Abstract
The hippocampus is thought to promote gradual incorporation of novel information into long-term memory by binding, reactivating, and strengthening distributed cortical-cortical connections. Recent studies implicate a key role in this process for hippocampally driven crosstalk with the (ventro)medial prefrontal cortex (vmPFC), which is proposed to become a central node in such representational networks over time. The existence of a relevant prior associative network, or schema, may moreover facilitate this process. Thus, hippocampal-vmPFC crosstalk may support integration of new memories, particularly in the absence of a relevant prior schema. To address this issue, we used functional magnetic resonance imaging (fMRI) and prior schema manipulation to track hippocampal-vmPFC connectivity during encoding and postencoding rest. We manipulated prior schema knowledge by exposing 30 participants to the first part of a movie that was temporally scrambled for 15 participants. The next day, participants underwent fMRI while encoding the movie's final 15 min in original order and, subsequently, while resting. Schema knowledge and item recognition performance show that prior schema was successfully and selectively manipulated. Intersubject synchronization (ISS) and interregional partial correlation analyses furthermore show that stronger prior schema was associated with more vmPFC ISS and less hippocampal-vmPFC interregional connectivity during encoding. Notably, this connectivity pattern persisted during postencoding rest. These findings suggest that additional crosstalk between hippocampus and vmPFC is required to compensate for difficulty integrating novel information during encoding and provide tentative support for the notion that functionally relevant hippocampal-neocortical crosstalk persists during off-line periods after learning.
Keywords: declarative memory, memory consolidation, resting state, functional connectivity, functional MRI
The formation of long-term memory traces involves a gradual integration of newly acquired information into neocortical associative networks (1, 2). The hippocampus is thought to promote this process by binding, reactivating, and strengthening connections between distributed neocortical representations, thus gradually reducing hippocampal dependence of the memory trace (3–5). Recent findings, however, show a concomitant increase in dependence on the (ventral) medial prefrontal cortex (vmPFC) (6–9) that may develop rapidly depending on contextual factors (10). These findings suggest that the binding role of the hippocampus may be transferred to the vmPFC (6, 11, 12) and implicate hippocampal–neocortical interactions in early stages of long-term memory formation (13).
Several lines of animal and human research suggest that coupling between these regions occurs at different stages of long-term memory formation. During encoding, neurons in medial prefrontal cortex (PFC) have been shown to exhibit unit activity that is phase locked to hippocampal theta oscillations (14). Moreover, functional connectivity between these regions as measured using functional MRI (fMRI) in humans has been shown to predict subsequent memory (15). Furthermore, there is evidence of post-encoding reactivation of memory traces within similar circuits. For instance, task-related neuronal spiking patterns are spontaneously “replayed” during postlearning off-line periods such as awake resting (16, 17) and sleep (9, 18, 19). Such replay patterns have been found in the hippocampus and neocortical regions (20), including the (v)mPFC (9, 12). Evidence for a functional relevance of such reactivation processes is moreover accumulating (21–23). The hippocampus and the vmPFC may thus form a neural circuit for reactivation of memory traces that is crucial for the integration of novel information into neocortical networks.
The existence of a relevant prior associative network, or schema, contributes to learning speed and improves subsequent memory performance for schema-related information (24–26). These notions suggest that a prior schema facilitates incorporation of schema-related information into neocortical networks and, therefore, increases speed of hippocampal independence (13, 27). In rats, such an effect has been reported (10). Moreover, if long-term memory formation involves connectivity between the hippocampus and the vmPFC, then it can be hypothesized that when novel encountered information is consistent with prior schema, the hippocampus and vmPFC need less interaction to be able to integrate this information. In contrast, when novel information is inconsistent with prior schema, compensatory mechanisms will be necessary to integrate the information. In line with this notion, vmPFC activity during encoding has been shown to depend on prior schema (28, 29). However, no studies to date have directly investigated such effects of prior schema on hippocampal-vmPFC connectivity during encoding or thereafter.
