on CPT-1 to inhibit its activity and shut down the delivery of fatty acids for oxidation (13). Thus malonyl CoA serves as a critical regulator of the balance between de novo lipogenesis and fatty acid oxidation.
The importance of malonyl CoA and fatty acid oxidation in energy balance is underscored by the gene knockout of Acc2. Systemic deletion of Acc2 has been reported to cause continuous fatty acid oxidation, reduced fat mass, and increased energy expenditure with an associated hyperphagia (1, 14). Mice lacking Acc2 displayed dramatic reductions of malonyl CoA in both skeletal muscle and heart. This reduction of malonyl CoA has been suggested to relieve the inhibition of CPT-1 and allow unrestrained delivery of fatty acids for oxidation. Moreover, Acc2 inactivation has been reported to blunt the development of obesity and insulin resistance associated with high fat feeding (15). The finding that deletion of Acc2 could reduce fat mass and increase energy expenditure, even in the setting of increased food intake, has made Acc2 an important target for pharmaceutical intervention against obesity and its comorbidities (16, 17).
In rodents Acc2 is found predominantly in skeletal muscle and heart, but also in liver and the central nervous system. Although its expression in white fat is low, Acc2 deletion has also been shown to have beneficial metabolic effects in white adipose tissue examined ex vivo (18). It is not known if the metabolic benefits of Acc2 inactivation can be attributed to the loss of Acc2 from a single tissue or if these effects require Acc2 inactivation in several peripheral tissues and/or the central nervous system. Identifying the critical sites of Acc2 action will be important for understanding the mechanism by which increased fatty acid oxidation alters energy expenditure. To dissect the site-specific effects of Acc2 deletion, we generated a loxP-flanked Acc2 allele in mice to allow for tissue-specific deletion of Acc2.
We hypothesized that selective deletion of Acc2 from skeletal muscle, its major site of expression, would recapitulate the leanness and increased energy expenditure previously noted with global Acc2 deletion. To our surprise, deletion of Acc2 from skeletal muscle had no effect on body weight, food intake, or body composition. Moreover, when Acc2 was inactivated in the germline and bred to homozygosity, mice lacking Acc2 in all tissues had similar body weights, body composition, and food intake as compared to control mice on both chow and high fat diets. These data raise questions regarding the potential efficacy of selectively targeting Acc2 for pharmacologic inhibition in an effort to treat obesity and its complications.
Results
Generation of a loxP-Flanked Acc2 Allele and Cre-Mediated Acc2 Deletion.
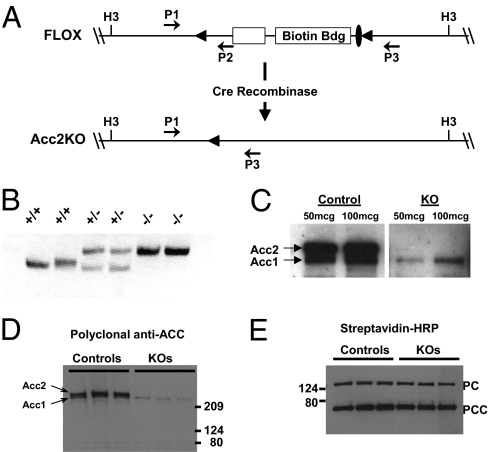
Recombineering techniques (19) using a bacterial artificial chromosome were used to place LoxP sequence elements around the exon harboring the biotin binding motif and an upstream exon within the Acc2 genomic locus. The biotin binding motif was targeted previously for systemic Acc2 deletion (15). The exon immediately upstream of the catalytic domain was included in our targeting strategy so that splicing of the remaining exons following Cre-mediated deletion would introduce a frameshift and nonsense mutation after Asp865 in any translated protein. Schematic representation of the targeting strategy is shown in Fig. 1A.
Fig. 1.
Conditional targeting of Acc2. (A) The Hind3 (H3) fragment of the Acc2 genetic locus containing the biotin-binding domain (Biotin Bdg) flanked by loxP sites is shown. Filled triangles represent loxP sequence elements. A single Frt sequence element (filled oval) remains in the final allele after Flpe recombinase-mediated deletion of the G418 selection cassette. Cre recombinase mediated recombination between the loxP sites results in the deletion of both exons. (B) PCR was used to demonstrate the deletion of the loxP-flanked exons using primers P1, P2, and P3 as shown in A. Predicted PCR fragment sizes: WT band = 350 bp; Acc2KO band = 425 bp. (C) Immunoblotting of muscle lysates from Acc2KO animals using Streptavidin conjugated to HRP. (D) Immunoblotting using Cell Signaling Technologies polyclonal total ACC antibody (#3662). (E) Immunoblotting of lower molecular weight species using streptavidin-HRP. PC = pyruvate carboxylase and PCC= propionyl CoA carboxylase.
