Abstract
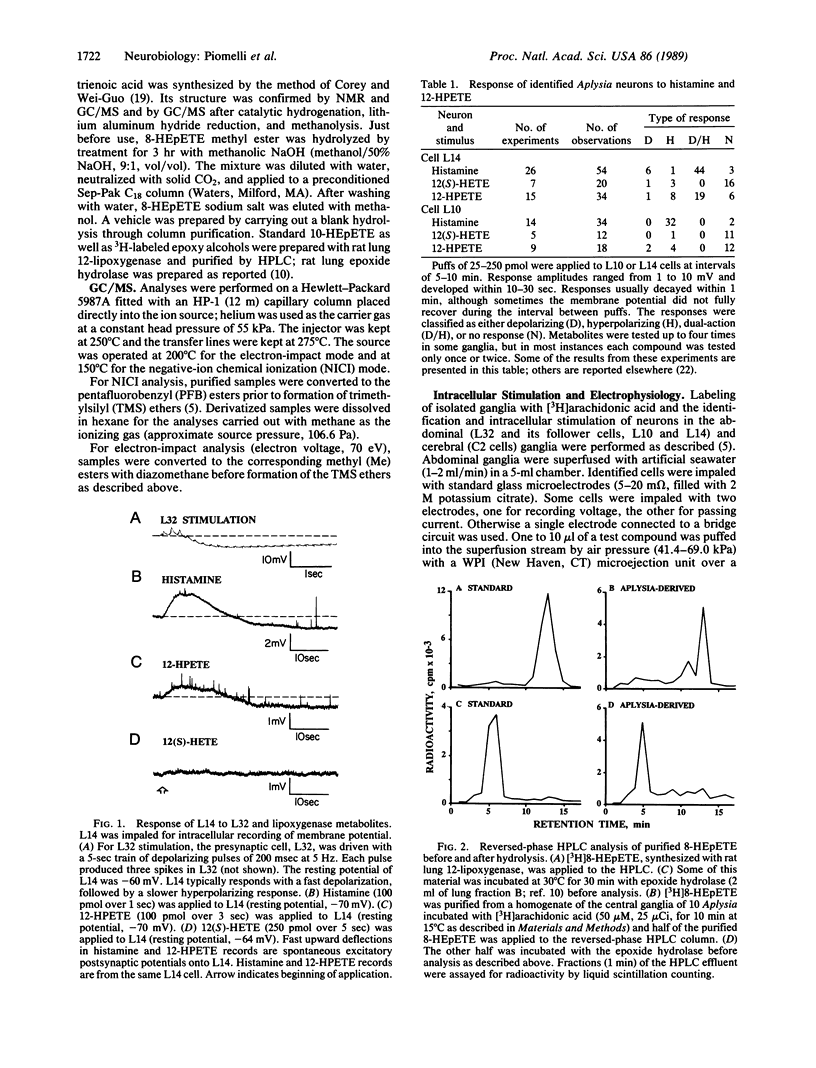
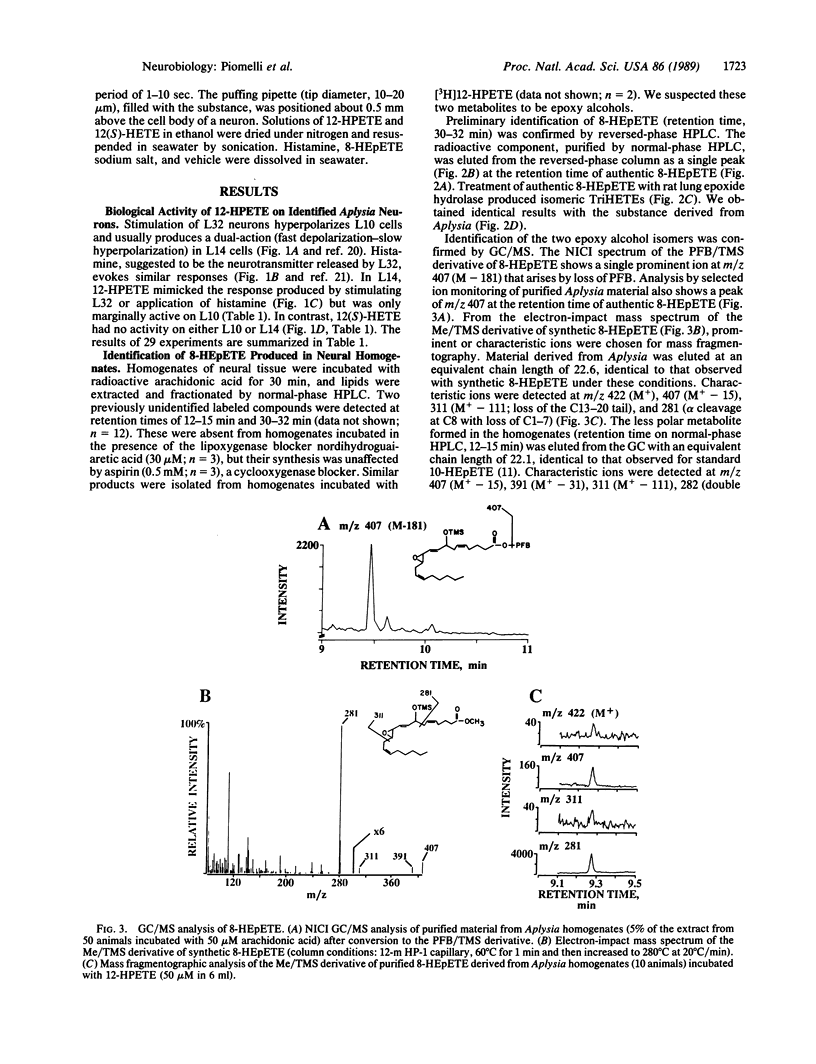
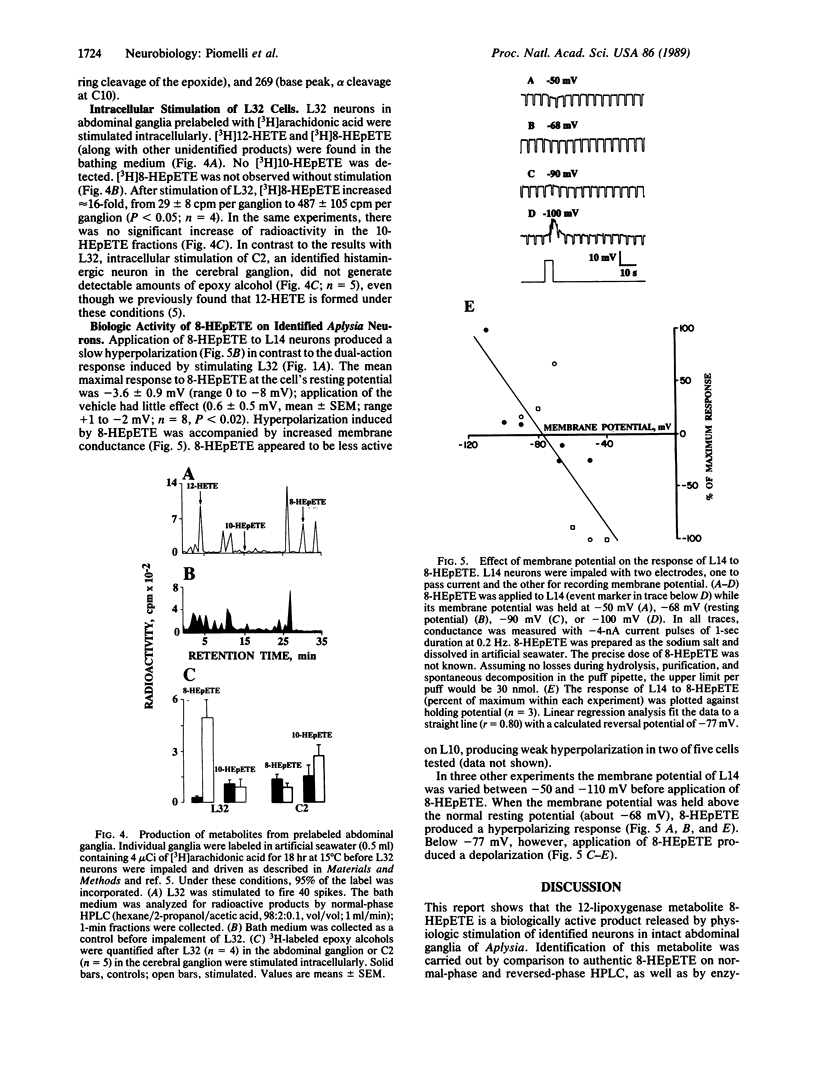
In Aplysia neural tissue, the release and metabolism of arachidonic acid are stimulated by histamine or by activation of the identified L32 nerve cell circuit of the abdominal ganglion. Previously we found that histamine and intracellular stimulation of L32 cells, which are putatively histaminergic neurons, cause the production of 12-hydroxy-5,8,10,14-icosatetraenoic acid (12-HETE), a product of the 12-lipoxygenase pathway formed through 12-hydroperoxy-5,8,10,14-icosatetraenoic acid (12-HPETE). 12-HPETE, but not 12(S)-HETE, mimics the dual-action response of L14 ink motor neurons to histamine and stimulation of L32. 12-HPETE can also be further metabolized to 8-hydroxy-11,12-epoxy-5,9,14-icosatrienoic acid (8-HEpETE) which was identified by HPLC, enzymatic hydrolysis, and GC/MS. Production of 8-HEpETE is specific, as its positional isomer 10-hydroxy-11,12-epoxy-5,8,14-icosatrienoic acid is not formed after physiologic stimulation. 8-HEpETE can elicit the late component (hyperpolarization) of the dual-action response in L14 cells, suggesting that it may be a second messenger in Aplysia.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant R. W., Bailey J. M. Isolation of a new lipoxygenase metabolite of arachidonic acid, 8. 11, 12-trihydroxy-5,9,14-eicosatrienoic acid from human platelets. Prostaglandins. 1979 Jan;17(1):9–18. doi: 10.1016/0090-6980(79)90071-6. [DOI] [PubMed] [Google Scholar]