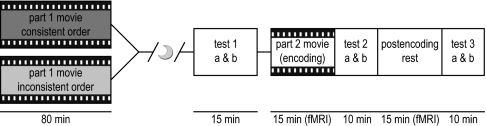
We therefore investigated schema-dependent hippocampal-vmPFC connectivity during encoding and postencoding rest in humans by using fMRI. One day before scanning, we manipulated schema knowledge by exposing participants to the first 80 min of a movie either in the correct (consistent schema group, n = 15) or in a temporally scrambled order (inconsistent schema group, n = 15; Fig. 1). Procedures on the following day were identical for both groups: First, participants were tested for schema knowledge (i.e., understanding of the storyline of the movie) and item recognition memory for still frames from the movie to control for item memory. Participants then underwent fMRI while watching the last 15 min of the movie in correct order and during an equally long period of postencoding rest. In between and after these two scans, participants completed an item recognition memory test for still frames and a multiple-choice questionnaire assessing schema knowledge. Importantly, these tests only probed knowledge of the last part of movie. We adopted this “natural viewing” paradigm, because it does not dictate the pace of stimulus encoding (30) thus allowing for assessment of spontaneously coinciding fluctuations in both conditions. For fMRI analysis, two recent model-free analysis methods were applied. First, we calculated interregional partial correlations (see ref. 31) between the hippocampus and vmPFC during encoding and postencoding rest. As an additional control for the specificity of between-group differences in hippocampal-vmPFC connectivity, we applied the same analysis to connectivity of the hippocampus with regions comprising the ventral visual stream. Second, we assessed intersubject synchronization (cf. ref. 32) during exposure to the movie by using newly developed group-level cluster-based randomization tests to investigate schema modulation of stimulus-driven activity.
Fig. 1.
Experimental design. One day before scanning, the consistent schema group viewed the first 80 min of a movie in correct order, whereas the inconsistent schema group viewed a temporally scrambled version. The next day, both groups performed an item recognition memory test (test 1a) and a test with open schema-related questions (test 1b). Then, they viewed the final 15 min of the movie inside the MRI scanner. Interleaved by a 15-min resting state scan, participants then completed two similar sets of tests: item recognition memory tests (tests 2a and 3a) and multiple choice tests on the content of the movie (tests 2b and 3b).
This design allowed us to test several predictions. First, we expected that our prior schema manipulation would lead to impaired memory performance in the inconsistent schema group, but that this impairment would be specific to schema-related questions and not affect item recognition memory of still frames. Second, we predicted that hippocampal-vmPFC connectivity would be stronger in the inconsistent schema group, and negatively related to prior schema knowledge within this group, because participants in this group would need to compensate for their inconsistent prior schema. Third, we expected these effects to persist during postencoding rest. Finally, we conjectured that prior schema might also affect stimulus-driven activity and, therefore, intersubject synchronization during movie viewing in our main regions of interest (ROIs).
Results
Memory Performance.
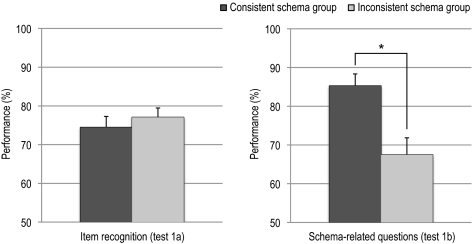
As expected, memory performance on open schema-related questions about the first part of the movie (see Fig. 1, test 1b) revealed a group difference (t(28) = 3.32; P = 0.002, higher for the consistent schema group; Fig. 2), demonstrating successful prior schema manipulation. In contrast, item recognition memory scores (hits minus false alarms) did not differ significantly (t(28) = 0.73, n.s.) between groups, indicating that there is no evidence that the group difference in prior schema strength can be explained by a difference in item processing.
Fig. 2.
Mean memory performance (and SEM) on tests regarding the first 80 min of the movie for the two experimental groups. For the item recognition memory test (test 1a), percentages represent percentage hits minus percentage false alarms. For schema-related open questions (test 1b), percentages of correct responses are shown. The item recognition memory test did not show a group difference, whereas the open schema questions test did, indicating a successful and specific manipulation of schema knowledge. SEM, standard error of the mean; *, P < 0.01.
Next, we investigated whether this difference in prior schema affected encoding of novel information that was equal for both groups. Specifically, we tested whether our prior schema manipulation would translate into a performance difference between groups in multiple choice tests (tests 2b and 3b; see Fig. 1 for design) probing content of the final 15 min of the movie seen inside the MRI scanner. In a repeated measures ANOVA on the number of correctly answered questions (Table S1) with TIME [multiple choice tests completed right after encoding (test 2b) versus after a subsequent 15 min rest period (test 3b)] as within subject factor and GROUP (consistent versus inconsistent schema) as between subject factor, we found no main effect of GROUP (F(1, 28) < 1) or interaction of GROUP by TIME (F(1, 28) < 1). Moreover, similar tests on the two item recognition memory tests performed in the MRI scanner (tests 2a and 3a, see Fig. 1 for design) yielded similar null results: No significant main effect (F(2,28) < 1) or interaction involving TIME (F(2,28) = 3.15, P = n.s.) was found. Thus, despite the fact that one group had a significantly poorer prior schema, the two groups were able to memorize information about the last part of the movie to an equal degree.
Neuroimaging Results: Interregional Partial Correlations.