To demonstrate functional inactivation of Acc2, mice harboring the floxed Acc2 allele were bred to Zp-3 Cre transgenic mice (Jax #003394) (20) to delete the Acc2 biotin binding domain in the germline. Global deletion of the loxP-flanked exons was confirmed by PCR of mouse tail DNA using primers flanking the targeted region of the Acc2 gene (Fig. 1B). Animals heterozygous for the Acc2 deleted allele were bred to homozygosity to generate mice lacking Acc2 (Acc2KO mice). Deletion of Acc2 protein (approximately 280 kDa) was demonstrated by Western blot analysis of muscle lysates from wild-type control mice and Acc2KO mice using streptavidin-conjugated HRP (streptavidin-HRP) to bind the biotin containing domains of Acc1 and Acc2 (Fig. 1C). To exclude the possibility that our targeting strategy generated a truncated form of Acc2, Western blot analysis of muscle lysates was performed using a polyclonal antibody that recognizes the peptide region flanking Lys653 in the mouse Acc2 protein; this site lies upstream of the targeted region (Fig. 1D). Importantly, no truncated isoform of Acc2 was revealed (predicted molecular mass of approximately 100 kDa), suggesting that deletion of the loxP-flanked exons caused protein instability and degradation of any potential Acc2 isoform. Similar results were seen using polyclonal antibodies directed against phosphorylated peptides upstream of the targeted region. The protein blot was then stripped and reprobed with streptavidin-HRP to examine other biotin-containing enzymes present in the lysate (Fig. 1E). In the absence of Acc2 protein, there was no up-regulation of other biotin containing enzymes (pyruvate carboxylase and propionyl CoA carboxylase).
Selective Deletion of ACC2 in Skeletal Muscle.
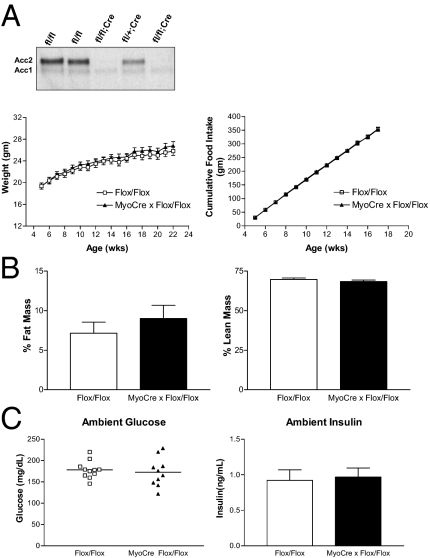
Given the predominant expression of Acc2 in skeletal muscle and the importance of muscle in total body energy consumption, Acc2 was selectively deleted from this tissue using a skeletal muscle-specific Cre recombinase transgenic animal in which Cre expression is driven by the mouse myogenin promoter and Mef2c enhancer (21). Deletion of Acc2 protein was confirmed by immunoblotting of muscle extracts from mice with muscle-specific deletion of Acc2 and littermate controls (Fig. 2A). Male mice were then individually housed and food intake and body weight were monitored weekly. To our surprise, selective deletion of Acc2 from skeletal muscle had no effect on bodyweight, food intake, or body composition (Fig. 2B). In addition, there was little effect on ambient glucose and insulin levels (Fig. 2C). Thus, even though skeletal muscle is one of the sites of highest Acc2 expression, selective deletion of Acc2 from this tissue had little effect on overall energy balance.
Fig. 2.
Phenotypic analyses of mice lacking Acc2 in skeletal muscle. (A) Immunoblot analyses of 50 mcg muscle lysates from mice homozygous for the floxed Acc2 allele without (fl/fl) and with (fl/fl; Cre) the muscle-specific Cre (MyoCre) transgene and mice heterozygous for the floxed allele with the muscle-specific Cre transgene (fl/+; Cre). Blots were probed with the anti-ACC polyclonal antibody. (B) Bodyweight and food intake were assessed weekly. Body composition analysis was performed at 22 weeks. Data are represented as % fat mass (fat mass/body mass × 100) and % lean mass (lean mass/body mass × 100). (C) Ambient blood glucose and insulin levels were determined 2 hr following the removal of food. Flox/Flox n = 11; MyoCre x Flox/Flox n = 10.
Characterization of Global Acc2 Knockout Mice.
