Abstract
Fluorescent bisretinoids, such as A2E and all-trans-retinal dimer, form as a by-product of vitamin A cycling in retina and accumulate in retinal pigment epithelial (RPE) cells as lipofuscin pigments. These pigments are implicated in pathological mechanisms involved in several vision-threatening diseases including age-related macular degeneration. Efforts to understand damaging events initiated by these bisretinoids have revealed that photoexcitation of A2E by wavelengths in the visible spectrum leads to singlet oxygen production and photooxidation of A2E. Here we have employed liquid chromatography coupled to electrospray ionization mass spectrometry together with tandem mass spectrometry (MS/MS), to demonstrate that A2E also undergoes photooxidation-induced degradation and we have elucidated the structures of some of the aldehyde-bearing cleavage products. Studies in which A2E was incubated with a singlet oxygen generator yielded results consistent with a mechanism involving bisretinoid photocleavage at sites of singlet molecular oxygen addition. We provide evidence that one of the products released by A2E photodegradation is methylglyoxal, a low molecular weight reactive dicarbonyl with the capacity to form advanced glycation end products. Methylglyoxal is already known to be generated by carbohydrate and lipid oxidation; this is the first report of its production via bisretinoid photocleavage. It is significant that AGE-modified proteins are detected in deposits (drusen) that accumulate below RPE cells in vivo; drusen have been linked to age-related macular degeneration pathogenesis. Whereas various processes play a role in drusen formation, these findings are indicative of a contribution from lipofuscin photooxidation in RPE.
Keywords: advanced glycation end products, lipofuscin, photofragmentation, photooxidation, retinal pigment epithelial cells
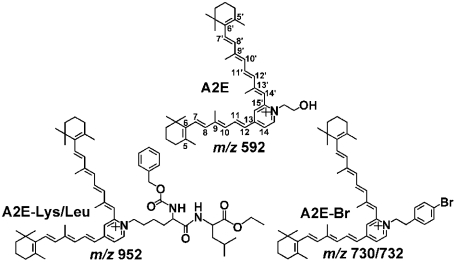
Fluorescent bisretinoid pigments are amassed as lipofuscin in retinal pigment epithelial (RPE) cells in association with aging although individuals vary with respect to the extent of accumulation (1). The excessive deposition of these compounds in RPE cells is also considered to lead to retinal degeneration in early onset blinding disorders associated with mutations in the genes encoding ABCA4 (ATP-binding cassette subfamily A member 4) (2, 3), ELOVL4 (4), and BEST-1 (5). Moreover, the deposition of these pigments likely also contributes to the etiology of age-related macular degeneration (AMD) (6, 7). Whereas these bisretinoids constitute a complex mixture, all appear to originate from reactions of all-trans-retinal and some have been identified, including A2E (Fig. 1) and its isomers (8); A2-dihydropyridine-phosphatidylethanolamine (A2-DHP-PE) and A2-dihydropyridine-ethanolamine (A2-DHP-E) (9); and all-trans-retinal dimer, all-trans-retinal dimer-phosphatidylethanolamine (all-trans-retinal dimer-PE), and all-trans-retinal dimer-ethanolamine (all-trans-retinal dimer-E) (10, 11). Structural features common to all of these pigments are the alternating single and double carbon–carbon bonds that originate from an aromatic head group, and extend along the two sidearms of the molecules and into the terminal β-ionone rings. These extended conjugation systems allow for absorption and excitation by light in the short wavelength region of the visible spectrum (A2E: λmax, 439 nm; A2-DHP-PE: λmax 490 nm; all-trans-retinal dimer: λmax, 430 nm). In the case of all-trans-retinal dimer-PE and all-trans-retinal dimer-E, an additional red-shift to 510 nm is associated with protonation of the Schiff base nitrogen (11).
Fig. 1.
Structures of A2E, A2E-Lysine/Leucine (A2E-Lys/Leu) and A2E-bromine (A2E-Br).
Photochemical reactions initiated by these bisretinoid pigments likely contribute to the adverse effects of RPE lipofuscin accumulation. Specifically, A2E and all-trans-retinal dimer are photosensitizers for the production of singlet oxygen; of these two bisretinoids all-trans-retinal dimer is the more efficient producer (11, 12). Both of these bisretinoids also chemically quench singlet oxygen with the result that polyoxidized forms of these compounds are generated. The participation of singlet oxygen in the photooxidation of A2E and all-trans-retinal dimer is indicated by the finding that exposure to a singlet oxygen generator produced the same forms of oxidized A2E as was observed with irradiation by short wavelength visible light. Moreover, photooxidation is potentiated when the lifetime of singlet oxygen is extended in the presence of deuterium oxide (12, 13). Further evidence comes from the inhibitory effects of known quenchers/scavengers of singlet oxygen (14). At least in the case of A2E, electron spin resonance spectroscopy indicates that superoxide anion is also generated following 430 nm irradiation of the compound (15). The oxygen-containing moieties ensuing from the photoexcitation of A2E and all-trans-retinal dimer include epoxides, furanoid oxides, and cyclic peroxides (11–13, 16, 17). Oxidized forms of A2E and all-trans-retinal dimer have been detected in human and mouse RPE cells (11, 13).
