Abstract
Dendritic epidermal T cells (DETC) express an invariant Vγ3/Vδ1 T-cell receptor, appear in fetal epidermis, and form a population of resident epidermal T cells. Their temporal development in the thymus has been studied extensively. However, little is known about the mechanisms involved in the embryonic trafficking of DETC from thymus to epidermis. We demonstrate that DETC in adult skin, as well as the DETC precursors in fetal thymus, express E and P selectin ligands (E- and P-lig). Mice deficient in α1,3 fucosyltransferases IV and VII (FTIV/VII) cannot synthesize the carbohydrate motifs that form key elements of these selectin ligands. The numbers of DETC in the epidermis of FTIV/VII−/− mice were dramatically reduced compared with normal mice. However, the development of DETC precursors in fetal thymus was identical in normal and FTIV/VII−/− mice, suggesting that the DETC precursors produced in FTIV/VII−/− mice could not traffic effectively to skin because they lack E- and P-lig. We tested this hypothesis by daily injection of blocking antibodies against E and P selectin into pregnant mice. Mice born from dams treated with anti-selectin antibodies, but not those born from dams treated with isotype control, had significantly diminished numbers of DETC. To test the role of chemokine receptors in DETC skin homing, we examined skin from CCR4−/− and CCR10−/− mice, respectively. DETC were significantly reduced in CCR4−/− mice but were present at normal levels in CCR10−/− mice. Our results present evidence for the crucial role of trafficking molecules in embryonic migration of DETC precursors to skin.
Keywords: dendritic epidermal T cells, skin T cells, T cell trafficking
T cells orchestrate immune and inflammatory responses, are central to host defense against pathogens, and bear T-cell receptors (TCR) that are heterodimers of either α and β or γ and δ TCR genes. T cells with γδ TCR are much less abundant than αβ TCR cells in the adult thymus, blood, and peripheral lymphoid organs but are more abundant in epithelial layers of tissues, such as skin, intestine, lung, and genitourinary tract (1–4). Murine dendritic epidermal T cells (DETC) are a unique population of γδ T cells that reside in murine epidermis. They have a dendritic morphology in situ and express a distinctive invariant Vγ3/Vδ1 TCR that recognizes a putative self-antigen expressed on the damaged, stressed, or transformed keratinocytes (5). In the past decade, significant advances have been made in understanding the roles of DETC in tissue homeostasis and wound healing (6–8). Recently, DETC were shown to contribute to the regression of cutaneous tumors through NKG2D ligation and to regulate autoimmune skin disease (9, 10). DETC also may be important mediators in bridging innate and adaptive immunity of skin through communication with keratinocytes and Langerhans cells (11).
The thymus is a unique site that provides an inductive environment for T-lineage commitment and V(D)J recombination at the γδ and αβ TCR loci. Vγ3/Vδ1 T cells are the first TCR-bearing cells to arise in the fetal thymus and selectively home to the skin between embryonic day (ED) 16 and 18 (12, 13). It initially was postulated that the Vγ3 TCR itself may be essential for the final skin localization of DETC precursors. However, Vγ3−/− and Vδ1−/− mice had normal complements of DETC (5, 10). More recent studies (14–16) highlight the importance of positive selection in the precisely timed skin migration of DETC precursors. Although some key molecules, such as the transcription factor Krueppel-like factor 2 and the cell-surface molecule sphingosine-1-phosphate 1 (S1P1), have been defined in T-cell emigration from the thymus (17), the mechanisms underlying the seeding of DETC precursors to embryonic skin still remain unknown. A recent study (18) showed that, after positive selection, Vγ3 TCR+ thymocytes express mRNA for some chemokine receptors associated with adult T cells’ skin homing, including CCR4 and CCR10, but this finding has not been explored further.
Transendothelial migration of lymphocytes through postcapillary venules and their subsequent tissue localization involve a tightly controlled series of events that differ somewhat in distinct tissues. Naive αβ T cells enter lymph nodes from blood through interactions with specialized endothelium that require L selectin and CCR7 (19). Although naive T cells do not enter into peripheral tissues, their activation by cognate antigen in lymph nodes endows newly formed effector/memory cells with tissue-homing properties. For example, activation of naive T cells by antigen in lymph nodes draining skin leads to expression of ligands for E and P selectin (E-lig and P-lig), new chemokine receptors (e.g., CCR4), and higher levels of αLβ2 integrin 1 (LFA-1) (20–22). This collection of cell-surface receptors favors the extravasation of these effector/memory T cells in dermal microvascular endothelium and defines them as skin-homing T cells. Although E and P selectin, CCL17 (CCR4 ligand), and intercellular adhesion molecule 1 (ICAM-1; αLβ2 ligand) are expressed on dermal microvasculature in inflamed skin, low levels of these receptors also are expressed in unperturbed skin vessels of both mice and humans (23), thus permitting skin homing in both resting and inflammatory conditions.
The initial step in mature T-cell migration into skin involves tethering and rolling of T cells on endothelium and is mediated by E-lig and P-lig interactions with E and P selectins, respectively (24). Appropriate glycosylation of cell-surface proteins is critical to the generation of functional E-lig and P-lig. A critical set of enzymes that mediate the generation of these carbohydrate ligands are the α1,3 fucosyltransferases (FTs). Both FTIV and FTVII are expressed in skin-homing effector/memory T cells, and mice deficient in both these enzymes cannot generate E-lig and P-lig on T cells (25). Subsequent to their tethering and rolling on the endothelial surface, skin-homing T cells must be activated by through chemokine receptors, and CCR4 has emerged as a critical receptor in this process. Mice deficient in CCR4 show impairment of skin homing by mature effector/memory T cells (26). CCR10 has also been implicated in T-cell skin homing, particularly to the epidermis (21, 27, 28).