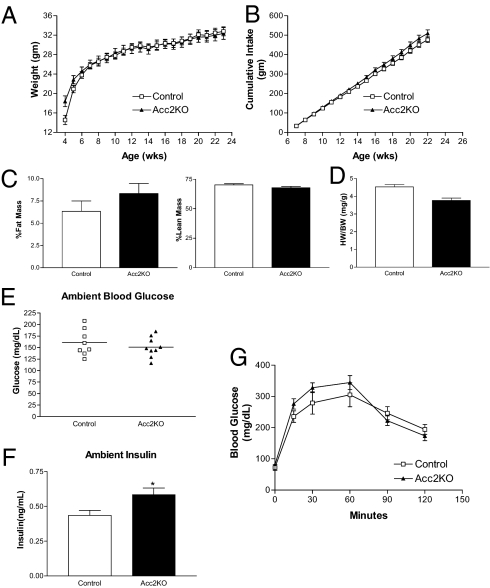
Because muscle specific deletion of Acc2 had little phenotypic effect, we sought to confirm the previously published physiologic effects of global Acc2 deletion. Male Acc2KO mice and littermate controls were singly housed and allowed free access to regular chow and water. Bodyweight and food intake were determined weekly. Similar to what we found with mice lacking Acc2 in skeletal muscle, there was no difference in either bodyweight or food intake on regular chow between Acc2KO animals or wild-type controls (Figs. 3 A and B). Body composition determined at 11 weeks of age showed no differences in percent body fat or percent lean mass (Fig. 3C). Heart size was reduced in the Acc2KO mice (Fig. 3D), which is consistent with previously published results and provides additional objective support for the deletion of Acc2 (22). Ambient blood glucose was unchanged and ambient insulin levels were slightly increased in Acc2KO mice. The response to an i.p. glucose load was unaffected in Acc2KO animals (Figs. 3 E–G). Taken together, these data suggest minimal metabolic effects from global deletion of Acc2.
Fig. 3.
Phenotypic analysis of global Acc2KO mice fed regular chow diet. (A and B) Bodyweight and food intake were determined weekly. (C) Body composition data are represented as % fat mass and % lean mass. (D) Wet heart weights were measured and are expressed per g animal body weight. (E and F) Ambient blood glucose and insulin levels were obtained from tail bleeds within 2 hr of food withdrawal. (G) Glucose tolerance test using glucose 2 g/kg injected i.p. following an overnight fast (16 h). There was no difference in body weights between Acc2KOs (34.1 ± 1.4 g, n = 9) and controls (32.8 ± 0.9 g, n = 7), P = 0.49.
Biochemical Analyses of ACC Activity and Malonyl CoA Levels.
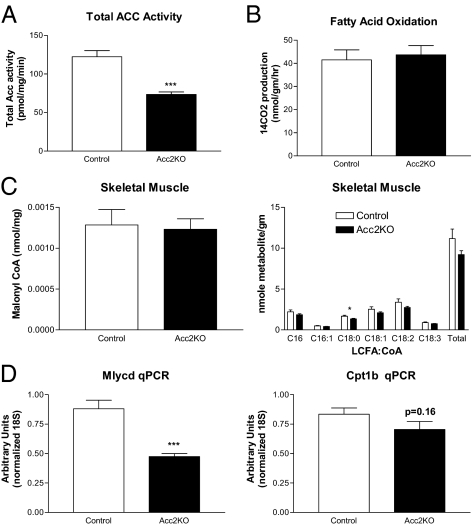
Given the minimal difference in bodyweight, food intake, and body composition between Acc2KO mice and controls, we directly measured ACC activity, fatty acid oxidation rates, and malonyl CoA levels in skeletal muscle. Total ACC activity in skeletal muscle extracts of Acc2KO mice compared to controls was reduced approximately 40% (Fig. 4A). However, there was no difference in fatty acid oxidation rates in ex vivo soleus muscle between Acc2KO mice and controls (Fig. 4B). Malonyl CoA, the reaction product of Acc2, was analyzed using mass spectroscopy on samples of skeletal muscle harvested from fed animals. Surprisingly, malonyl CoA levels were also unchanged in skeletal muscle of Acc2KO mice as compared to controls(Fig. 4C). As might be predicted from the malonyl CoA measurements, long chain fatty acyl CoAs demonstrated minimal changes between the two groups, suggesting little change in fatty acid flux in skeletal muscle. Interestingly, when examined in heart (the other site of significant Acc2 expression), malonyl CoA levels were decreased by approximately 30% and subsets of fatty acyl CoAs (C16:1 and C18:1) were selectively reduced (Fig. S1). Serum triglycerides, nonesterified fatty acids and β-hydroxybutyrate concentrations were measured and showed no difference between Acc2KO mice and controls (Table S1). Following an overnight fast, there was a trend toward increased serum β-hydroxybutyrate in Acc2KO mice (2.5 mM ± 0.16 vs. 2.0 mM ± 0.27; P = 0.16, n = 5), but this did not achieve statistical significance.
Fig. 4.
Biochemical assessment of the effect of Acc2 deletion in skeletal muscle. (A) Total ACC activity was measured in soleus muscle extracts from control (n = 5) and Acc2KO (n = 5) mice and is expressed as citrate-dependent activity per mg protein lysate. (B) Fatty acid oxidation was estimated by the average rate of 14CO2 production from 14C-palmitate by each soleus in the mouse (control n = 5; Acc2KO n = 5). (C) Malonyl CoA levels and long chain fatty acyl-CoAs were measured by mass spectroscopy in gastrocnemius of fed animals (Controls n = 11, Acc2KO n = 12). (D) Quantitative PCR for malonyl CoA decarboxylase and carnitine palmitoyl transferase from Acc2KO (n = 8) and control (n = 7) gastrocnemius mRNA. Data are presented as arbitrary units relative to ribosomal 18S ribosomal RNA.