By utilizing liquid chromatography (LC) coupled to electrospray ionization mass spectrometry (ESI-MS) together with tandem mass analysis (MS/MS), we have characterized photooxidation-induced cleavage products of A2E and we have shown that photolysis occurs at sites of singlet oxygen addition. The detection of these photo-products was facilitated by utilizing A2E that was tagged either by introducing a bromine label to the pyridinium head-group of A2E (A2E-bromine; A2E-Br) or by labeling A2E with a dipeptide linked to the pyridinium ring (A2E-lysine/leucine; A2E-Lys/Leu) (Fig. 1). These nonphysiological tags signaled cleavage products that carried the pyridinium head group of A2E. By observing similar cleavage products using multiple strategies, we demonstrated the reproducibility of our findings.
Results
Photooxidation of A2E, A2E-Br, and A2E-Lys/Leu.
Fast atom bombardment ionization mass spectrometry (FAB-MS) analysis revealed that 430 nm irradiation of A2E, A2E-Br and A2E-Lys/Leu (Fig. 1), lead to spectra characterized by m/z peaks at 592, 730/732, and 952 attributable to A2E, A2E-Br, and A2E-Lys/Leu, respectively, together with a series of m/z + n*16 peaks indicating starting compounds modified by addition or insertion of oxygen atoms (Fig. S1). These results confirmed that tagged-A2E underwent photooxidation in the same fashion as A2E. The mass spectra generated after A2E, A2E-Br, or A2E-Lys/Leu were incubated with the singlet oxygen generator, endoperoxide of 1,4-dimethylnaphthalene, also reflected profiles indicative of oxidation (Fig. S1).
LC/ESI-MS and ESI-MS Analysis of Photooxidation-Associated Cleavage Products of A2E.
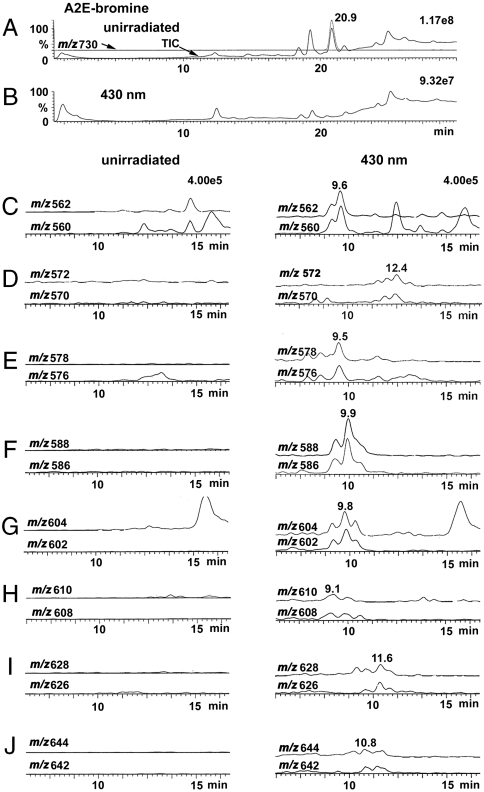
The mixture of lower molecular weight photo-induced cleavage products generated by irradiation of tagged A2E (A2E-Br) (Fig. 1) was subsequently studied by LC/MS (Fig. 2) using an electrospray ionization (ESI) source operating in positive ion mode. Because bromine imparts a characteristic isotope pattern in the mass-spectrum—two peaks of almost equal height separated by 2 m/z units due to isotopes 79Br and 81Br—the bromine tag was indicative of cleavage products that included the pyridinium head group of A2E-Br. Fig. 2 shows an overlay of total ion chromatogram and mass chromatogram of samples of A2E-Br that were unirradiated and irradiated for 10 min and monitored at m/z 730 (Fig. 2 A and B). The peak at 20.9 min is attributable to A2E-Br; this signal was greatly diminished by 430 nm irradiation. Importantly, selected molecular ion monitoring (Fig. 2 C–J) revealed 8 peaks (m/z 562/560, 572/570, 578/576, 588/586, 604/602, 610/608, 628/626, and 644/642) that carried the bromine tag and were lower in mass than m/z 730; these peaks were also not present in unirradiated samples of A2E-Br and eluted earlier than A2E-Br, consistent with oxidized products that are more polar than the parent compound. It should be noted that in some cases multiple peaks of equal abundance were detected at a given mass but different retention times. Some of these peaks are likely cis/trans isomers, isobaric with the major components and others could reflect isomers generated by photocleavage at one or other of the side-arms of A2E-Br. LC/ESI-MS analysis of irradiated samples of A2E-Lys/Leu (m/z 952) revealed five m/z signals (m/z 782, 792, 808, 824, 892) (Fig. S2) that matched photocleavage products generated with A2E-Br, after the mass of the Br and Lys/Leu tags were accounted for (Table 1).
Fig. 2.
LC/ESI-MS analysis of photooxidation-induced cleavage of bromine-tagged A2E. (A2E-Br; m/z 730) (A) Overlay of total ion chromatogram and mass chromatogram for m/z 730 recorded from unirradiated sample of A2E. (B) Total ion chromatogram of irradiated (430 nm; 10 min) sample of A2E. (C–J) Mass chromatograms at m/z 560/562, 570/572, 576/578, 586/588, 602/604, 608/610, 626/628, and 642/644 for unirradiated (Left) and irradiated (Right) samples of A2E-Br. Molecular ion monitoring was performed for bromide isotopes Br-79 (Upper Traces) and Br-81 (Lower Traces). Mass chromatograms were acquired in positive ESI mode and recorded as a function of retention time.
Table 1.
