Abstract
Purpose
Adjuvant radiation therapy (ART) for stage I seminoma can cause adverse late effects and alternative postorchiectomy management strategies have been developed. This study evaluated ART trends in the United States and the impact of clinical and sociodemographic factors on ART recommendations.
Methods
Of men diagnosed with stage I seminoma from 1990 through 2004, 3,125 were identified using the Surveillance, Epidemiology, and End Results cancer registry. A multivariable logistic regression analysis was performed to assess whether there was a significant association between diagnosis year, diagnosis age, race, county education level, region, tumor size, tumor category, and the recommendation for ART.
Results
There was a significant association (P < .001) between later year of diagnosis and a decrease in ART recommendation. Compared with men diagnosed in 1990 to 1994, men diagnosed in 1995 to 1999, and 2000 to 2004 were less likely to have ART (adjusted odds ratio [OR], 0.63; 95% CI, 0.48 to 0.84; and OR, 0.49; 95% CI, 0.37 to 0.63, respectively). There also was a significant association (P < .001) between county education level and ART recommendation. Men residing in counties with the highest education level were more likely to receive ART than men residing in counties with the lowest education level (OR, 2.12; 95% CI, 1.59 to 2.82). Also, men older than 30 years were more likely to receive ART than men age 30 or younger (OR, 1.26; 95% CI, 1.03 to 1.55).
Conclusion
ART recommendations for stage I seminoma are declining. Men in less educated regions and the youngest men were less likely to receive a recommendation for ART.
INTRODUCTION
Consensus has not been reached among oncologists in the United States on the postoperative treatment of men with stage I testicular seminoma. Currently, men are offered active surveillance, adjuvant radiation therapy (RT) or single-agent chemotherapy.1 Adjuvant RT effectively prevents relapse in nearly all men with stage I seminoma and, therefore, has been the standard of care for decades. Although most men do not experience adverse effects from RT, receiving RT places men at risk for radiation-induced malignancy2-9 and potentially for cardiac disease.10,11 Given the very high cure rates and the fact that many men are diagnosed with testicular cancer at a young age (ie, < 30), patients may live long enough to develop the late toxicities of RT.
Awareness of the potential for adverse late effects from RT led first to the evaluation of active surveillance, with results published in the early 1990s,12-16 and later to the evaluation of single-agent carboplatinum17 as alternative therapies for the postoperative management of stage I seminoma. With a median follow-up time of more than 12 years in some series,18 there is long-term data to support active surveillance as a standard postorchiectomy treatment option for men with stage I seminoma. The first report of a randomized trial, published in 2005,19 showed that single-agent carboplatinum may be as effective as RT, but longer follow-up is needed to determine long-term efficacy and toxicity. Without adjuvant therapy, approximately 15% to 20% of men with stage I seminoma will develop recurrent disease.20,21 When recurrences are detected early, salvage rates are nearly 100%. The primary benefit of active surveillance is that it avoids unnecessary treatment and the risk of treatment-related adverse effects in the 80% to 85% of men who do not recur. However, men who select the active-surveillance approach must be compliant with a surveillance protocol that requires close follow-up with regular office visits, laboratory studies, and abdominal computed tomography scans.1,22
We hypothesized that an increased awareness of the potential adverse effects from RT and the development of alternative approaches after orchiectomy have decreased recommendations for the use of adjuvant RT in the United States. Therefore, the purpose of this study was to estimate and describe adjuvant RT recommendation trends for patients with stage I seminoma from 1990 to 2004 in the United States using the Surveillance Epidemiology and End Results (SEER) database. In addition, we assessed whether specific clinical and sociodemographic factors were associated with the recommendation for adjuvant RT.
METHODS
Data Source
The SEER program of the National Cancer Institute assembles information on cancer incidence and survival in the United States. The SEER program registries routinely collect data on patient demographics, primary tumor site, tumor morphology and stage at diagnosis, first course of treatment, and follow-up for vital status. The registries participating in the SEER program during the 1990s captured approximately 97% of the incident cases.23 The public use data contain information on whether or not a subject received a recommendation for RT. However, it contains neither the radiation details (such as dose) nor information on systemic treatment. The population residing within the areas served by the nine SEER cancer registries is more affluent, has lower unemployment rates, and is more urban than the remainder of the United States population.24 The patients are linked to county-level sociodemographic information from the 1990 and 2000 census. The catchments for the nine registries used in this analysis comprise 10% of the United States population.
This study was exempt from institutional review board review.