The fact that gene knockout of Acc2 had minimal effects on skeletal muscle malonyl CoA levels and fatty acid oxidation rates suggests compensatory changes that blunt the effect of Acc2 deletion in skeletal muscle. Quantitative PCR of muscle RNA from Acc2KO and control mice was performed on a subset of genes involved in fatty acid oxidation. Whereas there was no change in expression of Acc1, Pgc1α, or Pparα mRNA, expression of malonyl CoA decarboxylase (Mlycd), the enzyme that degrades malonyl CoA to acetyl CoA, was significantly reduced. Muscle Cpt1 mRNA tended to be lower in Acc2KO muscle, but this did not achieve statistical significance (Fig. 4D). Reductions in malonyl CoA decarboxylase and Cpt1 may play in role in maintaining normal malonyl CoA levels and fatty acid oxidation rates in the setting of Acc2 deletion.
Comprehensive Metabolic Monitoring of Acc2KO Mice.
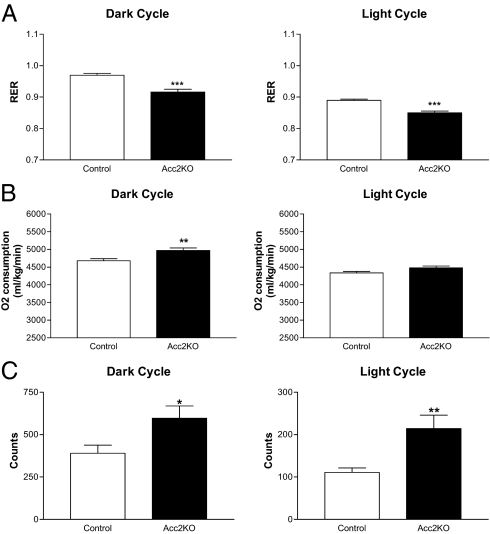
To further assess the physiologic impact of Acc2 deletion, another cohort of older Acc2KO (n = 8) and control mice (n = 8) was subjected to comprehensive metabolic monitoring. Acc2KO mice demonstrated a decrease in respiratory exchange ratio (RER) during both the dark and light cycles, implying a shift toward increased use of fat relative to carbohydrate as fuel (Fig. 5A). Oxygen consumption was also analyzed as an indirect measure of energy expenditure. Acc2KO mice exhibited a slight increase in oxygen consumption during the dark cycle (Acc2KO: 4968 ± 73 mL/kg/min vs. controls: 4680 ± 60 mL/kg/min; P = 0.005). There was a nonsignificant trend toward increased oxygen consumption in the light cycle as well (Acc2KO: 4478 ± 55 mL/kg/min vs. controls: 4335 ± 46 mL/kg/min; P = 0.055) (Fig. 5B). Interestingly, Acc2KO mice displayed increased physical activity in both the dark and light cycles as compared to controls (Fig. 5C). It is not clear if this increase in activity underlies the changes measured in RER and oxygen consumption.
Fig. 5.
Comprehensive Laboratory Animal Monitoring (CLAMS) of Acc2KO mice. Mice of both genotypes (n = 8) were acclimatized to monitoring cages for 2 days before analyses. (A) RER is presented as the average value measured every 39 min over an 8-hr block in the dark cycle (n = 13) or 10-hr period in the light cycles (n = 16) for each genotype. (B) Oxygen consumption is expressed per kg/min in both the dark and light cycles. (C) Activity in the XY plane is expressed as counts and is averaged over an 8-hr block in the dark cycle and 10-hr block in the light cycle.
Metabolic Challenge of Acc2KO Mice.