In the present study, we found that both DETC and their thymic precursors in WT mice expressed E-lig and P-lig. FTIV/VII double-deficient (FTIV/VII−/−) mice had normal number of DETC thymic precursors, but these cells did not express E-lig or P-lig, and adult FTIV/VII−/− mice had significantly reduced DETC in epidermis, suggesting that these cell-surface molecules were used by DETC to traffic to skin. We also were able to block DETC trafficking to epidermis by treating pregnant WT dams from ED14 to 20 with antibodies to E and P selectins. Finally, we also found that DETC, as well as a subset of their thymic precursors, expressed CCR4. Compared with WT mice, DETC were significantly reduced in CCR4−/− mice but were present at normal levels in CCR10−/− mice. These results reveal that embryonic trafficking of naive DETC precursors from fetal thymus to skin appears to depend on pathways similar to those used by adult effector/memory T cells; that is, E-lig, P-lig, and CCR4.
Results
DETC in Normal Skin Expressed E-Lig and P-Lig.
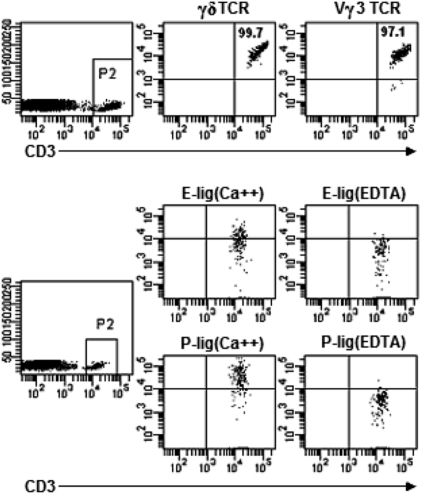
Single-cell suspensions were prepared from mouse epidermis to detect DETC. Consistent with published studies on DETC (5, 7), we found that nearly all CD3+ T cells in epidermis (99.7%) were γδ TCR+ (Fig. 1). The vast majority of CD3+ T cells (97.1%) were Vγ3 TCR+ (Fig. 1). This finding confirmed that DETC are the principal CD3+ T-cell population in the intact epidermis. We next asked if DETC expressed E-lig or P-lig, using E selectin/Fc or P selectin/Fc chimeric molecules. As shown in Fig. 1, DETC of adult WT mice expressed both E-lig and P-lig. This binding was calcium dependant, consistent with selectin binding.
Fig. 1.
DETC in normal WT C57BL/6 mice express E-lig and P-lig. Epidermal cell suspensions prepared from 8-week-old WT mice were stained with fluorescence-conjugated anti-CD3, anti-γδ TCR, and anti-Vγ3 TCR, followed with analysis by flow cytometry. The numbers in quadrants indicate the percentages in CD3+ T cells. E- and P-lig expression was examined by incubating skin cells with rmCD62E/Fc or rmCD62P/Fc chimera in HBSS buffer containing 2 mM calcium. HBSS buffer supplemented with 5 mM EDTA was used for the controls. Data are representative of at least three independent experiments.
DETC Were Diminished Significantly in FTIV/VII−/− Mice.
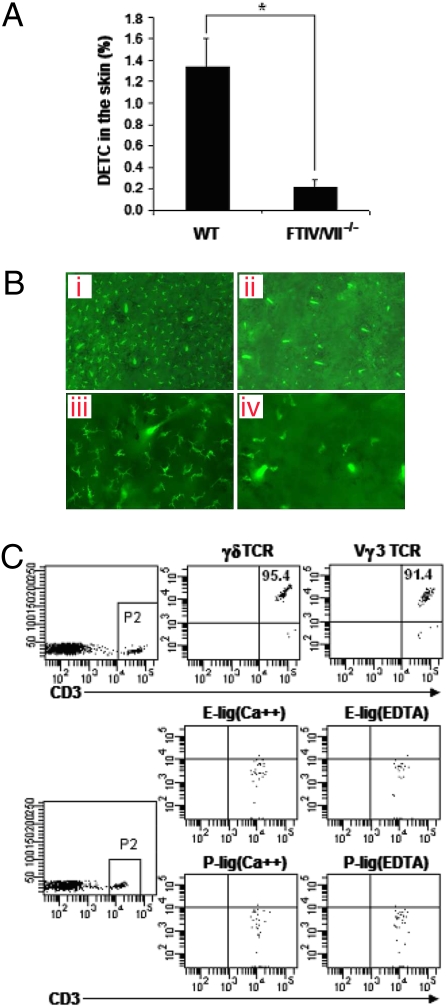
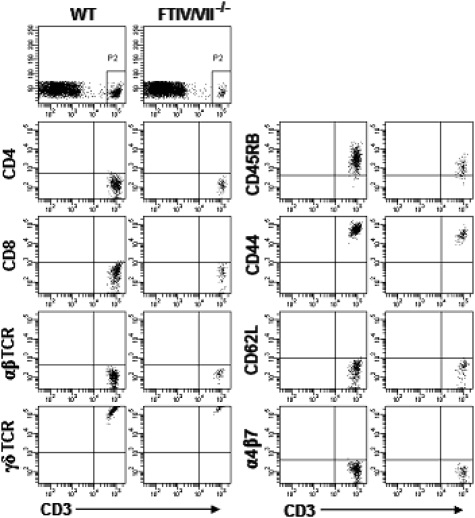
We next compared epidermal cell suspensions of FTIV/VII−/− mice with WT mice. We found that DETC frequency was much lower in FTIV/VII−/− mice than in age-matched adult WT mice (Fig. 2A). In an effort to compare DETC in WT and FTIV/VII−/− skin, we separated epidermal sheets and labeled them with FITC-conjugated anti-Vγ3 TCR mAb. Immunofluorescence microscopy showed that these Vγ3 TCR+ T cells exhibited dendritic cell-like morphology. DETC of FTIV/VII−/− mice were dramatically decreased compared with WT mice (Fig. 2B). As expected, the few DETC within the epidermis of FTIV/VII−/− mice did not express E-lig and P-lig (Fig. 2C). However, like those in WT mice, these DETC in FTIV/VII−/− mice showed a memory CD44highCD45RB+ phenotype. They also did not express L selectin (CD62L) or α4β7, one of the gut-homing markers, again like DETC in WT mice (Fig. 3). Therefore, E-lig/P-lig double deficiency resulted in a remarkable decrease in the number of DETC but did not influence the phenotype of these DETC. These intriguing phenomena prompted us to explore further the thymic development of DETC precursors in both WT and FTIV/VII−/− mice.