Mass values (m/z) for A2E, photooxidized A2E (+16 m/z) and photooxidation-induced cleavage products. Corresponding m/z values for A2E and two forms of tagged A2E: A2E-Br and A2E-Lys/Leu
| A2E[M]+ | A2E-Br[M]+ | A2E-Lys/Leu[M]+ | |
| m/z | 592 | 730/732 | 952 |
| oxidation (+16) | 608 | 746/748 | 968 |
| 624 | 762/764 | 984 | |
| 640 | 778/780 | 1000 | |
| 656 | 794/796 | 1016 | |
| 672 | 810/812 | 1032 | |
| 688 | 826/828 | 1048 | |
| 704 | 842/844 | 1064 | |
| 720 | 858/860 | 1080 | |
| 736 | 874/876 | 1096 | |
| photocleavage | 422 | 560/562 | 782 |
| 432 | 570/572 | 792 | |
| 438 | 576/578 | 798 | |
| 448 | 586/588 | 808 | |
| 464 | 602/604 | 824 | |
| 470 | 608/610 | 830 | |
| 488 | 626/628 | 848 | |
| 504 | 642/644 | 864 |
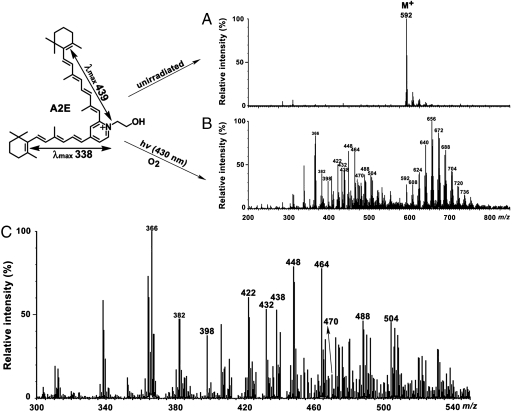
Guided by our findings from LC/ESI-MS, we turned to direct-injection ESI-MS to test the reproducibility of the photo-induced cleavage pattern we had observed. Accordingly, ESI-MS analysis of photo-induced fragments generated from untagged A2E (Fig. 3), revealed 8 photoproducts (m/z 422, 432, 438, 448, 464, 470, 488, and 504) corresponding to the photocleavage products generated with A2E-Br (Table 1). Several other lower molecule weight species were also detected.
Fig. 3.
ESI mass spectral analysis of A2E (untagged). Samples of A2E (m/z 592) were unirradiated (A) and irradiated at 430 nm (hv) for 30 min (B); expanded view of panel B in the range of m/z 300–540 (C). The series of peaks at m/z 608, 624, 640, 656, 672, 688, 704, 720, and 736 reflect photooxidation (m/z + n ∗ 16; n = 1,2,…,9) at carbon–carbon double bonds (B). Lower mass peaks (< m/z592) correspond to A2E fragments forming due to photooxidation-induced cleavage (panels B and C). Peaks from m/z 422 to 504 that are labeled in bold, correspond to photocleavage products also identified by LC-MS. (Upper Left) Structure of A2E. Absorbance peaks at 439 nm and 338 nm can be assigned to the long and short arms of the molecule, respectively.
Characterization of A2E Photocleavage Products by Tandem Mass Spectrometry.
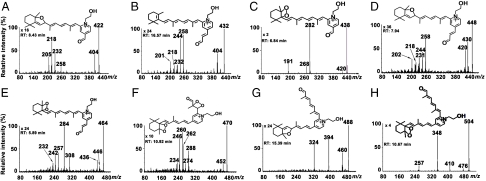
For structural characterization of 8 photooxidation-induced A2E cleavage products identified by LC/ESI-MS (m/z 422, 432, 438, 448, 464, 470, 488, and 504), tandem mass spectrometric analysis (MS/MS) was performed in the ESI positive-ion mode (Fig. 4). The MS/MS fragmentation processes leading to structural assignments for the photocleavage products corresponding to the precursor molecular ions (Fig. 4 A–H, Insets) involved rearrangements, eliminations, cyclizations, and bond cleavages; these MS/MS mechanisms are presented in Fig. S3, Fig. S4, Fig. S5, and Fig. S6 and are described in SI Text. In assigning structures to the precursor molecular ions, we were informed both by our previous studies of A2E (13), and by mass spectrometry principles. Specifically, our earlier work indicated that the first oxidation would occur at the C5–C6 double bond on the β-ionone ring of the short arm and that the next oxidation would be at the C5′–C6′ double bond on the long arm. In addition, the presence of a stable 5,8/5′,8′-monofuran would be consistent with previously described mechanisms (13, 17) whereas the C–C single bond associated with the peroxide at the 5, 6 (or 5′, 6′) position in the cyclohexenyl ring would remain intact despite O–O bond photocleavage (13). In addition, the structures proposed for the precursor molecular ions were guided by mass spectrometry principles (18): (i) direct MS/MS cleavage at double bonds does not occur because of the high energy requirements (19); (ii) MS/MS fragmentation processes generally involve a loss of water from a substituted ethanol group and a loss of CO from an aldehyde; in the case of an aldehyde conjugated to the pyridinium ring, no loss of CO is observed; (iii) even-mass fragment ions containing the positive charge of the pyridinium group would form via homolytic cleavages at charge remote sites; (iv) odd-mass product ion peaks would be radicals due to charge remote radical homolytic cleavages wherein the charge resides on the pyridinium group and a free radical resides at the fragmentation site; (v) homolytic charge remote site reactions would involve hydrogen and/or methyl rearrangements; (vi) oxygens could be added to aldehyde structures to satisfy elemental compositions and peroxides could undergo losses of atomic oxygen; and (vii) the C12–C13 and C14′–C15′ single bonds of A2E would be strengthened by the C11–C12 and C13′–C14′ double bonds, respectively, that are conjugated with double bonds situated in the aromatic ring (20, 21). Importantly, the proposed structures of the precursor and product ions of the 8 photocleavage products, were also supported by exact-mass measurements performed utilizing ESI-Fourier transform-ion cyclotron resonance (FT-ICR) MS and MS/MS. The most probable elemental compositions for these ions were obtained with a high degree of confidence: Based on the difference between the experimental mass and the predicted mass, the errors were within ± 2.7 ppm (Table S1).