Description of Study Cohort and Treatment
A total of 3,547 men diagnosed with American Joint Committee on Cancer (AJCC) stage I testicular seminoma between January 1, 1990, and December 31, 2004, were identified in the SEER database (Nine Registries Public Use Data, 1973-2004).25 The men were classified as having RT recommended or not having RT recommended. If a patient refused RT, it was assumed RT was recommended. Twelve men were excluded because their tumor was not resected or because their surgical status was not known. Five men were excluded from the analysis because tumor category was not documented. Twelve men were excluded because radiation information was not recorded or because the use of radioactive isotopes, an unconventional form of RT, was recommended. Fourteen men were excluded because race was not known. An additional 379 men were excluded because tumor size was not recorded. This left 3,125 men with complete data who comprised the study cohort. The baseline characteristics of the study cohort are described in Table 1.
Table 1.
Patient Characteristics of the Study Cohort
| Characteristic | Patients (men) |
|
|---|---|---|
| No. | % | |
| No. of patients | 3,125 | |
| Year of diagnosis | 1990-2004 | |
| Median age at diagnosis, years | 36 | |
| Range | 13-82 | |
| Proportion of county (age > 25) with less than a high school education, % | 15.4 | |
| Range | 3.7-42.2 | |
| Race | ||
| White | 2,876 | 92.0 |
| Nonwhite | 249 | 8.0 |
| SEER region | ||
| South | 258 | 8.3 |
| West | 1,644 | 52.6 |
| Midwest | 782 | 25.0 |
| Northeast | 441 | 14.1 |
| Tumor category | ||
| T1 | 2,451 | 78.4 |
| T2 | 604 | 19.3 |
| T3/T4 | 70 | 2.2 |
| Tumor size, cm | ||
| ≤ 4 | 1,872 | 59.9 |
| > 4 | 1,253 | 40.1 |
Abbreviation: SEER, Surveillance Epidemiology and End Results.
Statistical Analyses
Primary end point.
The primary end point of this study was the proportion of men for whom adjuvant RT was recommended within specific time periods between 1990 and 2004. Descriptive statistics were used to characterize the study cohort at baseline.
Logistic regression: description of covariates.
Explanatory variables included diagnosis year (to study trends in RT recommendations), tumor characteristics (size and tumor category), clinical characteristics (age at diagnosis and treatment region), and sociodemographic factors (race and percent of people in the county of residence age 25 or older with less than a high-school education). Diagnosis year, age at diagnosis, and percent of county age 25 or older with less than a high-school education were considered as continuous variables. Tumor size was categorized as ≤ 4 cm or larger than 4 cm. SEER extent of disease (EOD; 1990 to 2003) and cancer-specific (CS; 2004) coding was used to classify tumors as T1 (EOD/CS 10, 40), T2 (EOD/CS 15, 20, 30, 31, 45), or T3/4 (EOD/CS 50, 60, 70, 75). The cancer registries were categorized into regions: West (San Francisco, CA; Hawaii; New Mexico; Seattle, WA; Utah; San Jose, CA; and Los Angeles, CA); Midwest (Detroit, MI; Iowa), Northeast (Connecticut), and South (Atlanta, GA; rural GA). The proportion of adults within the county with less than a high-school education was obtained from 1990 and 2000 census data. The information closest to the year of diagnosis was used in the analysis. Education level is one of the most widely used indicators of socioeconomic position in public health research in the United States.26 The proportion of adults in the county of residence with less than a high-school education was selected as the sociodemographic indicator because health literacy is associated with education level27 and because county education level highly correlates with percentage of the population below the poverty level.28
Logistic regression: odds ratio.
Univariable and multivariable logistic regression analyses were performed to determine if there was an association between year of diagnosis, age at diagnosis, race, county education level, SEER region, tumor size, tumor category, and the recommendation for adjuvant RT. Diagnosis year was categorized into three equal time periods (1990 to 1994, 1995 to 1999, 2000 to 2004) and the proportion of adults in the county with less than a high-school education was categorized into quartiles. Multivariable logistic regression analyses of the adjusted odds of RT recommendation were then performed using education-level quartiles and 5-year diagnosis groups. Results are presented as odds ratios (OR) with 95% CI and P values. Two-sided P values less than .05 were considered statistically significant. To further investigate the impact of age, the adjusted OR of recommending adjuvant RT to men 30 years of age or younger, the lowest age quartile, was compared with that of men older than 30. For the purpose of illustration, the adjusted OR of recommending adjuvant RT during years 1995 to 2000 and during years 2001 to 2004 relative to years 1990 to 1994 was plotted on a bar graph with vertical bars encompassing the 95% CI. All statistical analyses were conducted using SAS version 9.1.3 (SAS Institute, Cary, NC).