Fig. 2.
DETC are significantly diminished in E-lig/P-lig double-deficient mice. (A) Total skin cells of 8-week-old WT or FTIV/VII−/− C57BL/6 mice were stained with fluorescence-conjugated anti-CD3 and anti-Vγ3 TCR mAbs. The frequency of DETC was analyzed by flow cytometry. *, P < 0.05. (B) Epidermal sheets were peeled from dorsal halves through incubation in 20 mM EDTA for 2–3 h. They then were labeled overnight at 4 °C with FITC-conjugated anti-Vγ3 TCR mAb in PBS containing 2% BSA. Finally, PBS-washed epidermal sheets were mounted on glass slides, and DETC were visualized by immunofluorescence microscope. a and c are WT epidermis sheets; b and d are FTIV/VII−/− epidermis sheets; a and b: low-magnification micrograph (×100); c and d: high-magnification micrograph (×200). (C) E-lig and P-lig expression on DETC of 8-week-old FTIV/VII−/− mice was examined using the binding assay described in Fig. 1. Data are representative of at least three independent experiments.
Fig. 3.
Equivalent phenotype of DETC in WT and E-lig/P-lig double-deficient mice. Epidermal cells prepared from 8-week-old WT or FTIV/VII−/− mice were labeled with fluorescence-conjugated mAbs. Given that the vast majority of CD3+ cells in epidermis are Vγ3 TCR+ DETC, the CD3+ cell population thus was gated for DETC phenotype analysis.
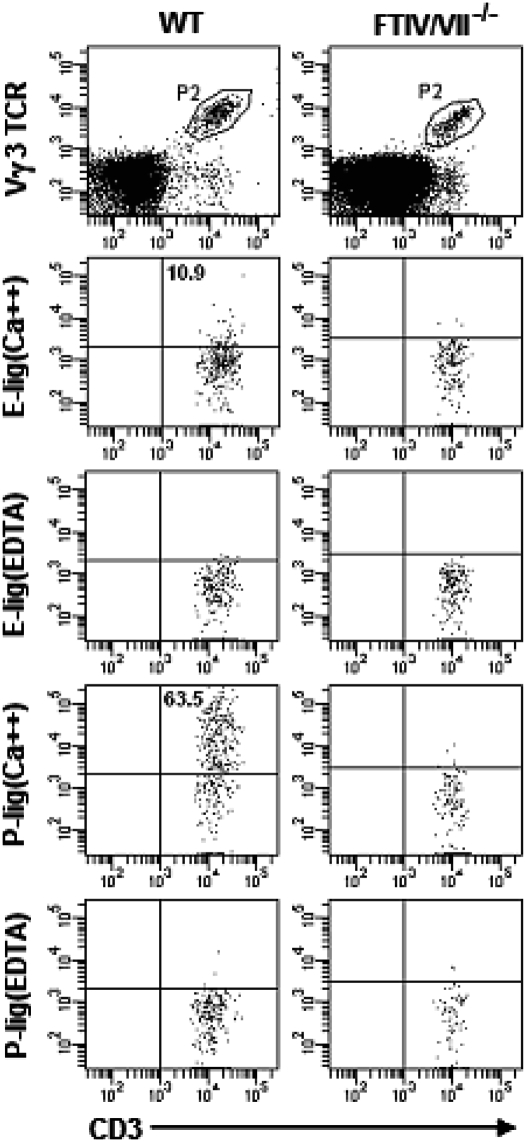
Fetal Thymic DETC Precursors Express E-lig and P-Lig.