Fig. 4.
ESI-MS/MS spectra and proposed structures of photooxidation-induced cleavage products of A2E. Product ion spectra of [M]+ ions at m/z 422 (A), 432 (B), 438 (C), 448 (D), 464 (E), 470 (F), 488 (G), and 504 (H). Proposed MS/MS mechanisms operating in the formation of the structurally informative product ions are presented in Fig. S3, Fig. S4, Fig. S5, and Fig. S6. The retention times indicated were obtained from LC/ESI-MS. × n, abundance multiplication factor versus the parent ion.
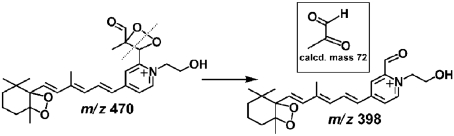
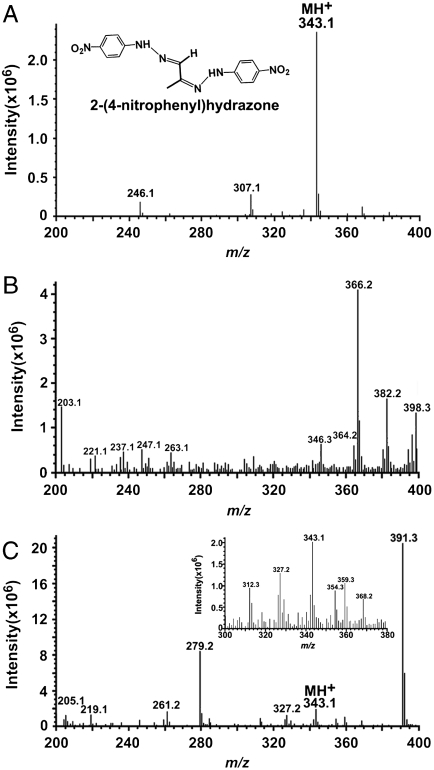
Included within the complex mixture of photocleavage products was a species at m/z 470 (RT 10.98 min) that was present in low abundance (Fig. 4F). The proposed structure of this product revealed an unstable photo-fragment that originated from an A2E molecule that had undergone peroxide formation at at least 3 sites on the long arm (Fig. 5): (i) at the C5′–C6′ double bonds on the β-ionone ring where the first oxidation is known to occur (13); (ii) at the C11′–C12′ double bond where cleavage occurred producing the aldehyde group; and (iii) at the C13′–C14′ double bond. We expect that the m/z 470 photocleavage product was of low abundance because further cleavage would occur following opening of the C13′–C14′ peroxide ring. Photocleavage here would result in a photo-fragment of mass 398 Da together with a dicarbonyl molecule (methylglyoxal; calculated molecular weight 72 Da) (Fig. 5). Indeed, this photocleavage mechanism likely occurs because a species at m/z 398 is detectable by ESI-MS (Fig. 3). Further corroboration was obtained by nanoelectrospray FT-ICR MS, as the experimental mass of this product ion was shown to be 398.2329, consistent with the predicted elemental formula (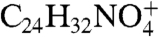 ) (theoretical mass 398.2326; error, 0.1 ppm). It should be mentioned that methylglyoxal is highly toxic and is an agent involved in formation of advanced glycation end (AGE) products (22, 23). To obtain additional evidence for methylglyoxal release following A2E photo-oxidation, we trapped this volatile fragment with 4-nitrophenylhydrazine (4-NPH), a compound well known for reacting with carbonyl groups. As shown in Fig. 6, reaction of 4-NPH with commercially available methylglyoxal, yielded the expected 2-(4-nitrophenyl)hydrazone product (Fig. S7A) at m/z 343.1 ([M + H]+). Pooled samples of irradiated A2E were then incubated/not incubated with 4-NPH and evaluated by ESI-MS. Accordingly, we observed a production peak at m/z 343.1 (Fig. 6C) that in the absence of 4-NPH was not produced (Fig. 6B); this peak could be explained by the facile reaction of 4-NPH with the released photo-product methylglyoxal. Incubation with 4-NPH also eliminated peaks at m/z 366, 382, and 398 (Fig. 6C) that were observed with irradiated A2E in the absence of 4-NPH (Fig. 6B and Fig. 3C). The eradication of these peaks by reaction with 4-NPH confirmed that these photo-fragments were aldehyde-bearing. Subsequent analysis by nanoelectrospray FT-ICR MS established that the experimental mass of the nitrophenyl hydrazone product was 343.11484, consistent with the predicted elemental formula (
) (theoretical mass 398.2326; error, 0.1 ppm). It should be mentioned that methylglyoxal is highly toxic and is an agent involved in formation of advanced glycation end (AGE) products (22, 23). To obtain additional evidence for methylglyoxal release following A2E photo-oxidation, we trapped this volatile fragment with 4-nitrophenylhydrazine (4-NPH), a compound well known for reacting with carbonyl groups. As shown in Fig. 6, reaction of 4-NPH with commercially available methylglyoxal, yielded the expected 2-(4-nitrophenyl)hydrazone product (Fig. S7A) at m/z 343.1 ([M + H]+). Pooled samples of irradiated A2E were then incubated/not incubated with 4-NPH and evaluated by ESI-MS. Accordingly, we observed a production peak at m/z 343.1 (Fig. 6C) that in the absence of 4-NPH was not produced (Fig. 6B); this peak could be explained by the facile reaction of 4-NPH with the released photo-product methylglyoxal. Incubation with 4-NPH also eliminated peaks at m/z 366, 382, and 398 (Fig. 6C) that were observed with irradiated A2E in the absence of 4-NPH (Fig. 6B and Fig. 3C). The eradication of these peaks by reaction with 4-NPH confirmed that these photo-fragments were aldehyde-bearing. Subsequent analysis by nanoelectrospray FT-ICR MS established that the experimental mass of the nitrophenyl hydrazone product was 343.11484, consistent with the predicted elemental formula (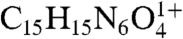 ; theoretical mass 343.11493; error, 0.26 ppm) and confirmatory of the reaction of 4-NPH with the photo-product methylglyoxal.