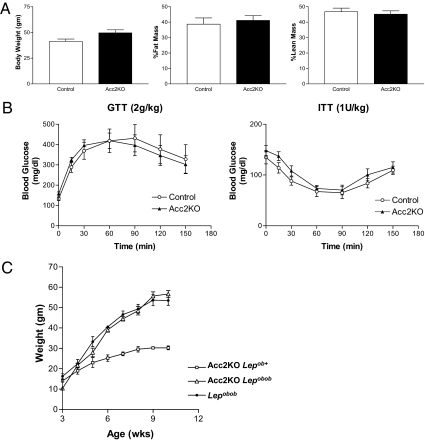
As we found no beneficial effect of Acc2 deletion on overall energy balance in mice fed a regular chow diet, Acc2KO mice and controls were metabolically challenged with high fat feeding. After 11 weeks of high fat (58%) feeding, the average weight gain for Acc2KO mice was equivalent with that of controls (Acc2KO: 0.77 g/wk; n = 8 vs. control: 0.72 g/wk; n = 9, P = 0.67). There was no difference in ambient glucose (Acc2KO: 148 ± 8.8 mg/dL, n = 8 vs. Control 146 ± 8.1 mg/dL, n = 9; P = 0.88) or insulin levels (Acc2KO: 1.62 ± 0.43 ng/mL, n = 8 vs. control 1.21 ± 0.23 ng/mL, n = 9; P = 0.40) after 11 weeks of high fat feeding. To exclude the possibility that 11 weeks of high fat feeding was an inadequate metabolic challenge to alter energy balance, a separate cohort of mice were exposed to prolonged high fat (45%)/high sucrose (35%) feeding for 36 weeks. Acc2KO mice and controls exhibited similar bodyweights and body composition (Fig. 6A). When examined by i.p. glucose and insulin tolerance tests, there was no detectable difference between Acc2KO mice and controls in either glucose handling or insulin sensitivity under these conditions (Fig. 6B). Given the variable response of different mouse strains to high fat diet-induced obesity, the Acc2KO allele was bred into a leptin deficient background to drive massive obesity. Even in the setting of leptin deficiency, Acc2 deletion had no effect on body weight gain (Fig. 6C). Matched weight gains on high fat diets and in leptin deficiency suggest that the differences in RER, oxygen consumption and activity measured in Acc2KO mice are not sufficient to alter energy balance enough to protect against diet-induced or genetic obesity.
Fig. 6.
Effects of high fat diet and leptin deficiency on energy balance in Acc2KO mice. Mice were fed a high fat (45%)/high sucrose (35%) diet for 36 weeks. (A) Bodyweight and body composition (percent fat and lean mass): Acc2KO n = 9; controls n = 7. (B) Glucose tolerance test (2 g/kg) after a 6-hr fast and insulin tolerance test (1U regular insulin/kg) after a 6-hr fast. (C) Bodyweight gain of Acc2KO mice with (open triangles; n = 10) or without (open squares; n = 8) leptin deficiency as compared to leptin deficient controls (filled circles; n = 4).
Discussion
Acc2 is considered a key regulator of fatty acid oxidation because of its ability to generate malonyl CoA, the physiologic inhibitor of CPT-1, and alter the flux of long chain fatty acids into mitochondria for oxidation. Previous reports have suggested that inhibition of Acc2 results in continuous fatty acid oxidation, increased energy expenditure, and reduction of body fat mass, all in the setting of significant hyperphagia (1, 2). Deletion of Acc2 was also shown to prevent the development of diet-induced obesity and its resultant glucose intolerance and insulin resistance (15). Given these positive metabolic effects, Acc2 has become an attractive drug target for obesity therapy (23).
We sought to dissect the tissue-specific effects of Acc2 deletion to explore the mechanisms by which changes in fatty acid oxidation might alter energy balance. In stark contrast to previously published data, we find that mice genetically engineered to lack Acc2 specifically in skeletal muscle or in all tissues were equivalent to controls in body weight, food intake, and body composition on a chow or high fat diet. Acc2 deletion did decrease total ACC activity in skeletal muscle. However, direct measurements of fatty acid oxidation rates and total malonyl CoA in skeletal muscle, the major site of Acc2 expression, were unchanged in this Acc2 knockout model. This indicates that compensation of some sort maintains malonyl CoA levels in the face of Acc2 deficiency. One potential compensatory mechanism might be alterations in Acc1 activity. Acc1 is found in skeletal muscle, but its expression is low relative to Acc2. Acc1 mRNA is not altered by Acc2 deletion, but Acc1 protein appears to be slightly decreased (not increased) with the loss of Acc2 (Figs. 1 C and D). Thus, compensation at the level of Acc1 protein does not occur with Acc2 deletion. Remarkably, even though Acc2 knockout dramatically reduces total ACC protein in muscle, ACC activity is reduced by only approximately 40%. This discrepancy suggests that some adaptation with regards to residual Acc1 activity might be occurring in response to Acc2 deletion. ACC activity can be regulated allosterically by citrate, malonyl CoA, and acylated-long chain fatty acids as well as by multimerization of ACC proteins (11). Our studies do not rule out the possibility that changes in posttranslational or allosteric regulation of Acc1 might partially compensate for the loss of Acc2. Importantly, we do find that Acc2 deletion is associated with a decrease in malonyl CoA decarboxylase expression. Reductions in malonyl CoA decarboxylase, an enzyme that degrades malonyl CoA, would be expected to increase malonyl CoA levels (24). Thus, alterations in malonyl CoA decarboxylase may be responsible in part for blunting the metabolic effects of Acc2 deletion in skeletal muscle.