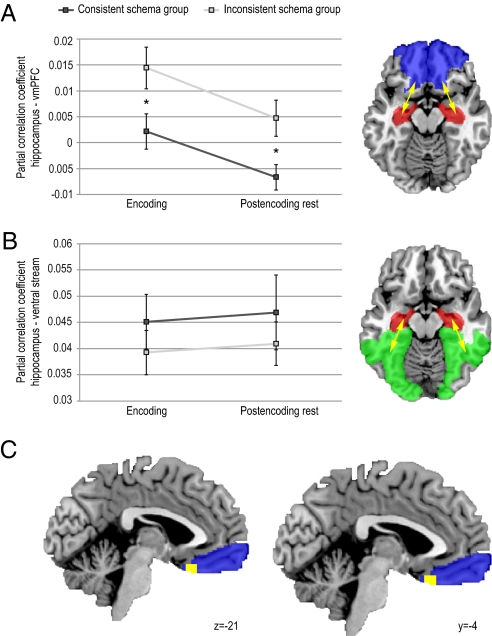
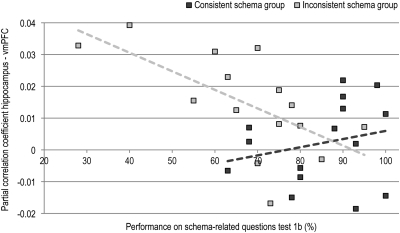
For the fMRI data, we first calculated interregional partial correlations (31). We performed anatomical parcellation of the fMRI data based on a previously described template (i.e., the automatic anatomical labeling template, AAL; ref. 33). Subsequently, we extracted averaged regional time courses for each region, and used these to calculate a partial correlation matrix containing pairwise correlation coefficients between regions after partialling out any variance explained by time courses of any of the other regions (see Experimental Procedures for more details). This procedure was repeated for every participant and condition (encoding and postencoding rest). Based on our a priori ROIs, we restricted our subsequent group analyses to partial correlations between the hippocampus and eight areas comprising ventral and medial parts of the prefrontal cortex (Fig. 3A), treating these subregions as repeated measures. Thus, partial correlation coefficients were entered (after Fisher z transformation) into a mixed factorial ANOVA with TIME (encoding versus postencoding rest) and SUBREGION (eight different vmPFC subregions) as within subjects factors, and GROUP (consistent versus inconsistent schema) as between subjects factor. As hypothesized, this ANOVA revealed a main effect of GROUP (F(1,28) = 13.97, P = 0.001), with stronger overall interregional partial correlations between hippocampus and vmPFC for the inconsistent schema group (Fig. 3A). Moreover, we found stronger overall interregional partial correlations during encoding than during postencoding rest (main effect of TIME: F(1,28) = 6.75, P = 0.015). These effects of prior schema, however, did not differ between the encoding and postencoding rest conditions: We found no significant interaction between GROUP and TIME and, moreover, GROUP main effects remained significant when testing the encoding (F(1,28) = 5.48, P = 0.027) and postencoding rest condition (F(1,28) = 7.27, P = 0.012) separately. In further agreement, the strength of prior schema (i.e., performance on test 1b before scanning) for the inconsistent schema group was negatively correlated with hippocampal connectivity to the vmPFC during encoding (r(13) = −0.64, P = 0.011), whereas no such effect was found in the consistent schema group (r(13) = 0.23, n.s.; Fig. 4). Thus, participants that saw the scrambled movie but were able to reconstruct the storyline had connectivity patterns comparable with the participants that did see the movie in the correct order. Finally, we found a main effect of SUBREGION (F(1,28) = 3.67, P = 0.009), indicating that overall, connectivity strength with the hippocampus differed between subregions of the vmPFC. However, because no significant interactions of SUBREGION with any of the other factors were found, we did not perform any further tests specific to subregions. In sum, our hypothesis of schema-dependent connectivity between hippocampus and vmPFC during encoding was confirmed: Participants that had an inconsistent prior schema needed more, likely compensatory, connectivity between hippocampus and vmPFC to reach a same level of performance. Moreover, this effect persisted during postencoding rest.
Fig. 3.
Interregional partial correlations and intersubject synchronization analyses. (A and B) Means (and SEM) of interregional partial correlations of the hippocampus with the vmPFC (A) and with ventral visual stream regions (B) for both experimental groups during encoding and postencoding rest. Connectivity between the hippocampus and vmPFC was stronger for the inconsistent schema group both during encoding and during postencoding rest. In contrast, this effect was not found for hippocampal connectivity with ventral visual stream regions. Main ROIs are depicted in red (hippocampus), blue (vmPFC), and green (visual stream). (C) ISS difference between groups (in yellow), with stronger ISS in the consistent schema group in the vmPFC (blue; statistical parametric map thresholded at P < 0.001, uncorrected, for visualization purposes; peak voxel coordinates in MNI152 space [−4,24,−21], corrected pcluster = 0.05). *, P < 0.05.
Fig. 4.