; theoretical mass 343.11493; error, 0.26 ppm) and confirmatory of the reaction of 4-NPH with the photo-product methylglyoxal.
Fig. 5.
Photochemical mechanism proposed for conversion of m/z 470 photocleavage product to m/z 398 photo-product. Cleavages at the O–O bond and C–C single bond at the C13′–C14′ peroxide ring yields an aldehyde-bearing photo-product with m/z 398 and a smaller dicarbonyl molecule (methylglyoxal) with calculated molecular weight 72 (Inset).
Fig. 6.
Detection of methylglyoxal by derivatization with 4-nitrophenylhydrazine (4-NPH). ESI-MS spectra in the range m/z 200–400. (A) Incubation of 4-NPH with methylglycoxal (obtained commercially) in ethanol yielded the m/z 343.1 product 2-(4-nitrophenyl)hydrazone (inset). (B) Cleavage products of A2E in photooxidized sample (irradiation at 430 nm). 4-NPH. The presence of 2-(4-nitrophenyl)hydrazone (m/z 343.1) is indicative of the reaction of 4-NPH with methylglyoxal in the mixture of A2E photodegradation products. Inset, MS profile expanded between m/z 300 and 380.
LC/ESI-MS Following Incubation of A2E with a Singlet Oxygen Generator.
Photocleavage along the side-arms of A2E could occur at sites where endoperoxide (O–O) moieties form subsequent to addition of singlet molecular oxygen at carbon–carbon double bonds. Evidence in support of this mechanism was obtained from samples of A2E-bromine incubated with the singlet oxygen generator endoperoxide of 1,4-dimethylnaphthalene. As presented in SI Text and Fig. S8, LC/ESI-MS analysis of the resulting photocleavage products revealed 5 peaks (m/z 572/570, 578/576, 604/602, 610/608, and 644/642) that were lower in mass than the parent ion A2E-Br (m/z 730/732). These bromine isotope doublet peaks also corresponded to molecular ions (m/z 432, 438, 464, 470, and 504) detected in samples of A2E (untagged) that were irradiated at 430 nm (Fig. 3 and Table 1). Cleavage at the peroxide sites would result in the formation of aldehyde (-CHO) functional groups (Fig. S7B). Consistent with this mechanism, the 1H-NMR spectrum of irradiated samples of A2E exhibited a broad resonance at 9.3 ppm indicative of a proton signal specific to an aldehyde moiety; this signal was not present in a sample of unirradiated A2E. These data are consistent with a previous report of aldehyde formation following A2E oxidation (17).
Discussion
The extensive conjugation systems situated along the side-arms of A2E are linked through a pyridinium ring that houses a nitrogen carrying an inherent positive charge. This system renders the compound amenable to ESI in the positive ionization mode. Accordingly, we have utilized ESI-MS to identify products of photocleavage occurring within the polyenic structures that comprise the side-arms of A2E. Taken together, the results reported here are consistent with a mechanism by which A2E photocleavage occurs at sites of singlet molecular oxygen addition to carbon–carbon double bonds (Fig. S7B). At each of these sites, opening of both O–O and C–C bonds gives rise to two fragments each bearing an aldehyde moiety. Aldehydes are reactive species that form Schiff bases with amino groups and can be expected to play havoc in biological systems. Photocleavage products bearing the pyridinium ring moiety carried a positive charge and were readily monitored. Equally interesting, however, are the complementary noncharged residual photo-products (Fig. S7B) that would be released upon photofragmentation; some of these would bear a β-ionone ring. However, because the fragments originating distal to the cleavage site are uncharged, they were undetectable by ESI-MS. Nevertheless, it is clear that numerous photo-products of a variety of molecular weights were generated (Fig. 3), with many neutral photocleavage products having molecular weights complementing the detectable products (Fig. 5). In addition, because our research indicates that atomic oxygen can also insert at the carbon–carbon double bonds (12, 15) (Fig. 3), many of these photo-fragments could carry furanoid or epoxide moieties (Fig. 4). Because we have identified the oxidized forms of A2E and all-trans-retinal dimer in human and mice eyes, it is clear that these photooxidation processes are ongoing in vivo (11, 13).