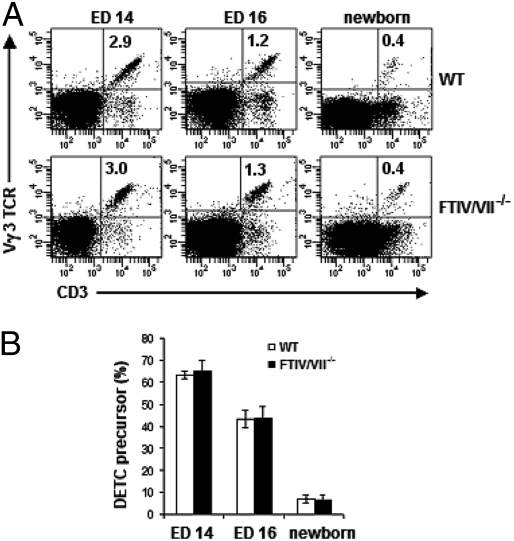
CD3+ Vγ3/Vδ1 T cells first can be detected in fetal thymus at ED14 and expand significantly at ED15 before leaving the thymus at ED16 (12, 13). We therefore analyzed the expression of E-lig and P-lig on ED15 thymocytes. As shown in Fig. 4, we found CD3+ Vγ3 TCR+ thymocytes of WT mice expressed E-lig and P-lig as well. In contrast, we did not detect the expression of E-lig or P-lig on DETC precursors in the fetal thymus of FTIV/VII−/− mice. Further, most of E-lig/P-lig+ DETC precursors in thymus of WT mice also expressed CD24 and CD122 (IL-2Rβ) (Fig. S1). We next asked whether the deficiencies of E-lig and P-lig altered the development of these cells in the thymus. To address this question, ED14, ED16, or newborn thymuses from WT or FTIV/VII−/− mice were harvested for analysis. As shown in Fig. 5 A and B, the percentages of DETC precursors in the fetal thymus at different time-points were comparable between age-matched WT and FTIV/VII−/− mice. These data strongly suggested that the E-lig and P-lig expressed on the surface of thymic DETC precursors may have enabled them to gain access to skin and ultimately to epidermis, a process that did not occur in FTIV/VII−/− mice.
Fig. 4.
Thymic DETC precursors in normal WT C57BL/6 mice express E-lig and P-lig. Fetal thymocyte suspensions of WT or FTIV/VII−/− mice at ED15 were prepared. E-lig and P-lig expression was examined using the binding assay described in Fig. 1. The numbers in quadrants indicate the percentages in DETC precursors.
Fig. 5.
E-lig/P-lig double deficiency does not impair DETC development in the fetal thymus. (A) Fetal or newborn thymocyte suspensions prepared at various EDs (above plots) were labeled with fluorescence-conjugated anti-CD3 and anti-Vγ3 TCR mAbs. Results are representative of three experiments at each time-point. The numbers in quadrants indicate the percentages of DETC precursors of total thymocytes. (B) Summary data (mean ± SD) on the frequency of DETC precursors of CD3+ thymocytes. Profiles are representative of three independent experiments.
E/P Selectin Blockade Impairs the Migration of DETC Precursors from Thymus to the Skin.
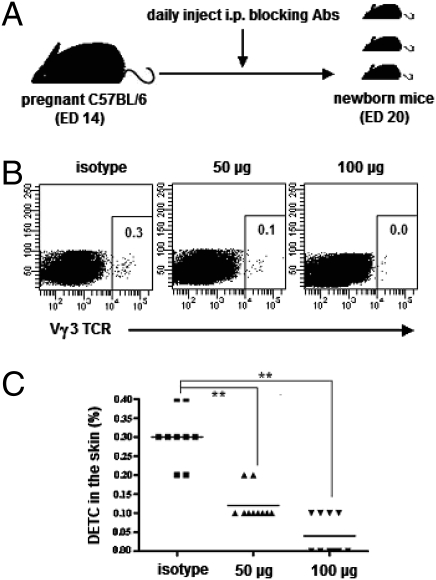
To confirm our hypothesis further, specific antibodies against E selectin and P selectin were used in an attempt to block the interactions between selectins and their ligands in vivo. The pregnant WT dams were injected with the same dose of each blocking antibody (50 μg or 100 μg) from ED14 until birth (Fig. 6A). The results are shown in Fig. 6 B and C. The DETC frequency in neonatal skin was markedly reduced after injection of blocking mAbs. This reduction was dose-dependent. These data provide further evidence that E-lig and P-lig expressed on DETC precursors in the fetal thymus functionally mediate DETC skin localization through binding to E selectin and P selectin, which are constitutively expressed in the vascular endothelium of skin, respectively.
Fig. 6.
Blocking E selectin and P selectin in vivo reduces DETC number in neonatal skin. (A) Experimental design. From ED14 to ED20 (birth), the pregnant WT C57BL/6 mice were injected i.p. daily with combined mAbs (100 μg or 50 μg each) against E selectin and P selectin or with isotype-matched control mAbs. The neonatal back skins were harvested for the preparation of single-cell suspensions by enzymatic digestion. (B) Cells then were labeled for DETC frequency analysis by flow cytometry. (C) Summary data on the percentages of DETC in neonatal skin after blocking E selectin and P selectin. **, P < 0.01. Results are representative of two independent experiments.
The Frequency of DETC Also Was Significantly Reduced in CCR4−/− Mice but Not in CCR10−/− Mice.