Interregional partial correlations between hippocampus and vmPFC as a function of prior schema knowledge during encoding. Data from the inconsistent and consistent schema groups are shown separately. These measures revealed a significant negative correlation within the inconsistent schema group (r(13) = −0.607, P < 0.05) but not within the consistent schema group (r(13) = 0.234, n.s.), indicating that those participants that were able to reconstruct the storyline had connectivity patterns comparable with participants that saw the first part of the movie in correct order.
To further test whether these effects were specific to hippocampal connectivity with the vmPFC, we applied the same analysis to another set of regions, namely a set of eight regions in the extrastriate/inferotemporal cortex that comprise the ventral stream, which is regarded to be importantly involved in perceptual identification of objects (34). First, we averaged the partial correlation coefficients (after Fisher z transformation) over the eight subregions for both connectivity to vmPFC and the ventral stream, and entered these into a repeated measured ANOVA with PATHWAY (vmPFC versus ventral stream) and TIME (encoding versus postencoding rest) as within subject factors, and GROUP (consistent versus inconsistent schema) as between subjects factor. This ANOVA yielded a significant main effect of PATHWAY (F(1,28) = 124.42, P < 0.001, higher for the ventral stream), and a PATHWAY * GROUP (F(1,28) = 6.29, P = 0.018) interaction but, importantly, no GROUP main effect or main effect or interaction involving TIME (see Fig. 3B). Further testing revealed that the PATHWAY * GROUP interaction was indeed carried by a GROUP effect for the vmPFC (described above): No GROUP effect was present for ventral stream (F(1,28) = 1.00, P = 0.33). Thus, prior schema had no effect on connectivity between the hippocampus and the ventral visual stream.
Neuroimaging Results: ISS.
The second model-free analysis method that we applied was an extension of an earlier described voxel-wise intersubject synchronization (ISS) method (32). In this method, BOLD signal time courses are correlated across participants in a voxel-wise fashion to obtain an estimate of regional synchronization of brain activity. This method is particularly applicable to data acquired during natural viewing of real-world stimuli such as movies, in which BOLD activity cannot be modeled, but can be assumed to exhibit meaningful temporal coherence across participants. Notably, such synchronization of the BOLD signal across participants does not necessarily reflect an increase in neural activity, but rather indicates that activity is more tightly coupled to sensory input. Our method (see Experimental Procedures for details) allowed us to quantify and test main effects of ISS in both conditions and to make a statistical comparison between the two movie conditions, both using cluster-based randomization tests. Results of the ISS main effect analysis revealed significant ISS in large parts of the brain, among which the entire occipital lobe extending ventrally into inferior temporal cortex and medial temporal lobe, and parts of the frontal lobe (both P < 0.001, whole brain corrected at cluster-level) for both groups. When comparing groups, a significant cluster in vmPFC with peak voxel coordinates [−4, 24, −21] was found to exhibit stronger ISS in the consistent schema group (P = 0.05, corrected at cluster-level for a reduced search region; see Fig. 3C). No effects were found for the opposite contrast, and in the hippocampus and the ventral stream for either contrast. In sum, differential ISS was found only in the vmPFC, with a consistent schema resulting in stronger synchronization when encoding novel information related to this schema.
Discussion
Using an experimental approach involving a manipulation of prior schema knowledge and a model-free functional MRI design, this study shows that connectivity between the hippocampus and the vmPFC is enhanced when novel information is encoded that does not fit a consistent prior associative schema. We found this effect both as a between-group effect and within the group that did not have a consistent prior schema, where those participants that had the least schema knowledge had the strongest connectivity. Interestingly, the pattern of differential connectivity between groups persisted during a postlearning rest period. Moreover, decreased ISS in the vmPFC was found for the inconsistent schema group during encoding. Manipulation of prior schema thus leads to modulations in and between memory-related brain structures, both while acquiring novel information and during an off-line period immediately thereafter.
Memory performance measures clearly indicate that prior schema knowledge was selectively manipulated in the inconsistent schema group. This group had lower scores on the schema test before scanning, but did not show any impairment in item recognition memory. On the second day, performance on neither content-related questions nor item recognition memory tests dif-fered between groups. These final tests only probed the newly encoded final part of the movie, which was identical for both groups. We therefore interpret differences in brain activity observed during movie encoding and postencoding rest as related to compensation of an inconsistent prior associative schema.
The present study adds to a body of evidence associating the vmPFC with integration and comprehension of knowledge. For instance, previous studies have shown that schema manipulation affects vmPFC activity during processing of a storyline (28). In line with this, several studies have related narrative comprehension to (more dorsal) medial PFC activity (35, 36). Also, vmPFC activity has recently been linked to representation of conceptual knowledge (37). Here, we show that ISS in the vmPFC was larger in the group that had a more consistent prior schema. It is important to note that enhanced ISS does not necessarily imply an increase in activity. Rather, it points toward a more direct coupling between activity in this region and sensory input, in this case, the movie that was presented synchronously across subjects. Because elaborative processes in prefrontal regions would likely yield activity that is not directly coupled to the input and therefore does not synchronize across subjects (32), our finding of enhanced ISS related to larger prior schema suggests that prior schema facilitates processing in this region.