Metabolic compensation that maintains malonyl CoA levels and de novo lipogenesis in the setting of liver-specific Acc1 deletion has been reported and calls into question the model of strict compartmentalization of malonyl CoA pools and ACC activity between the cytosol and mitochondria (25). In another study, antisense oligonucleotides were used to down-regulate Acc1 and Acc2 in liver and white fat. Targeting of individual isoforms had little effect on overall metabolism; however, when both Acc1 and Acc2 were reduced, hepatic steatosis and liver insulin resistance were ameliorated (26). In our model, the associated down-regulation of malonyl CoA decarboxylase transcription in response to Acc2 deletion supports the concept that other enzymatic components in fatty acid metabolism pathways can compensate for the loss of Acc2. Whether the deletion of both Acc isoforms would be sufficient to override metabolic compensation, drive fatty acid oxidation, and promote leanness remains to be determined.
The alterations in fatty acid utilization (as measured by RER), oxygen consumption, and activity seen in Acc2KO mice implicate Acc2 activity in these physiologic processes. However, as malonyl CoA levels, fatty acid oxidation rates, and long chain fatty acyl CoAs are unchanged in muscles of Acc2KO mice, it seems unlikely that skeletal muscle is the tissue mediating these effects. Given the significant effect of Acc2 deletion on cardiac malonyl CoA levels (Fig. S1), it is intriguing to speculate that changes in cardiac metabolism might result in measurable differences in systemic fuel utilization. As Acc2 is also expressed in the central nervous system, it is possible that deletion of Acc2 from the central nervous system might contribute to the modest changes in physical activity and/or oxygen consumption. Importantly, the differences in RER, oxygen consumption, and activity are insufficient to promote leanness and improved glucose tolerance on regular or high fat diets or protect against the obesity associated with leptin deficiency.
While our manuscript was under review, Hoehn et al. (27) reported the limited metabolic effects of Acc2 deletion in a rodent model. Similar to our findings, deletion of Acc2 had no effect on fat mass, food intake, body weight, or glucose homeostasis on regular chow or after 5 weeks of high fat feeding. Our study significantly extends the metabolic challenge (11 and 36 weeks of high fat feeding and leptin deficiency) and shows no beneficial effect of Acc2 deletion on energy balance. For reasons that are not clear, we did not observe the changes in ex vivo muscle fatty acid oxidation rates or malonyl CoA levels described by Hoehn et al. (27); however, it should be noted that total body fat utilization (as measured by RER) is similarly increased in the two models. Nevertheless, both studies demonstrate minimal effects of Acc2 deletion on overall energy balance.
The molecular explanation for the phenotypic differences between our model of Acc2 deletion and the original model published by Abu-Elheiga et al. (1) is not clear. Although the Acc2 biotin-binding site was deleted in both models, different targeting strategies were employed. Our conditional allele is designed to delete the enzyme active site and inactivate any translated protein product from the allele. Indeed, our immunoblotting data confirms the complete absence of Acc2 protein or a potential truncated isoform. We are confident that full length Acc2 has been deleted and that no residual truncated isoform from the targeting strategy remains. This is corroborated by the reduction of total ACC activity in skeletal muscle, the reduction in heart size and the decrease in cardiac malonyl CoA levels. On the other hand, the targeting strategy employed in the previous study (1) only replaced the biotin binding motif-containing exon. RNA splicing across the targeting cassette would leave the mRNA in frame and may have left a mutated, but otherwise intact protein lacking a catalytic domain. If produced, such a protein might potentially have “dominant negative” activity toward Acc1.
Given the magnitude of the metabolic effects (protection against obesity and improved glucose tolerance) previously ascribed to Acc2 deletion, it seems unlikely that background strain differences between these models would completely mask any metabolic benefit of Acc2 deletion in our Acc2KO animals. Moreover, mice with muscle-specific Acc2 deletion and mice with global Acc2 deletion are in different mixed genetic backgrounds. In neither case can we detect significant effects on energy balance associated with the loss of Acc2. Despite the decrease in ACC activity in our model, compensation occurs to maintain malonyl CoA levels and blunt changes in fatty acid oxidation rates. At a minimum, this involves down-regulation of malonyl CoA decarboxylase.
Whether more robust alterations in malonyl CoA levels and fatty acid oxidation rates in muscle could affect overall energy balance remains to be determined. Overall our findings suggest that selective inhibition of Acc2 alone will likely have little effect in treating obesity and its associated glucose intolerance. Future research will need to focus on the mechanisms underlying the down-regulation of malonyl CoA decarboxylase expression, which, if countered, might enhance the potential therapeutic effect of Acc2 inhibitors.
Materials and Methods
Generation of Mice with a Conditional Acc2 Allele.
The Acc2 targeting construct was created using recombineering techniques in a 129SvEV/AB2.2 bacterial artificial chromosome containing the Acc2 gene (clone bMQ103o12; Sanger Institute, Cambridge, UK). LoxP sites were positioned upstream of the preceding exon and downstream of the biotin binding domain exon using an Frt-flanked kanamycin resistance cassette. The targeting construct was electroporated into 129 ES cells and G418 resistant clones were selected under standard conditions. Appropriately targeted ES cells were identified by PCR and Southern blotting and were then injected into blastocysts and were implanted into pseudopregnant females by the Beth Israel Deaconess Medical Center's transgenic core. To generate the loxP-flanked (“floxed”) Acc2 allele, the Frt-flanked selection cassette was removed by breeding mice heterozygous for the Acc2 floxed allele to mice expressing Flp-recombinase (JAX Strain #003946). Deletion of the selection cassette was confirmed by PCR.