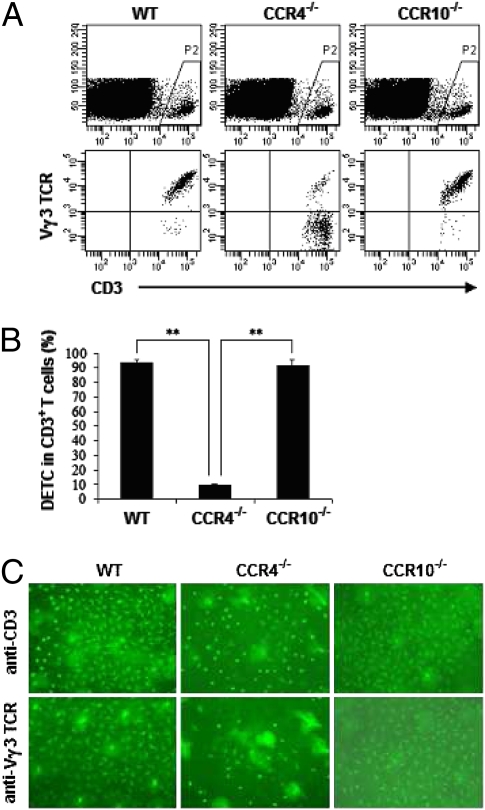
CCR4 and CCR10 are two relevant chemokine receptors that have been investigated extensively in T-cell skin homing (21, 26 –28). To test the possible role of CCR4 or CCR10 in DETC skin localization, CCR4−/− and CCR10−/− mice were used in our subsequent experiments. As before, DETC frequency within the epidermis of these mice was analyzed. We observed that DETC were significantly diminished in CCR4−/− mice, whereas CCR10−/− mice showed normal levels of DETC (Fig. 7 A–C). We next focused on CCR4 expression in WT mice and found that the majority of adult DETC in skin (96.0%) and a subset of thymic DETC precursors at ED15 (4.8%) expressed CCR4 (Fig. S2). In newborn skin, the frequency of DETC in CCR4−/− mice also was significantly reduced as compared with WT mice (Fig. S3). Taking these findings together, we conclude that CCR4, but not CCR10, is important for DETC skin localization.
Fig. 7.
DETC are diminished in CCR4−/− but not in CCR10−/− mice. (A) Epidermal cells were prepared from 8-week-old WT, CCR4−/−, or CCR10−/− C57BL/6 mice. Cells then were washed thoroughly and labeled with fluorescence-conjugated anti-CD3 and anti-Vγ3 TCR mAbs for DETC analysis by flow cytometry. (B) Summary data on the percentages of DETC in CD3+ T cells. **, P < 0.01. (C) DETC on epidermal sheets were visualized by labeling with FITC-conjugated anti-CD3 or anti-Vγ3 TCR mAb (×100). One of three independent experiments with similar results is shown.
Discussion
It has been more than 20 years since two groups independently discovered Thy-1+ DETC within the murine epidermis (29, 30), but the molecular mechanisms for DETC trafficking to the skin have been unclear. Here we show that DETC within the epidermis of adult mice express E-lig and P-lig, as judged by binding to E selectin- and P selectin-IgG chimeric molecules in a calcium-dependant fashion. Fetal thymic precursors of DETC, defined as ED15 cells bearing Vγ3/Vδ1 TCR, also express E-lig and P-lig, albeit at slightly lower levels. It may be that cells that exit from the thymus into blood have optimally high levels of E-lig and P-lig. The expression of E-lig and P-lig on naive γδ T cells has not been reported previously, and expression of these ligands in αβ T cells is limited mostly to effector/memory T cells that have been activated in lymph nodes draining skin.
At least three glycosylated cell-surface proteins on leukocytes have been reported to function as E-lig: hematopoietic cell E-lig/L-lig (HCELL, a CD44 glycoform expressed on hematopoietic progenitors) and CD43 and P selectin glycoprotein ligand 1 (PSGL-1) expressed on T lymphocytes. Only PSGL-1 has been identified as a leukocyte P-lig (25). The binding motif for E selectin is an α2,3-sialylated, α1,3-fucosylated glycan, the prototype of which is sialyl Lewisx. There are at least five distinct α1,3 fucosyltransferase loci in the human and murine genomes, but only two are expressed in leukocytes or their progenitors. These two are FTVII and FTIV, and both play a key role in the fucosylation of E-lig (24). Mice deficient in either of these α1,3 fucosyltransferases have some residual E-lig activity, but mice deficient in both FTIV and FTVII show completely absent E-lig and P-lig activity. T cells develop normally in these mice, but secondary lymphoid organs are atrophic, and skin-specific immunity is greatly impaired (31).
We examined the skin of FTIV/VII−/− mice for DETC and found a very significant decrease in the number of DETC in their epidermis. Interestingly, the few DETC that were in epidermis had Vγ3/Vδ1 invariant TCR and were negative for E-lig and P-lig, suggesting that other pathways could compensate partially for this deficiency. Indeed, the normal expression of CCR4 and αLβ2 on T cells from FTIV/VII−/− mice is likely to have facilitated this impaired recruitment to the epidermis. The fate of the Vγ3/Vδ1 cells generated in the thymus of these mice is unknown, because we did not investigate their migration to other tissues. However, the observations described earlier led us to hypothesize that DETC precursors in ED16 thymus used E-lig and P-lig to migrate to skin and to initiate the process by which they would extravasate in dermal vessels and migrate to epidermis.