Our findings furthermore show that a lack of prior schema leads to enhanced partial connectivity of this region with the hippocampus during learning. A large body of behavioral literature has demonstrated that information that is not consistent with prior associative knowledge is less easily comprehended, integrated, and remembered (24–26). Our finding may thus reflect increased allocation of neural resources to integrative mnemonic processes. In agreement, a previous study showed enhanced hippocampal-neocortical connectivity during successful memory encoding (15). An interesting parameter, however, is the directionality of such interactions (38): If such interactions indeed promote integration into cortical-cortical networks, one would expect directional dominance toward the neocortex. Because of limited temporal resolution, human studies using fMRI cannot provide a definite answer to this question. However, rodent studies have shown that activity of mPFC neurons is phase locked to hippocampal theta oscillations during learning (14, 39, 40), and that mPFC activity is delayed by ≈50 ms with respect to these oscillations (14). Thus, it appears that hippocampal activity may drive information transfer to the neocortex already during learning. These observations, however, cannot exclude the possibility that such processes are themselves triggered by a cortically cued retrieval process. Hippocampal–neocortical interactions during encoding may therefore be best understood as a reciprocal process in which retrieval and integration processes are intricately intertwined.
Additionally, we found schema-dependent differences in hippocampal-vmPFC connectivity to persist during a postencoding resting period, suggesting that a lack of prior schema resulted in increased spontaneous reprocessing of newly encoded information. Spontaneous reoccurrence of prior task-related brain activity has been observed in a number of domains. For instance, learning experiences have been shown to modulate brain activity in a subsequent unrelated cognitive task (41). Moreover, numerous studies have shown that functionally relevant brain networks remain active even in the absence of an explicit task (42–44) and are thought to subserve offline reprocessing of prior experiences (41, 45–48). The present findings therefore suggest that persistence of hippocampal-neocortical connectivity patterns may be functionally relevant (49, 50) and relate to spontaneous reactivation of newly formed memory traces. Consistent with such notions, rodent studies have repeatedly demonstrated replay of learning-related hippocampal neuronal spiking patterns during subsequent off-line periods. Such replay phenomena have been found to occur during sleep (9, 18–20) but also during postencoding waking states (16, 17). Notably, similar effects have been reported in the mPFC (9, 12), suggesting that concerted reactivation occurs in hippocampal and mPFC circuits. A recent study has moreover shown that PFC cell firing during sleep follows hippocampal cell firing with a delay of ≈100 ms and is driven by hippocampal sharp wave-ripple bursts during slow wave sleep (SWS; ref. 38). Thus, spontaneous postlearning hippocampal–neocortical interactions, at least during SWS, may also exhibit directional dominance toward the neocortex. In humans, comparable reactivation of hippocampal memory-related activity has been observed during SWS by using fMRI (23). Additionally, intracranial recordings during sleep and waking states shortly after learning have shown functionally relevant ripples in the medial temporal lobe (21). The apparent directionality of hippocampal-neocortical reactivations is in accordance with models of systems consolidation. These models assume reverse temporal gradients over the course of consolidation for involvement of the hippocampus (less over time) and the mPFC (more over time; refs. 6–8). Furthermore, it has been postulated that the vmPFC becomes a central node in newly established cortical-cortical networks (4, 6–8, 11, 12). Finally, the existence of a prior schema has also been shown to accelerate such systems consolidation processes in rats (10). Thus, although such processes cannot be observed directly, our finding of prior schema-dependent hippocampal-neocortical connectivity during postencoding rest is consistent with the view that hippocampally driven reactivations of distributed memory representations during off-line periods facilitate gradual incorporation of information into neocortical associative networks.
An alternative account of our findings could be that connectivity differences are driven by differences in attention or arousal. However, such an explanation would not seem very plausible. First, attention differences would likely yield memory performance differences after viewing the last part of the movie (particularly for the schema-related tests), whereas we found differences neither in item recognition nor in schema-related knowledge. Second, a difference in attention and arousal would likely lead to differences in perceptual processing and, thus, altered connectivity between the hippocampus and perceptual areas. To rule out this possibility, we repeated the interregional partial correlation analysis for hippocampal connectivity to regions comprising the ventral visual stream, which carry visual information to the hippocampus (34, 51) and are affected by attention (52, 53). This analysis yielded no connectivity differences between groups. A comparison between the two hippocampal connectivity pathways moreover confirmed that the prior schema effect was significantly larger for the hippocampal-vmPFC pathway. We furthermore examined ISS effects in the ventral stream, which revealed strong ISS for both groups, but no group differences. Third, an attentional account would not readily explain our finding that partial connectivity differences persist during postencoding rest without attentional requirements. Finally, it should be noted that our measure of partial connectivity assesses the amount of unique variance shared by two regions by partialing out any variance explained by signal fluctuations in other regions (as defined in the AAL template; refs. 31, 33), and is thus a highly specific connectivity measure. In sum, there is no compelling reason to assume that the observed differences in hippocampal-vmPFC connectivity merely reflect unspecific differences in arousal or attention between the two groups.