- Byrne J. H. Neural circuit for inking behavior in Aplysia californica. J Neurophysiol. 1980 Apr;43(4):896–911. doi: 10.1152/jn.1980.43.4.896. [DOI] [PubMed] [Google Scholar]
- Derewlany L. O., Pace-Asciak C. R., Radde I. C. Hepoxilin A, hydroxyepoxide metabolite of arachidonic acid, stimulates transport of 45Ca across the guinea pig visceral yolk sac. Can J Physiol Pharmacol. 1984 Dec;62(12):1466–1469. doi: 10.1139/y84-243. [DOI] [PubMed] [Google Scholar]
- Eisenstadt M., Goldman J. E., Kandel E. R., Koike H., Koester J., Schwartz J. H. Intrasomatic injection of radioactive precursors for studying transmitter synthesis in identified neurons of Aplysia californica. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3371–3375. doi: 10.1073/pnas.70.12.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashida H., Brown D. A. Two polyphosphatidylinositide metabolites control two K+ currents in a neuronal cell. 1986 Sep 25-Oct 1Nature. 323(6086):333–335. doi: 10.1038/323333a0. [DOI] [PubMed] [Google Scholar]
- Kretz R., Shapiro E., Bailey C. H., Chen M., Kandel E. R. Presynaptic inhibition produced by an identified presynaptic inhibitory neuron. II. Presynaptic conductance changes caused by histamine. J Neurophysiol. 1986 Jan;55(1):131–146. doi: 10.1152/jn.1986.55.1.131. [DOI] [PubMed] [Google Scholar]
- Kretz R., Shapiro E., Kandel E. R. Presynaptic inhibition produced by an identified presynaptic inhibitory neuron. I. Physiological mechanisms. J Neurophysiol. 1986 Jan;55(1):113–130. doi: 10.1152/jn.1986.55.1.113. [DOI] [PubMed] [Google Scholar]