Skin immune responses are impaired in mice deficient in both E and P selectin but are intact in either E selectin−/− or P selectin−/− mice, suggesting that ligands for both selectins mediate homing of adult T cells to skin (32). Therefore, we elected to use both E selectin- and P selectin-blocking antibodies together to test the hypothesis that DETC precursors used E-lig and P-lig to localize in skin. We injected pregnant mice daily with different amounts of blocking monoclonal antibodies to E and P selectin from ED14 until birth. The data show clearly that pups born from dams treated with E and P selectin antibodies had many fewer DETC in epidermis than pups born from dams injected with isotype control antibodies. Antibody treatment did not influence the generation of ED16 thymic Vγ3/Vδ1 DETC precursors. Taken together, these data support the hypothesis that embryonic trafficking of DETC from thymus to skin utilizes E-lig and P-lig.
Although endothelial selectins mediate the initial tethering and rolling of lymphocytes under flow, β2 integrins on lymphocytes must be activated before lymphocytes undergo firm adhesion and transendothelial migration. This process typically is achieved by engagement of chemokine receptors expressed on leukocytes. For example, naive T cells use L selectin to roll on the surface of endothelial venules in lymph node. The secondary lymphoid tissue chemokine (SLC; CCL21) is displayed on the surface of venules in this microenvironment, and the engagement of CCR7 on the naive T cell triggers a G protein-coupled activation of αLβ2, which then binds to constitutively expressed ICAM-1 (33). This cascade of events is required for extravasation of naive T cells into lymph node, a process that occurs constantly throughout life. The specific combination of ligands and receptors also is critical, because although neutrophils express L selectin (and can roll on lymph node venules), they do not express CCR7 and thus are not activated in this setting (34). Therefore, lymph nodes allow only a select population of lymphocytes to enter from blood.
Extravasation of memory T cells in human skin is thought to involve cutaneous lymphocyte antigen (CLA), which is identified by an antibody directed against its sialyl-Lex–like motif. Both PSGL-1 and CD43 can function as CLA in human T cells (25). Skin-homing memory T cells use CLA to tether and roll on E selectin that is constitutively expressed on dermal microvasculature. The activation of αLβ2 on these cells and their subsequent binding to ICAM-1 also are mediated by chemokine receptors. The most convincing evidence for a skin-selective chemokine receptor implicates CCR4 in this process. CCR4 is uniformly expressed on skin-homing memory effector T cells in mouse and human, and at least one of its ligands (CCL17) has been identified as being expressed constitutively on dermal microvasculature (23). However, CCR10 also has been implicated as important for skin T-cell homing, as have CCR8 and CCR6 (28). Although mice deficient in CCR6 and CCR8 were not available to us, we did examine the epidermis of both CCR4−/− and CCR10−/− mice. Compared with WT mice, the total CD3+ γδ TCR+ T-cell population within the epidermis of CCR4−/− or CCR10−/− mice was indistinguishable. However, we found that CCR4−/− mice had an obvious deficiency in Vγ3/Vδ1 DETC, whereas CCR10−/− mice showed normal numbers of Vγ3/Vδ1 DETC. This finding was somewhat surprising, because the ligand for CCR10, CCL27/CTACK, is produced by epithelial tissues, including epidermis, and was a logical candidate for the chemokine that might attract T cells to epidermis (21). However, we cannot rule out the possibility that CCR10−/− DETC precursors trafficked efficiently to skin but did not proliferate effectively in situ. Although CCR4 appears to be required for DETC skin localization, it is present only on a fraction of ED15 DETC precursors. At this point, it is not clear whether more DETC precursors will develop CCR4 at later ED or whether expression is up-regulated once these cells leave the thymus. Alternatively, it may be that only the fraction of the ED15 DETC precursors that express CCR4 actually traffic to, or survive in, skin.
A recent report (18) suggested that Vγ3/Vδ1 DETC precursors in thymus are positively selected, perhaps by intrathymic encounter with their cognate ligand. These investigators demonstrated that ED16 Vγ3/Vδ1 up-regulated CD122, the β chain of the IL-15 and IL-2 receptors. IL-15 appears to be essential for the survival and expansion of DETC in epidermis (35). This same study demonstrated that, at the level of mRNA transcripts, these cells also strongly up-regulated CCR10 and down-regulated CCR6. CCR4 expression did not appear to be affected. S1P1 expression also was increased. These investigators concluded that, upon positive selection, the thymic microenvironment prepared DETC precursors for exit from the thymus (increased S1P1, decreased CCR6), migration to the epidermis (increased CCR10), and survival in situ (CD122). Selectin ligands were not studied. In our study, we demonstrated skin-homing DETC precursors not only are E-lig/P-lig+CD24+CD122+ but also are CCR4+. Our results support the idea that skin-homing receptors develop in the embryonic thymic microenvironment but are consistent with E-lig, P-lig, and CCR4 being the critical molecules that confer the potential for skin homing.