The findings of this study raise a number of important issues that should be addressed in future research. First, an important limitation of the present experimental design is that it did not allow us to directly observe whether increased hippocampal-vmPFC connectivity led to integration of information into neocortical long-term memory networks. Future studies using a similar schema manipulation should therefore test behaviorally whether enhanced hippocampal-neocortical connectivity will lead to stronger consolidation strength over a longer time interval. Moreover, it should be shown in humans that retrieval of information encoded in the presence of a relevant prior schema would exhibit less hippocampal dependence (10) but stronger vmPFC recruitment in combination with cortical-cortical connectivity (54). Second, it will be highly informative to track the time course of postlearning hippocampal-vmPFC connectivity. For instance, it should be investigated whether this connectivity decreases over time and whether it is reinstated during sleep (38). Finally, investigation of schema building periods over longer periods of time may provide crucial information regarding schema acquisition itself and may yield important applications in educational strategies.
In conclusion, this study demonstrates enhanced hippocampal-vmPFC connectivity during and shortly after successful encoding of novel information when no consistent prior associative schema is present. These findings converge with a growing body of evidence suggesting that the incorporation of novel information into neocortical long-term memory networks is facilitated by hippocampal-neocortical crosstalk that extends from encoding into early stages of consolidation.
Experimental Procedures
Participants.
Thirty-one native Dutch right-handed healthy students [12 men, age 18–31 (mean 22.17), randomly divided into two groups] participated in this study. All had normal or corrected-to-normal vision, no hearing problems, no current depression (score below 11 on the Beck Depression Inventory, BDI; ref. 55), and no history of neurological or psychiatric disease. All stated that they had not seen the movie used in this study before. They were paid for participation and were notified that they could earn extra money for better performance. Possible confounding factors [age, gender, hours of sleep, time of day, and English language skills (tested by means of the Oxford placement test; ref. 56)] did not differ significantly over groups. One participant had to be excluded for falling asleep during the rest period. Therefore, the final groups consisted of 15 individuals each. Ethical approval was obtained from the institutional review board (CMO Region Arnhem-Nijmegen, The Netherlands) and all participants gave written informed consent. More specific information regarding the design and movie used can be found in SI Experimental Procedures.
Memory Tests.
Before MRI scanning, participants were tested on their memory about the first part of the movie. These tests consisted of an item recognition test where participants had to indicate whether a certain scene had been present in the first part of the movie or not (test 1a), and open questions where schema-related knowledge was tested (test 1b). Memory of (exclusively) the final part of the movie was tested inside the scanner by using similar item recognition memory tests and multiple choice questions probing schema-related knowledge. These tests were performed directly after the movie (test 2), and after a 15-min resting state fMRI scan (test 3), in counterbalanced order across participants. More specific information regarding the memory tests used and the statistical analyses can be found in SI Experimental Procedures.
fMRI Data Preprocessing.
Raw fMRI data were preprocessed by using SPM5 (www.fil.ion.ucl.ac.uk/spm). First, motion correction was performed by using iterative rigid body realignment to minimize the residual sum of squares between the first and all further functional scans, and subsequent rigid body coregistration to corresponding individual T1 images by using mutual information optimization. Subsequently, data were spatially normalized into a common space, defined by the Montreal Neurological Institute (MNI) 152 T1 image (voxel size = 3.5 × 3.5 × 3.5), and smoothed by convolving the data with an 8-mm FWHM 3D kernel (used only for the ISS analysis). The first 11 scans were excluded, which left 409 scans per condition (movie and rest) for analysis.
Interregional Partial Correlation Analysis.
Interregional partial correlation analysis was performed by using in-house software written in Matlab (Mathworks) in accordance with the method described by Salvador et al. (31). This procedure determines unique interregional connectivity by partialling out the contributions to interregional pairwise correlations of the regional time courses of a set of control regions. It effectively circumvents some of the concerns with the validity and interpretation of interregional correlations observed in BOLD fMRI data, for example, the possibility that pairwise interregional correlations may be driven by third regions. Additionally, head movements or undersampling (and aliasing) of physiological pulsations have been argued to inflate interregional correlations (57, 58). However, such effects are unlikely to be regionally specific and are therefore strongly attenuated when controlling for a large number of control regions (31). Specific implementation of this method is explained in SI Experimental Procedures.