RESULTS
Descriptive Characteristics of the Study Cohort
Among the 3,125 men with stage I testicular seminoma identified in the SEER registry, 78% had T1 disease, 19% had T2 disease, and 2% had T3 or T4 disease (Table 1). Sixty percent of the seminomas were ≤ 4 cm and 40% were larger than 4 cm. The median age at diagnosis was 36 years (range, 13 to 82) and the majority of the patients were white (92%). Adjuvant RT was recommended for 81% of the men during the entire period. Among the 2,537 for whom adjuvant RT was recommended, 2,479 received RT, 18 refused RT, and it was not known if the other 40 men received RT.
Adjusted Odds of Recommending Adjuvant RT
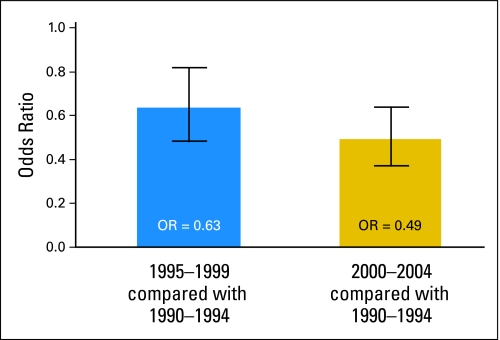
After adjusting for age at diagnosis, race, SEER region, tumor category, tumor size, county education level, and diagnosis year, there was a significant association between later year of diagnosis and a decrease in the recommendation for adjuvant RT (OR, 0.93; 95% CI, 0.91 to 0.96; P < .001; Table 2). Specifically, as shown in Figure 1, compared with men diagnosed from 1990 through 1994, men diagnosed from 1995 through 2000 and from 2001 through 2004 were less likely to receive a recommendation for adjuvant RT with adjusted OR of 0.63 (95% CI, 0.48 to 0.84) and 0.49 (95% CI, 0.37 to 0.63), respectively. Seventy-five percent of men diagnosed in 2004 received an adjuvant RT recommendation, down from 85% of men diagnosed in 1990.
Table 2.
Unadjusted and Adjusted Odds Ratios of Recommending Adjuvant RT for Stage I Seminoma for Each Clinical and Patient Characteristic
| Covariate | No. of Patients (men) | RT (%) |
Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Recommended | Not Recommended | Odds Ratio | 95% CI | P | Odds Ratio | 95% CI | P | ||
| Year of diagnosis (per year increase) | 3,125 | 0.96 | 0.94 to 0.98 | < .001 | 0.93 | 0.91 to 0.96 | < .001 | ||
| Median age at diagnosis (per year increase), years | 3,125 | 36 | 35 | 1.01 | 1.00 to 1.02 | .111 | 1.01 | 1.00 to 1.02 | .127 |
| Range | 17-81 | 13-82 | |||||||
| Proportion of county (age > 25) with less than a high school education (per % increase) | 3,125 | 15.4 | 15.6 | 0.96 | 0.95 to 0.98 | < .001 | 0.95 | 0.93 to 0.96 | < .001 |
| Range | 3.7-41.5 | 6.3-42.2 | |||||||
| Race | |||||||||
| White | 2,876 | 81.0 | 19.0 | 1.0* | — | 1.0* | — | ||
| Nonwhite | 249 | 83.1 | 16.9 | 1.16 | 0.82 to 1.63 | .413 | 1.20 | 0.84 to 1.71 | .322 |
| SEER region | |||||||||
| South | 258 | 78.7 | 21.3 | 1.0* | — | 1.0* | — | ||
| West | 1,644 | 83.2 | 16.9 | 1.34 | 0.97 to 1.85 | .080 | 1.43 | 1.03 to 1.98 | .034 |
| Midwest | 782 | 80.7 | 19.3 | 1.13 | 0.80 to 1.60 | .483 | 1.36 | 0.95 to 1.95 | .089 |
| Northeast | 441 | 76.2 | 23.8 | 0.87 | 0.60 to 1.26 | .449 | 1.02 | 0.70 to 1.48 | .935 |
| Tumor category | |||||||||
| T1 | 2,451 | 80.8 | 19.2 | 1.0* | — | 1.0* | — | ||
| T2 | 604 | 83.0 | 17.1 | 1.15 | 0.91 to 1.46 | .232 | 1.27 | 0.99 to 1.62 | .057 |
| T3/T4 | 70 | 78.6 | 21.4 | 0.87 | 0.49 to 1.55 | .638 | 0.86 | 0.48 to 1.56 | .620 |
| Tumor size, cm | |||||||||
| ≤ 4 | 1,872 | 80.3 | 19.7 | 1.0* | — | 1.0* | — | ||
| > 4 | 1,253 | 82.4 | 17.6 | 1.15 | 0.96 to 1.38 | .141 | 1.12 | 0.92 to 1.35 | .257 |
Abbreviations: RT, radiation therapy; SEER, Surveillance Epidemiology and End Results.