In mice that have accumulated RPE lipofuscin bisretinoids while raised under cyclic light, subsequent dark-rearing does not result in the addition or loss of these compounds (24). On the other hand, it is clear that considerable degradation of the bisretinoids can occur as a consequence of photo-oxidation; over time this process could result in considerable loss of bisretinoid pigment even as newly formed pigment is being deposited. Consequently, measurements of A2E, all-trans-retinal dimer compounds and A2-DHP-PE in the eye at any given time, do not likely reflect the true burden placed on the RPE cells by the deposition of these bisretinoids throughout the life of an individual.
Products of A2E photooxidative cleavage are implicated as agents that perturb the RPE cell and its surroundings. For instance, we have shown that cellular injury can be induced by photooxidized A2E under conditions that eliminate singlet oxygen or other reactive forms of oxygen as the immediate cause of damage (25). It is significant that the current work has revealed that photocleavage of A2E can produce a low molecular weight product (m/z 72), methylglyoxal; this reactive dicarbonyl can alter molecular structure and function by forming AGE-adducts with proteins, phospholipids, and nucleotides. Methylglyoxal is known to form as a by-product of metabolic pathways such as glycolysis and lipid peroxidation (26, 27). Here we present evidence for an additional unique source—the bisretinoids of RPE lipofuscin. Because there are 3 methyl groups distributed along the side-arms of A2E and the double bonds both proximal and distal to these methyl groups have the potential to undergo photocleavage (Fig. 1), three moles of methylglyoxal per mole of A2E could be released in this way. AGE-modified proteins contributes to age-related inflammatory disease (28) and along with complement-related proteins, cholesterol, and other molecules, have been detected in aged Bruch’s membrane and drusen (29–31), the subRPE deposits that have been linked to AMD (32).
Diffusion of A2E photocleavage products such as methylglyoxal is suggested by the finding that photooxidation of intracellular A2E is associated with DNA damage even though the parent bisretinoid is housed in lysosomes (33). We have also previously observed that when cultured RPE cells that have accumulated A2E are irradiated (430 nm), the extracellular fibronectrin substrate on which the cells are grown becomes AGE-modified (34). The observation that the complement protein C3 can be activated in serum overlying irradiated-A2E-laden RPE cells is also consistent with the view that diffusible reactive cleavage products of A2E are generated by photooxidation (35, 36). Moreover, direct exposure of complement-containing serum to photooxidized forms of A2E or all-trans-retinal dimer also leads to C3 activation. These findings are significant because genetic association studies have demonstrated links between DNA sequence variants in complement factor H, factor B (BF)/complement component C2 and complement component C3, and protection or risk for AMD (37–41). Together these genetic studies implicate complement dysregulation in the presence of an activating agent such as the products of A2E photocleavage, as an underlying cause of AMD pathogenesis in a significant number of cases.
RPE bisretinoid lipofuscin is a target for therapies that aim to combat vision loss in atrophic AMD. Several approaches under investigation involve limiting bisretinoid formation (42–44). A number of reports, including from the Age-Related Eye Disease Study, have also demonstrated a role for antioxidant intake in protecting against AMD progression (45–47). Evidence from our work (14, 25, 48, 49) indicates that antioxidants may operate at least in part by suppressing RPE bisretinoid photooxidation. The current report also lends support to epidemiological studies that suggest a link between AMD and sunlight exposure (50, 51).
Methods
Synthesis.
A2E-Br (M.W. 730/732) and A2E-Lys/Leu (M.W. 952) were synthesized by incubating mixtures of all-trans-retinal and 4-bromophenethylamine in ethanol or all-trans-retinal and Cbz-Lys-Leu-OEt in ethanol espectively, as described in SI Text. 1H-NMR assignments of synthesized A2E-Lys/Leu and A2E-Br are provided in SI Text. For synthesis of 2-(4-nitrophenyl)hydrazone, 4-NPH was incubated with methylglyoxal in the presence of sulfuric acid (98%, 0.5 ml) (SI Text).
Photooxidation and Singlet Oxygen Oxidation.
A2E, A2E-Br, and A2E-Lys/Leu (100 μM in water with 1% DMSO) were irradiated (430 ± 20 nm, 1.3 mW/cm2, 10–30 min) or incubated with the singlet oxygen generator 1,4-endoperoxide of 1,4- dimethylnaphthalene (40 mM) (52) in deuterated methanol overnight at room temperature. To identify carbonyl groups within irradiated A2E, samples of the latter were incubated with 0.2 M 4-NPH as detailed in SI Text.
FAB-MS.
FAB-MS was performed as described in (SI) Text.
LC/ESI-MS and LC/ESI-MS/MS.
Analysis was performed on a Waters Alliance 2690 HPLC interfaced with a Waters, MicroMass ZQ mass spectrometer as described in SI Text. In addition, an Agilent 1100 HPLC system was used with a reversed phase column, linear gradients of H2O and acetonitrile with formic acid and ESI-MS data aquisition via a Waters-Micromass LCT Premier XE Time-of-Flight mass spectrometer in the positive ionization mode as described in SI Text. Tandem MS (ESI-MS/MS) employing collision-induced dissociation was performed using a Waters-Micromass Q-TOF Micro mass spectrometer in the positive ionization mode; details in SI Text. Fourier transform mass spectrometry was performed to obtain high-resolution exact mass, as described in SI Text.
Supplementary Material
Acknowledgments.
We thank Dr. Jiangao He for LC-MS assistance. This work was supported by National Institutes of Health Grant EY 12951 and a grant to the Department of Ophthalmology from Research to Prevent Blindness.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913112107/DCSupplemental.