ISS Analysis.
The second model-free fMRI data analysis method we used was a group-level extension of a voxel-wise ISS analysis method (cf. ref. 32). This procedure uses cross-correlations of time courses across participants to estimate (group differences in) regional synchronization across participants. Instead of using one brain as a model for activity patterns in another brain in a pairwise fashion (32), we calculated, for each voxel and for each participant, the correlations between this participant and the mean of other participants. Because of dependencies within these measures, these correlations were subjected to cluster-based randomization tests (59, 60) to test the null hypotheses that (i) time series data of a random set of participants can be sign permuted without affecting group-level ISS and (ii) participants can be randomly assigned to groups without affecting differential ISS between groups. Reduced search regions were used for statistical tests in regions of interest (hippocampus, vmPFC, and the ventral stream). Specific implementation of this method is explained in SI Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Elena Shumskaya and Robert Oostenveld for their support on the analysis methods. Furthermore, we thank Atsuko Takashima and the anonymous reviewers for their insightful comments on the manuscript. E.J.H. (451.07.019) and G.F. (918.66.613) were supported by grants from the Netherlands Organization for Scientific Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914892107/DCSupplemental.
References
- 1.Marr D. A theory for cerebral neocortex. Proc R Soc Lond B Biol Sci. 1970;176:161–234. doi: 10.1098/rspb.1970.0040. [DOI] [PubMed] [Google Scholar]
- 2.Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 3.Rasch B, Born J. Maintaining memories by reactivation. Curr Opin Neurobiol. 2007;17:698–703. doi: 10.1016/j.conb.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 5.Sutherland GR, McNaughton B. Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Curr Opin Neurobiol. 2000;10:180–186. doi: 10.1016/s0959-4388(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 6.Takashima A, et al. Declarative memory consolidation in humans: A prospective functional magnetic resonance imaging study. Proc Natl Acad Sci USA. 2006;103:756–761. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterpenich V, et al. Sleep-related hippocampo-cortical interplay during emotional memory recollection. PLoS Biol. 2007;5:e282. doi: 10.1371/journal.pbio.0050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gais S, et al. Sleep transforms the cerebral trace of declarative memories. Proc Natl Acad Sci USA. 2007;104:18778–18783. doi: 10.1073/pnas.0705454104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Euston DR, Tatsuno M, McNaughton BL. Fast-forward playback of recent memory sequences in prefrontal cortex during sleep. Science. 2007;318:1147–1150. doi: 10.1126/science.1148979. [DOI] [PubMed] [Google Scholar]
- 10.Tse D, et al. Schemas and memory consolidation. Science. 2007;316:76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- 11.Frankland PW, Bontempi B. Fast track to the medial prefrontal cortex. Proc Natl Acad Sci USA. 2006;103:509–510. doi: 10.1073/pnas.0510133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takehara-Nishiuchi K, McNaughton BL. Spontaneous changes of neocortical code for associative memory during consolidation. Science. 2008;322:960–963. doi: 10.1126/science.1161299. [DOI] [PubMed] [Google Scholar]
- 13.Wang SH, Morris RG. Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annu Rev Psychol. 2010;61:49–79. doi: 10.1146/annurev.psych.093008.100523. C41–44. [DOI] [PubMed] [Google Scholar]
- 14.Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 15.Ranganath C, Heller A, Cohen MX, Brozinsky CJ, Rissman J. Functional connectivity with the hippocampus during successful memory formation. Hippocampus. 2005;15:997–1005. doi: 10.1002/hipo.20141. [DOI] [PubMed] [Google Scholar]
- 16.Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- 17.Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: Effects of behavioral state, experience, and EEG dynamics. J Neurosci. 1999;19:4090–4101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- 19.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 20.Qin YL, McNaughton BL, Skaggs WE, Barnes CA. Memory reprocessing in corticocortical and hippocampocortical neuronal ensembles. Philos Trans R Soc Lond B Biol Sci. 1997;352:1525–1533. doi: 10.1098/rstb.1997.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008;131:1806–1817. doi: 10.1093/brain/awn103. [DOI] [PubMed] [Google Scholar]
- 22.Gerrard JL, Burke SN, McNaughton BL, Barnes CA. Sequence reactivation in the hippocampus is impaired in aged rats. J Neurosci. 2008;28:7883–7890. doi: 10.1523/JNEUROSCI.1265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasch B, Büchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 24.Bransford JD, Johnson MK. Contextual prerequisites for understanding - some investigations of comprehension and recall. J Verb Learn Verb Behav. 1972;11:717–726. [Google Scholar]