This group served as the reference group in the logistic regression analysis.
Fig 1.
Adjusted odds ratio of recommending radiation therapy for stage I seminoma relative to years 1990 to 1994. Vertical bars indicate 95% CIs.
A significant association was also noted between an increasing proportion of adults within the county with less than a high-school education and a decrease in the recommendation for adjuvant RT (OR, 0.95; 95% CI, 0.93 to 0.96; P < .001). Compared with men residing in counties with the highest proportion of adults with less than a high-school education (highest quartile), men residing in counties with the lowest proportion of adults with less than a high-school education (lowest quartile) were more likely to receive RT, with an adjusted OR of 2.12 (95% CI, 1.59 to 2.82; Table 3). From 1990 to 2004, 77% of men in the lowest quartile received a recommendation for adjuvant RT compared to 85% of men in the highest quartile.
Table 3.
Adjusted Odds Ratios of Recommending Adjuvant Radiation Therapy for Stage I Seminoma According to Year of Diagnosis, County Education Level, and Age Group Category
| Covariate | No. of Patients (men) | RT Recommended (%) | Adjusted Odds Ratio† | 95% CI | P |
|---|---|---|---|---|---|
| Year | |||||
| 1990-1994 | 706 | 85.3 | 1.0* | — | |
| 1995-1999 | 988 | 81.9 | 0.63 | 0.48 to 0.84 | .001 |
| 2000-2004 | 1,431 | 78.7 | 0.49 | 0.37 to 0.63 | < .001 |
| Proportion of county (age > 25) with less than a high school education, quartile | |||||
| 4 (least educated areas) | 717 | 77.3 | 1.0* | — | |
| 3 | 788 | 80.1 | 1.48 | 1.13 to 1.92 | .004 |
| 2 | 834 | 81.7 | 1.52 | 1.16 to 1.98 | .002 |
| 1 (most educated areas) | 786 | 85.4 | 2.12 | 1.59 to 2.82 | < .001 |
| Age group, years | |||||
| ≤ 30 | 782 | 78.4 | 1.0* | — | |
| > 30 | 2,343 | 82.1 | 1.26 | 1.03 to 1.55 | .024 |
Abbreviations: RT, radiation therapy; SEER, Surveillance Epidemiology and End Results.
This group served as the reference group in the logistic regression analysis.
Odds adjusted for race, SEER region, tumor category, and tumor size.
Recommendations for adjuvant RT were not affected by age at diagnosis when age was analyzed as a continuous variable (OR, 1.01; 95% CI, 1.00 to 1.02; P = .127). However, men older than 30 years were more likely to receive a recommendation for RT than men age 30 years or younger, with an adjusted OR of 1.26 (95% CI, 1.03 to 1.55; P = .024).
DISCUSSION
The results of this study show that recommendations for adjuvant RT for stage I testicular seminoma in the United States declined from 1990 to 2004. Adjuvant RT was recommended for 75% of men in 2004, down from 85% of men in 1990. This decline may have occurred because of an increasing awareness of the potential late effects of RT2,10,29,30 and the development of alternative therapies for the postoperative management of stage I seminoma.12-17 The youngest men were less likely to have RT recommended, perhaps because of heightened concern regarding radiation-induced second malignancy in younger men. During this period, men residing in counties with a higher education level were more likely to receive adjuvant RT than men residing in counties with a lower education level. Recommendations for adjuvant RT were not associated with tumor category or tumor size.
Several points require further consideration, first among them being how these findings compare with those of other studies. Steele et al31 assessed trends in testicular cancer treatment in the United States from 1985 to 1996 using the National Cancer Data Base. The proportion of patients receiving surgery and radiation for early-stage seminoma was relatively stable across the three sampled time points (75% in 1985 to 1986, 73% in 1990 to 1991, and 74% in 1995 to 1996). The authors did not evaluate the impact of patient age, tumor size, or sociodemographic status on adjuvant treatment for seminoma. Our study demonstrated a decline in adjuvant RT rates because it considers a later time period that coincides with the appreciation of RT late effects and increased acceptance of active surveillance. The difference between adjuvant RT rates in our study and the Steele et al study may reflect the difference in the data collection designs of the two registries, the difference in patient populations included in the two registries32 and the fact our study considered adjuvant RT recommendations rather than adjuvant RT administration. Another study by Tyldesley et al33 that coincided with the time period of our study found that the proportion of patients managed by active surveillance in British Columbia, Canada, increased from 10% in 1992 to 33% in 2002, consistent with the trend of decreasing adjuvant RT recommendations demonstrated in our study.