References
- 1.Delori FC, Goger DG, Dorey CK. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophth Vis Sci. 2001;42:1855–1866. [PubMed] [Google Scholar]
- 2.Shroyer NF, et al. The rod photoreceptor ATP-binding cassette transporter gene, ABCR, and retinal disease: from monogenic to multifactorial. Vision Res. 1999;39:2537–2544. doi: 10.1016/s0042-6989(99)00037-1. [DOI] [PubMed] [Google Scholar]
- 3.Delori FC, et al. In vivo measurement of lipofuscin in Stargardt’s disease—Fundus flavimaculatus. Invest Ophth Vis Sci. 1995;36:2327–2331. [PubMed] [Google Scholar]
- 4.Vasireddy V, et al. Elovl4 5-bp deletion knock-in mouse model for Stargardt-like macular degeneration demonstrates accumulation of ELOVL4 and lipofusin. Exp Eye Res. 2009;89:905–912. doi: 10.1016/j.exer.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marmorstein A, Cross HE, Peachey NS. Functional roles of bestrophins in ocular epithelia. Prog Retin Eye Res. 2009;28:206–226. doi: 10.1016/j.preteyeres.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal photobiology. Exp Eye Res. 2005;80:595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Sparrow JR. In: Retinal Degenerations: Biology, Diagnostics and Therapeutics. Tombran-Tink J, Barnstable CJ, editors. Totowa, NJ: Humana Press; 2007. pp. 213–236. [Google Scholar]
- 8.Parish CA, Hashimoto M, Nakanishi K, Dillon J, Sparrow JR. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc Natl Acad Sci USA. 1998;95:14609–14613. doi: 10.1073/pnas.95.25.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y, Fishkin NE, Pande A, Pande J, Sparrow JR. Novel lipofuscin bisretinoids prominent in human retina and in a model of recessive Stargardt disease. J Biol Chem. 2009;284:20155–20166. doi: 10.1074/jbc.M109.021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fishkin N, Sparrow JR, Allikmets R, Nakanishi K. Isolation and characterization of a retinal pigment epithelial cell fluorophore: An all-trans-retinal dimer conjugate. Proc Natl Acad Sci USA. 2005;102:7091–7096. doi: 10.1073/pnas.0501266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SR, et al. The all-trans-retinal dimer series of lipofuscin pigments in retinal pigment epithelial cells in a recessive Stargardt disease model. Proc Natl Acad Sci USA. 2007;104:19273–19278. doi: 10.1073/pnas.0708714104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Shabat S, et al. Formation of a nona-oxirane from A2E, a lipofuscin fluorophore related to macular degeneration, and evidence of singlet oxygen involvement. Angew Chem Int Edit. 2002;41:814–817. doi: 10.1002/1521-3773(20020301)41:5<814::aid-anie814>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Jang YP, Matsuda H, Itagaki Y, Nakanishi K, Sparrow JR. Characterization of peroxy-A2E and furan-A2E photooxidation products and detection in human and mouse retinal pigment epithelial cells lipofuscin. J Biol Chem. 2005;280:39732–39739. doi: 10.1074/jbc.M504933200. [DOI] [PubMed] [Google Scholar]
- 14.Sparrow JR, et al. Involvement of oxidative mechanisms in blue light induced damage to A2E-laden RPE. Invest Ophth Vis Sci. 2002;43:1222–1227. [PubMed] [Google Scholar]
- 15.Kim SR, Jockusch S, Itagaki Y, Turro NJ, Sparrow JR. Mechanisms involved in A2E oxidation. Exp Eye Res. 2008;86:975–982. doi: 10.1016/j.exer.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dillon J, Wang Z, Avalle LB, Gaillard ER. The photochemical oxidation of A2E results in the formation of a 5,8,5′,8′-bis-furanoid oxide. Exp Eye Res. 2004;79:537–542. doi: 10.1016/j.exer.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Keller LMM, Dillon J, Gaillard ER. Oxidation of A2E results in the formation of highly reactive aldehydes and ketones. Photochem Photobiol. 2006;82:1251–1257. doi: 10.1562/2006-04-01-RA-864. [DOI] [PubMed] [Google Scholar]
- 18.de Hoffmann E, Stroobant V. Mass Spectrometry Principles and Applications. Chichester, England: John Wiley & Sons, Inc; 2007. [Google Scholar]
- 19.Hsu F, Turk J. Elucidation of the double-bond position of long-chain unsaturated fatty acids by multiple-stage linear ion-trap mass spectrometry with electrospray ionization. J Am Soc Mass Spectr. 2008;19:1673–1680. doi: 10.1016/j.jasms.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan G, Li Q, Tan H, Ge T. Electrospray ionization ion-trap time of flight tandem mass spectrometry of two furofurans: Sesamin and gmelinol. Rapid Commun Mass Sp. 2007;21:3613–3620. doi: 10.1002/rcm.3243. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez-Diez TM, Zheng J. Detection of glutathione conjugates derived from 4-ipomeanol metabolism in bile of rats by liquid chromatography-tandem mass spectrometry. Drug Metab Dispos. 2004;32:1345–1350. doi: 10.1124/dmd.104.000406. [DOI] [PubMed] [Google Scholar]
- 22.Passarelli M, et al. Advanced glycation end product precursors impair ABCA1-dependent cholesterol removal from cells. Diabetes. 2005;54:2198–2205. doi: 10.2337/diabetes.54.7.2198. [DOI] [PubMed] [Google Scholar]
- 23.Sell DR, Strauch CM, Shen W, Monnier VM. Aging, diabetes, and renal failure catalyze the oxidation of lysyl residues to 2-aminoadipic acid in human skin collagen: evidence for metal-catalyzed oxidation mediated by alpha-dicarbonyls. Ann NY Acad Sci. 2008;1126:205–209. doi: 10.1196/annals.1433.065. [DOI] [PubMed] [Google Scholar]