- 25.Johnson-Laird PN. Mental Models: Towards a Cognitive Science of Language, Inference, and Consciousness. Cambridge, MA: Harvard Univ Press; 1983. [Google Scholar]
- 26.Zwaan RA, Radvansky GA. Situation models in language comprehension and memory. Psychol Bull. 1998;123:162–185. doi: 10.1037/0033-2909.123.2.162. [DOI] [PubMed] [Google Scholar]
- 27.Morris RG. Elements of a neurobiological theory of hippocampal function: The role of synaptic plasticity, synaptic tagging and schemas. Eur J Neurosci. 2006;23:2829–2846. doi: 10.1111/j.1460-9568.2006.04888.x. [DOI] [PubMed] [Google Scholar]
- 28.Maguire EA, Frith CD, Morris RG. The functional neuroanatomy of comprehension and memory: the importance of prior knowledge. Brain. 1999;122:1839–1850. doi: 10.1093/brain/122.10.1839. [DOI] [PubMed] [Google Scholar]
- 29.Mar RA. The neuropsychology of narrative: story comprehension, story production and their interrelation. Neuropsychologia. 2004;42:1414–1434. doi: 10.1016/j.neuropsychologia.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Hasson U, Furman O, Clark D, Dudai Y, Davachi L. Enhanced intersubject correlations during movie viewing correlate with successful episodic encoding. Neuron. 2008;57:452–462. doi: 10.1016/j.neuron.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salvador R, et al. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex. 2005;15:1332–1342. doi: 10.1093/cercor/bhi016. [DOI] [PubMed] [Google Scholar]
- 32.Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303:1634–1640. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- 33.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 34.Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 35.Hasson U, Nusbaum HC, Small SL. Brain networks subserving the extraction of sentence information and its encoding to memory. Cereb Cortex. 2007;17:2899–2913. doi: 10.1093/cercor/bhm016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yarkoni T, Speer NK, Zacks JM. Neural substrates of narrative comprehension and memory. Neuroimage. 2008;41:1408–1425. doi: 10.1016/j.neuroimage.2008.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumaran D, Summerfield JJ, Hassabis D, Maguire EA. Tracking the emergence of conceptual knowledge during human decision making. Neuron. 2009;63:889–901. doi: 10.1016/j.neuron.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wierzynski CM, Lubenov EV, Gu M, Siapas AG. State-dependent spike-timing relationships between hippocampal and prefrontal circuits during sleep. Neuron. 2009;61:587–596. doi: 10.1016/j.neuron.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:2187–2199. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paz R, Bauer EP, Paré D. Theta synchronizes the activity of medial prefrontal neurons during learning. Learn Mem. 2008;15:524–531. doi: 10.1101/lm.932408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peigneux P, et al. Offline persistence of memory-related cerebral activity during active wakefulness. PLoS Biol. 2006;4:e100. doi: 10.1371/journal.pbio.0040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 43.Damoiseaux JS, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith SM, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Curr Biol. 2009;19:1023–1027. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miall RC, Robertson EM. Functional imaging: Is the resting brain resting? Curr Biol. 2006;16:R998–R1000. doi: 10.1016/j.cub.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasson U, Nusbaum HC, Small SL. Task-dependent organization of brain regions active during rest. Proc Natl Acad Sci USA. 2009;106:10841–10846. doi: 10.1073/pnas.0903253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci USA. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 50.Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65:280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 52.De Weerd P, Peralta MR, 3rd, Desimone R, Ungerleider LG. Loss of attentional stimulus selection after extrastriate cortical lesions in macaques. Nat Neurosci. 1999;2:753–758. doi: 10.1038/11234. [DOI] [PubMed] [Google Scholar]
- 53.Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 54.Takashima A, et al. Shift from hippocampal to neocortical centered retrieval network with consolidation. J Neurosci. 2009;29:10087–10093. doi: 10.1523/JNEUROSCI.0799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 56.Allan D. Oxford placement test. Oxford: Oxford Univ Press; 1992. [Google Scholar]
- 57.Cordes D, Haughton V, Carew JD, Arfanakis K, Maravilla K. Hierarchical clustering to measure connectivity in fMRI resting-state data. Magn Reson Imaging. 2002;20:305–317. doi: 10.1016/s0730-725x(02)00503-9. [DOI] [PubMed] [Google Scholar]
- 58.Rombouts SA, Stam CJ, Kuijer JP, Scheltens P, Barkhof F. Identifying confounds to increase specificity during a “no task condition”. Evidence for hippocampal connectivity using fMRI. Neuroimage. 2003;20:1236–1245. doi: 10.1016/S1053-8119(03)00386-0. [DOI] [PubMed] [Google Scholar]
- 59.Hayasaka S, Nichols TE. Validating cluster size inference: Random field and permutation methods. Neuroimage. 2003;20:2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.