- Miyamoto T., Lindgren J. A., Hökfelt T., Samuelsson B. Regional distribution of leukotriene and mono-hydroxyeicosatetraenoic acid production in the rat brain. Highest leukotriene C4 formation in the hypothalamus. FEBS Lett. 1987 May 25;216(1):123–127. doi: 10.1016/0014-5793(87)80769-x. [DOI] [PubMed] [Google Scholar]
- Nugteren D. H. Arachidonate lipoxygenase in blood platelets. Biochim Biophys Acta. 1975 Feb 20;380(2):299–307. doi: 10.1016/0005-2760(75)90016-8. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak C. R. Arachidonic acid epoxides. Demonstration through [18O]oxygen studies of an intramolecular transfer of the terminal hydroxyl group of (12S)-hydroperoxyeicosa-5,8,10,14-tetraenoic acid to form hydroxyepoxides. J Biol Chem. 1984 Jul 10;259(13):8332–8337. [PubMed] [Google Scholar]
- Pace-Asciak C. R. Formation and metabolism of hepoxilin A3 by the rat brain. Biochem Biophys Res Commun. 1988 Feb 29;151(1):493–498. doi: 10.1016/0006-291x(88)90620-1. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak C. R., Granström E., Samuelsson B. Arachidonic acid epoxides. Isolation and structure of two hydroxy epoxide intermediates in the formation of 8,11,12- and 10,11,12-trihydroxyeicosatrienoic acids. J Biol Chem. 1983 Jun 10;258(11):6835–6840. [PubMed] [Google Scholar]
- Pace-Asciak C. R. Hemoglobin- and hemin-catalyzed transformation of 12L-hydroperoxy-5,8,10,14-eicosatetraenoic acid. Biochim Biophys Acta. 1984 May 11;793(3):485–488. doi: 10.1016/0005-2760(84)90267-4. [DOI] [PubMed] [Google Scholar]