- 24.Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci USA. 2000;97:7154–7159. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sparrow JR, et al. A2E-epoxides damage DNA in retinal pigment epithelial cells Vitamin E and other antioxidants inhibit A2E-epoxide formation. J Biol Chem. 2003;278:18207–18213. doi: 10.1074/jbc.M300457200. [DOI] [PubMed] [Google Scholar]
- 26.Thornalley PJ. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems—role in aging and disease. Drug Metabol Drug Interact. 2008;23:125–150. doi: 10.1515/dmdi.2008.23.1-2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price CL, Knight SC. Methylglyoxal: Possible link between hyperglycaemia and immune suppression? Trends Endocrinol Metab. 2009;20:312–317. doi: 10.1016/j.tem.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Yan SF, Ramasamy R, Schmidt AM. Receptor for AGE (RAGE) and its ligands-cast into leading roles in diabetes and the inflammatory response. J Mol Med. 2009;87:235–247. doi: 10.1007/s00109-009-0439-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crabb JW, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Handa JT, et al. Increase in advanced glycation end product pentosidine in Bruch’s membrane with age. Invest Ophth Vis Sci. 1999;40:775–779. [PubMed] [Google Scholar]
- 31.Curcio CA, Millican CL, Bailey T, Kruth HS. Accumulation of cholesterol with age in human Bruch’s membrane. Invest Ophth Vis Sci. 2001;42:265–274. [PubMed] [Google Scholar]
- 32.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 33.Sparrow JR, Zhou J, Cai B. DNA is a target of the photodynamic effects elicited in A2E-laden RPE by blue light illumination. Invest Ophth Vis Sci. 2003;44:2245–2251. doi: 10.1167/iovs.02-0746. [DOI] [PubMed] [Google Scholar]
- 34.Zhou J, et al. Mechanisms for the induction of HNE- MDA- and AGE-adducts, RAGE and VEGF in retinal pigment epithelial cells. Exp Eye Res. 2005;80:567–580. doi: 10.1016/j.exer.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Zhou J, Jang YP, Kim SR, Sparrow JR. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc Natl Acad Sci USA. 2006;103:16182–16187. doi: 10.1073/pnas.0604255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou J, Kim SR, Westlund BS, Sparrow JR. Complement activation by bisretinoid constituents of RPE lipofuscin. Invest Ophth Vis Sci. 2009;50:1392–1399. doi: 10.1167/iovs.08-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haines JL, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 38.Edwards AO, et al. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 39.Klein RJ, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gold B, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yates JR, et al. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357:553–561. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- 42.Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maiti P, et al. Small molecule RPE65 antagonists limit the visual cycle and prevent lipofuscin formation. Biochemistry. 2006;45:852–860. doi: 10.1021/bi0518545. [DOI] [PubMed] [Google Scholar]
- 44.Radu RA, et al. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophth Vis Sci. 2005;46:4393–4401. doi: 10.1167/iovs.05-0820. [DOI] [PubMed] [Google Scholar]
- 45.Christen WG, et al. Prospective cohort study of antioxidant vitamin supplement use and the risk of age-related maculopathy. Am J Epidemiol. 1999;149:476–484. doi: 10.1093/oxfordjournals.aje.a009836. [DOI] [PubMed] [Google Scholar]
- 46.AREDS. A randomized, placebo-controlled clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Leeuwen R, et al. Dietary intake of antioxidants and risk of age-related macular degeneration. J Am Med Assoc. 2005;294:3101–3107. doi: 10.1001/jama.294.24.3101. [DOI] [PubMed] [Google Scholar]
- 48.Jang YP, Zhou J, Nakanishi K, Sparrow JR. Anthocyanins protect against A2E photooxidation and membrane permeabilization in retinal pigment epithelial cells. Photochem Photobiol. 2005;81:529–536. doi: 10.1562/2004-12-14-RA-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou J, Gao X, Cai B, Sparrow JR. Indirect antioxidant protection against photooxidative processes initiated in retinal pigment epithelial cells by a lipofuscin pigment. Rejuv Res. 2006;9:256–263. doi: 10.1089/rej.2006.9.256. [DOI] [PubMed] [Google Scholar]
- 50.Tomany SC, Cruickshanks KJ, Klein R, Klein BEK, Knudtson MD. Sunlight and the 10-year incidence of age-related maculopathy. The Beaver Dam Eye Study. Arch Ophthalmol. 2004;122:750–757. doi: 10.1001/archopht.122.5.750. [DOI] [PubMed] [Google Scholar]
- 51.Fletcher AE, et al. Sunlight exposure, antioxidants, and age-related macular degeneration. Arch Ophthalmol. 2008;126:1396–1403. doi: 10.1001/archopht.126.10.1396. [DOI] [PubMed] [Google Scholar]
- 52.Turro NJ, Chow M-F, Rigaudyo J. Mechanism of thermolysis of endoperoxides of aromatic compounds: Activation parameters, magnetic field, and magnetic isotope effects. J Am Chem Soc. 1981;103:7218–7222. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.