Detection of Acc2 Protein.
Muscle protein extracts were prepared using a lysis buffer containing 20 mM Tris, 50 mM NaCl, 50 mM, NaF, 5 mM Na4P2O7, 250 mM sucrose, 1% Triton ×100 by volume and 2 mM DTT with the protease inhibitors aprotinin, leupeptin, benzamide, and PMSF added. Protein concentration was determined using the BioRad DC protein assay. Protein extracts were subjected to electrophoresis through precast denaturing gels (Novex 4% or 4–12% gradient) and then transferred to nitrocellulose. Membranes were probed with either streptavidin conjugated to HRP (Pierce) or anti-ACC polyclonal antibody (#3662; Cell Signaling Technologies).
Genotyping Mice for the Altered Acc2 Allele.
Genotyping was performed on mouse tail DNA. The genotyping strategy was based on primers flanking the 5′-lox P site and a reverse primer distal to the 3′-lox P site. 5′-forward primer (P1): TGGTGGCTCCATAATTTGTGGG; 5′-reverse primer (P2): TTCTCTTTGGTGAAGGTCTGGG 3′-reverse primer (P3): TCTTGGTCTTTGCTCCAAGCAAG.
Animal Housing and Phenotyping Conditions.
Mice were maintained in accordance with the Institutional Animal Care and Use Committee (IACUC) of the Beth Israel Deaconess Medical Center. All animal studies were approved by IACUC at the Beth Israel Deaconess Medical Center. Mice were housed in a 14/10 light/dark cycle under controlled temperature (22–25 °C) and given free access to food and water. Standard chow diet was from Purina. High fat diet was 58% total calories from fat (D12331; Research Diets); high fat/high sucrose diet was 45% fat, 35% sucrose (D12451; Research Diets). Body composition analysis was performed on unsedated mice using an Echo MRI machine.
Comprehensive Metabolic Monitoring.
Animals were acclimatized in metabolic cages for 2 days before metabolic monitoring in a Columbus Instruments Metabolic Monitoring unit in the Beth Israel Deaconess Medical Center mouse physiology core. Mice were housed under controlled conditions with a 14/10 light/dark cycle and were given free access to food and water. Automated monitoring was performed for two days following acclimatization.
Glucose and Insulin Tolerance Tests.
Mice were fasted (6-16 hr) before i.p. injection of glucose (2 g/kg) or insulin (1 U/kg). Glucose was assayed from tail blood at times 0, 15 min, 30 min, 60 min, 90 min, 120 min, and 150 min after the injection.
Biochemical Assays.
Blood glucose was measured using One Touch Ultra glucometer. Insulin levels were assayed using the Ultrasensitive Rat Insulin Elisa assay (Crystal Chem) with mouse insulin standards. Total ACC activity was measured in crude muscle extracts prepared from skeletal muscle using a radiolabeled bicarbonate fixation assay as previously described (28). Activity is presented as citrate dependent activity measured in the presence of 2 mM citrate. Malonyl CoA and long chain fatty acyl-CoAs were measured in extracts from gastrocnemius muscle of fed mice as previously described using liquid chromatography/tandem MS analysis (26). Fatty acid oxidation rates in ex vivo soleus muscles were estimated by liberation of 14CO2 from 14C-labeled palmitate as previously described (29) except that 2% BSA, 5 mM glucose, 0.2 mM palmitate, and 0.5 uCi/mL 14C-palmitate were used during the labeling incubation. Oxidation rates for each mouse were the average of both soleus muscle measurements.
Statistical Analysis.
All values are presented as mean ± SEM. Differences between groups were assessed by unpaired t test. Statistically significant differences are indicated with asterisks: *P < 0.05; **P < 0.01; ***P < 0.001.
Supplementary Material
Acknowledgments
We thank L. Christiansen, Z. Yang, and C. Moodie for technical support, Dr. E. Olson for providing myogenin-Cre mice, and Dr. B. Kahn for helpful scientific discussions. This work is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (P01DK56116 to B.B.L. and K08DK071561 to D.P.O.) and Takeda Pharmaceuticals.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913492107/DCSupplemental.