- Piomelli D., Feinmark S. J., Shapiro E., Schwartz J. H. Formation and biological activity of 12-ketoeicosatetraenoic acid in the nervous system of Aplysia. J Biol Chem. 1988 Nov 15;263(32):16591–16596. [PubMed] [Google Scholar]
- Piomelli D., Shapiro E., Feinmark S. J., Schwartz J. H. Metabolites of arachidonic acid in the nervous system of Aplysia: possible mediators of synaptic modulation. J Neurosci. 1987 Nov;7(11):3675–3686. doi: 10.1523/JNEUROSCI.07-11-03675.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D., Volterra A., Dale N., Siegelbaum S. A., Kandel E. R., Schwartz J. H., Belardetti F. Lipoxygenase metabolites of arachidonic acid as second messengers for presynaptic inhibition of Aplysia sensory cells. Nature. 1987 Jul 2;328(6125):38–43. doi: 10.1038/328038a0. [DOI] [PubMed] [Google Scholar]
- Sautebin L., Spagnuolo C., Galli C., Galli G. A mass fragmentographic procedure for the simultaneous determination of HETE and PGF2alpha in the central nervous system. Prostaglandins. 1978 Dec;16(6):985–988. doi: 10.1016/0090-6980(78)90115-6. [DOI] [PubMed] [Google Scholar]
- Walker I. C., Jones R. L., Wilson N. H. The identification of an epoxy-hydroxy acid as a product from the incubation of arachidonic acid with washed blood platelets. Prostaglandins. 1979 Aug;18(2):173–178. doi: 10.1016/0090-6980(79)90102-3. [DOI] [PubMed] [Google Scholar]
- Wittenberg B. A., Briehl R. W., Wittenberg J. B. Haemoglobins of invertebrate tissues. Nerve haemoglobins of Aphrodite, Aplysia and Halosydna. Biochem J. 1965 Aug;96(2):363–371. doi: 10.1042/bj0960363. [DOI] [PMC free article] [PubMed] [Google Scholar]