Taken together, our data strongly suggest that, during embryonic development, the seeding of epidermis with Vγ3/Vδ1 DETC derived from the thymus depends on interactions between E-lig and P-lig on these cells and E and P selectin expressed on dermal microvasculature. In addition, it appears that CCR4, but not CCR10, is required for this process. Human skin contains large numbers of effector/memory T cells that express E-lig and P-lig and CCR4. However, these cells are antigen experienced and have acquired E-lig and P-lig and CCR4 expression after their activation by antigen in lymph nodes that drain the skin interface with the environment. As such, they are exclusively CD45RO+ (36). Equivalent studies in the mouse are ongoing, but preliminary data suggest that the vast majority of αβ T cells recruited to skin are positive for both E-lig and P-lig and CCR4. Moreover, these cells can be demonstrated to reside in dermis for long periods of time and perhaps indefinitely. Neonatal mouse skin, however, is virtually devoid of αβ T cells, and the vast majority of T cells in neonatal mouse skin are Vγ3/Vδ1 DETC. Our study demonstrates that the mechanisms employed to recruit T cells to skin are similar, if not identical, in fetal and adult life.
Materials and Methods
Mice.
All mice, including C57BL/6, FTIV/VII −/− C57BL/6, CCR4−/− C57BL/6, and CCR10−/− C57BL/6 mice, were bred and housed at the animal facility of Harvard Institute of Medicine. All experiments were performed under the protocols approved by Harvard Medical School animal care and use committee. Eight-week-old mice were used for experiments.
Chimeras and Antibodies.
rmE-Selectin/Fc chimera and rmP-Selectin/Fc chimera were purchased from R&D Systems. The mCCL22/Fc chimera was a generous gift from Daniel J. Campbell, Benaroya Research Institute. APC anti-hFc-IgG was purchased from Jackson ImmunoResearch. All other antibodies and isotype controls were obtained from BD PharMingen.
Preparation of Skin/Epidermal Cells.
Hair-removed ears were split into dorsal and ventral halves, cut into small pieces, and then incubated at 37 °C in HBSS supplemented with 1 mg/mL collagenase A and 40 μg/mL DNase I for 2 h. To stop digestion, 10 mM EDTA in HBSS was added. Skin-cell suspensions were collected after filtrating through a 70-μm nylon cell strainer. For neonatal mice, back skins were harvested for skin-cell preparation. To prepare epidermal cells, the dermal sides were floated down and incubated in 20 mM EDTA for 2–3 h. Epidermal sheets then were peeled gently from the dermis and were digested for 30 min before filtration.
Preparation and Analysis of Epidermis Sheets.
The epidermal sheets were prepared as described above and incubated overnight at 4 °C with FITC-conjugated anti-CD3 or anti-Vγ3 TCR mAb at 1:50 ratio in PBS containing 2% BSA. The stained sheets were rinsed in PBS and mounted on glass slides with anti-fade mounting medium for fluorescence microscope (Nikon Eclipse E600, U-III Film Camera System).
Thymus Analysis and Blocking Antibody Treatment.
Plug day was set as ED0. From ED14 to ED20 (birth), pregnant mice were injected i.p. with combined mAbs (100 μg or 50 μg each) against E selectin and P selectin or with isotype-matched control mAbs. The thymuses from ED14, ED15, ED16, and newborn mice were removed to prepare single-cell suspensions.
Flow Cytometric Analysis.
Expression of surface markers was examined by staining cells with 0.5 μg/mL Ab in PBS containing 2% FCS and 0.2% NaN3 for 30 min at 4 °C. To detect E-lig and P-lig expression, nonspecific binding to Fc receptors was blocked by anti-mouse CD16/CD32. Cells then were incubated with 5 mg/mL of rmCD62E/Fc chimera or rmCD62P/Fc chimera in HBSS supplemented with 2 mM calcium, 5% FCS, and 1 mM Hepes buffer at 4 °C for 30 min. After washes, cells were incubated with APC anti-hFc-IgG and other cell-surface markers at 4 °C for an additional 30 min. HBSS buffer supplemented with 5 mM EDTA instead of calcium was used for the controls. Data were analyzed on a FACSCanto flow cytometer (BD Biosciences) using FACSDiva software.
Statistical Analysis.
The differences in the means between groups were compared using a one-tailed Student's t test. P ≤ 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
The CCR10−/− mice were the generous gift of Dr. Craig Gerard. We thank Lisa Liu for extensive discussion of the project and Tian Tian and Zachary Beck for their technical assistance. This work was supported by National Institutes of Health Grants R01 AI041707 and R37 AI025082 to T.S.K.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912943107/DCSupplemental.