References
- 1.Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- 2.Choi CS, et al. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci USA. 2007;104:16480–16485. doi: 10.1073/pnas.0706794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houmard JA. Intramuscular lipid oxidation and obesity. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1111–R1116. doi: 10.1152/ajpregu.00396.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bansode RR, Huang W, Roy SK, Mehta M, Mehta KD. Protein kinase C deficiency increases fatty acid oxidation and reduces fat storage. J Biol Chem. 2008;283:231–236. doi: 10.1074/jbc.M707268200. [DOI] [PubMed] [Google Scholar]
- 5.Jaworski K, et al. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat Med. 2009;15:159–168. doi: 10.1038/nm.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka T, et al. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci USA. 2003;100:15924–15929. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thupari JN, Landree LE, Ronnett GV, Kuhajda FP. C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity. Proc Natl Acad Sci USA. 2002;99:9498–9502. doi: 10.1073/pnas.132128899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drynan L, Quant PA, Zammit VA. Flux control exerted by mitochondrial outer membrane carnitine palmitoyltransferase over beta-oxidation, ketogenesis and tricarboxylic acid cycle activity in hepatocytes isolated from rats in different metabolic states. Biochem J. 1996;317:791–795. doi: 10.1042/bj3170791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eaton S. Control of mitochondrial beta-oxidation flux. Prog Lipid Res. 2002;41:197–239. doi: 10.1016/s0163-7827(01)00024-8. [DOI] [PubMed] [Google Scholar]
- 10.McGarry JD. Malonyl-CoA and carnitine palmitoyltransferase I: An expanding partnership. Biochem Soc Trans. 1995;23:481–485. doi: 10.1042/bst0230481. [DOI] [PubMed] [Google Scholar]
- 11.Brownsey RW, Boone AN, Elliott JE, Kulpa JE, Lee WM. Regulation of acetyl-CoA carboxylase. Biochem Soc Trans. 2006;34:223–227. doi: 10.1042/BST20060223. [DOI] [PubMed] [Google Scholar]
- 12.Abu-Elheiga L, et al. The subcellular localization of acetyl-CoA carboxylase 2. Proc Natl Acad Sci USA. 2000;97:1444–1449. doi: 10.1073/pnas.97.4.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saggerson D. Malonyl-CoA, a key signaling molecule in mammalian cells. Annu Rev Nutr. 2008;28:253–272. doi: 10.1146/annurev.nutr.28.061807.155434. [DOI] [PubMed] [Google Scholar]
- 14.Ruderman N, Flier JS. Cell biology. Chewing the fat—ACC and energy balance. Science. 2001;291:2558–2559. doi: 10.1126/science.1060277. [DOI] [PubMed] [Google Scholar]
- 15.Abu-Elheiga L, Oh W, Kordari P, Wakil SJ. Acetyl-CoA carboxylase 2 mutant mice are protected against obesity and diabetes induced by high-fat/high-carbohydrate diets. Proc Natl Acad Sci USA. 2003;100:10207–10212. doi: 10.1073/pnas.1733877100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harwood HJ., Jr Acetyl-CoA carboxylase inhibition for the treatment of metabolic syndrome. Curr Opin Investig Drugs. 2004;5:283–289. [PubMed] [Google Scholar]
- 17.Harwood HJ., Jr Treating the metabolic syndrome: Acetyl-CoA carboxylase inhibition. Expert Opin Ther Targets. 2005;9:267–281. doi: 10.1517/14728222.9.2.267. [DOI] [PubMed] [Google Scholar]
- 18.Oh W, et al. Glucose and fat metabolism in adipose tissue of acetyl-CoA carboxylase 2 knockout mice. Proc Natl Acad Sci USA. 2005;102:1384–1389. doi: 10.1073/pnas.0409451102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee EC, et al. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- 20.Lewandoski M, Wassarman KM, Martin GR. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr Biol. 1997;7:148–151. doi: 10.1016/s0960-9822(06)00059-5. [DOI] [PubMed] [Google Scholar]
- 21.Li S, et al. Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice. Proc Natl Acad Sci USA. 2005;102:1082–1087. doi: 10.1073/pnas.0409103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Essop MF, et al. Reduced heart size and increased myocardial fuel substrate oxidation in ACC2 mutant mice. Am J Physiol Heart Circ Physiol. 2008;295:H256–H265. doi: 10.1152/ajpheart.91489.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tong L, Harwood HJ., Jr Acetyl-coenzyme A carboxylases: Versatile targets for drug discovery. J Cell Biochem. 2006;99:1476–1488. doi: 10.1002/jcb.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koves TR, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Harada N, et al. Hepatic de novo lipogenesis is present in liver-specific ACC1-deficient mice. Mol Cell Biol. 2007;27:1881–1888. doi: 10.1128/MCB.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savage DB, et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006;116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoehn KL, et al. Acute or chronic upregulation of mitochondrial fatty acid oxidation has no net effect on whole-body energy expenditure or adiposity. Cell Metab. 2010;11:70–76. doi: 10.1016/j.cmet.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minokoshi Y, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 29.Bruce CR, Dyck DJ. Cytokine regulation of skeletal muscle fatty acid metabolism: Effect of interleukin-6 and tumor necrosis factor-alpha. Am J Physiol Endocrinol Metab. 2004;287:E616–E621. doi: 10.1152/ajpendo.00150.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.