References
- 1.Saito H, et al. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. Nature. 1984;309:757–762. doi: 10.1038/309757a0. [DOI] [PubMed] [Google Scholar]
- 2.Hayday AC, et al. Structure, organization, and somatic rearrangement of T cell gamma genes. Cell. 1985;40:259–269. doi: 10.1016/0092-8674(85)90140-0. [DOI] [PubMed] [Google Scholar]
- 3.Brenner MB, et al. Identification of a putative second T-cell receptor. Nature. 1986;322:145–149. [PubMed] [Google Scholar]
- 4.Nanno M, Shiohara T, Yamamoto H, Kawakami K, Ishikawa H. gammadelta T cells: Firefighters or fire boosters in the front lines of inflammatory responses. Immunol Rev. 2007;215:103–113. doi: 10.1111/j.1600-065X.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- 5.Payer E, Elbe A, Stingl G. Epidermal T lymphocytes—ontogeny, features and function. Springer Semin Immunopathol. 1992;13:315–331. doi: 10.1007/BF00200531. [DOI] [PubMed] [Google Scholar]
- 6.Jameson J, et al. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 7.Sharp LL, Jameson JM, Cauvi G, Havran WL. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol. 2005;6:73–79. doi: 10.1038/ni1152. [DOI] [PubMed] [Google Scholar]
- 8.Jameson J, Havran WL. Skin gammadelta T-cell functions in homeostasis and wound healing. Immunol Rev. 2007;215:114–122. doi: 10.1111/j.1600-065X.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 9.Strid J, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9:146–154. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- 10.Girardi M. Immunosurveillance and immunoregulation by gammadelta T cells. J Invest Dermatol. 2006;126:25–31. doi: 10.1038/sj.jid.5700003. [DOI] [PubMed] [Google Scholar]
- 11.Takashima A, Bergstresser PR. Cytokine-mediated communication by keratinocytes and Langerhans cells with dendritic epidermal T cells. Semin Immunol. 1996;8:333–339. doi: 10.1006/smim.1996.0044. [DOI] [PubMed] [Google Scholar]
- 12.Havran WL, Allison JP. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988;335:443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 13.Havran WL, Allison JP. Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature. 1990;344:68–70. doi: 10.1038/344068a0. [DOI] [PubMed] [Google Scholar]
- 14.Xiong N, Raulet DH. Development and selection of gammadelta T cells. Immunol Rev. 2007;215:15–31. doi: 10.1111/j.1600-065X.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- 15.Lewis JM, et al. Selection of the cutaneous intraepithelial gammadelta+ T cell repertoire by a thymic stromal determinant. Nat Immunol. 2006;7:843–850. doi: 10.1038/ni1363. [DOI] [PubMed] [Google Scholar]
- 16.Jensen KD, et al. Thymic selection determines gammadelta T cell effector fate: Antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinreich MA, Hogquist KA. Thymic emigration: When and how T cells leave home. J Immunol. 2008;181:2265–2270. doi: 10.4049/jimmunol.181.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong N, Kang C, Raulet DH. Positive selection of dendritic epidermal gammadelta T cell precursors in the fetal thymus determines expression of skin-homing receptors. Immunity. 2004;21:121–131. doi: 10.1016/j.immuni.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Mora JR, von Andrian UH. T-cell homing specificity and plasticity: New concepts and future challenges. Trends Immunol. 2006;27:235–243. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Austrup F, et al. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 21.Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marelli-Berg FM, Cannella L, Dazzi F, Mirenda V. The highway code of T cell trafficking. J Pathol. 2008;214:179–189. doi: 10.1002/path.2269. [DOI] [PubMed] [Google Scholar]
- 23.Chong BF, Murphy JE, Kupper TS, Fuhlbrigge RC. E-selectin, thymus- and activation-regulated chemokine/CCL17, and intercellular adhesion molecule-1 are constitutively coexpressed in dermal microvessels: A foundation for a cutaneous immunosurveillance system. J Immunol. 2004;172:1575–1581. doi: 10.4049/jimmunol.172.3.1575. [DOI] [PubMed] [Google Scholar]
- 24.Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4:325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 25.Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin Ther Targets. 2007;11:1473–1491. doi: 10.1517/14728222.11.11.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell JJ, O'Connell DJ, Wurbel MA. Cutting edge: Chemokine receptor CCR4 is necessary for antigen-driven cutaneous accumulation of CD4 T cells under physiological conditions. J Immunol. 2007;178:3358–3362. doi: 10.4049/jimmunol.178.6.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soler D, Humphreys TL, Spinola SM, Campbell JJ. CCR4 versus CCR10 in human cutaneous TH lymphocyte trafficking. Blood. 2003;101:1677–1682. doi: 10.1182/blood-2002-07-2348. [DOI] [PubMed] [Google Scholar]
- 28.Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: Chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- 29.Bergstresser PR, Tigelaar RE, Dees JH, Streilein JW. Thy-1 antigen-bearing dendritic cells populate murine epidermis. J Invest Dermatol. 1983;81:286–288. doi: 10.1111/1523-1747.ep12518332. [DOI] [PubMed] [Google Scholar]
- 30.Tschachler E, et al. Expression of Thy-1 antigen by murine epidermal cells. J Invest Dermatol. 1983;81:282–285. doi: 10.1111/1523-1747.ep12518326. [DOI] [PubMed] [Google Scholar]
- 31.Smithson G, et al. Fuc-TVII is required for T helper 1 and T cytotoxic 1 lymphocyte selectin ligand expression and recruitment in inflammation, and together with Fuc-TIV regulates naive T cell trafficking to lymph nodes. J Exp Med. 2001;194:601–614. doi: 10.1084/jem.194.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staite ND, Justen JM, Sly LM, Beaudet AL, Bullard DC. Inhibition of delayed-type contact hypersensitivity in mice deficient in both E-selectin and P-selectin. Blood. 1996;88:2973–2979. [PubMed] [Google Scholar]
- 33.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida R, et al. EBI1-ligand chemokine (ELC) attracts a broad spectrum of lymphocytes: Activated T cells strongly up-regulate CCR7 and efficiently migrate toward ELC. Int Immunol. 1998;10:901–910. doi: 10.1093/intimm/10.7.901. [DOI] [PubMed] [Google Scholar]
- 35.De Creus A, et al. Developmental and functional defects of thymic and epidermal V γ 3 cells in IL-15-deficient and IFN regulatory factor-1-deficient mice. J Immunol. 2002;168:6486–6493. doi: 10.4049/jimmunol.168.12.6486. [DOI] [PubMed] [Google Scholar]
- 36.Clark RA, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.