Second, the decline in adjuvant RT recommendations observed in this study may reflect an increased awareness of the potential severe adverse late effects of adjuvant RT and the development of alternative therapies for the postoperative management of stage I seminoma. Several studies published during this time period reported an increased risk of second malignancy after adjuvant RT2,5-9,11 and highlighted the increased infertility that can develop after adjuvant RT in a group that already has a risk of impaired infertility.29,30 However, radiation-induced infertility is not an issue if appropriate radiation technique is utilized.34-36 Also published during this time period were several studies advocating the benefits of active surveillance over adjuvant RT.12-16,21 The primary benefit of active surveillance is that it avoids unnecessary treatment and the risk of treatment-related adverse effects in the 80% to 85% of men who do not experience a recurrence. Men who select the active-surveillance approach must comply with a surveillance protocol that requires close follow-up. Evidence-based guidelines recommend an office visit, laboratory studies, and an abdominal computed tomography scan every 3 to 4 months for the first 3 years, every 6 months for the fourth year, and then annually for up to 10 years.1,22 However, some institutions use less intensive surveillance protocols.
Third, although RT recommendation rates declined during the study period, 75% of the men in this study received a recommendation for adjuvant RT in 2004 and at least 72% of the men received adjuvant RT. This finding suggests that providers in the United States are not readily adopting active surveillance, despite evidence of a significant risk of radiation-induced second malignancy after adjuvant RT2,3,5-9 and evidence from large cohorts of patients that active surveillance provides survival rates equivalent to those seen with the use of adjuvant RT.12,21 Literature on the adoption of active surveillance by practitioners in the United States is limited to one small study that surveyed 24 members of the Radiation Therapy Oncology Group Genitourinary Committee in 2001.37 Seventy-five percent of them reported that they routinely offer active surveillance and they estimated that less than 10% of their patients choose surveillance. It is possible that practitioners in the United States are offering active surveillance but patients are selecting adjuvant RT (the SEER database does not contain information regarding whether or not active surveillance was offered to patients). Little is known about how patients with stage I seminoma select postorchiectomy treatment. However, in general, patient treatment selection is influenced by how providers present the treatment options to the patient.
Fourth, there is increasing evidence of disparate treatments being offered to cancer patients in different sociodemographic groups. Most studies conclude that less adjuvant cancer therapy is given to those in lower socioeconomic groups.38,39 Similarly, our study found that men residing in counties with a lower education level were less likely to receive adjuvant RT for stage I seminoma than men residing in counties with a higher education level. The proportion of adults in the county with less than a high-school education was selected as the sociodemographic indicator for this study because this factor highly correlates with the percentage of adults below the poverty level28 and because education level is linked with health literacy.27 We speculate that patients with testicular seminoma living in less educated areas receive fewer RT recommendations because they have reduced access to health care resources or possibly lack an understanding of the need for adjuvant treatment. Alternatively, living in a less educated area may be associated with another factor that was not measured in this study, and this unmeasured factor may explain why men in less educated areas were less likely to receive a RT recommendation. Because patients in lower socioeconomic strata are less likely to receive post-treatment surveillance for other cancer sites,38,40 our findings raise concern that men in less educated regions may be receiving neither adjuvant RT nor active surveillance. However, this concern is speculative and cannot be evaluated using the SEER database.
Finally, the SEER database is a population-based cancer registry that had a relatively stable geographic catchment over the duration of the study period; therefore, the treatment trends should be reasonably generalizable to the United States as a whole. The limitations of this study are those common to observational studies utilizing the SEER registry. SEER contains information regarding whether or not RT was recommended but does not contain radiation dose, chemotherapy details, or active surveillance information. Provider factors, such as years in practice, and patient comorbidites, were not known and both could effect radiation recommendations. Also, patient preference was not taken into account, except for the handful of men who refused radiation. Since sociodemographic indicators are often colinear, only one sociodemographic indicator was analyzed in this study and county-level, rather than individual, education attainment was available. In addition, some of the characteristics now used to identify men with favorable prognostic factors, such as absence of rete testis invasion,21 were not specified in the public use database.
This study determined adjuvant RT recommendation trends for patients with stage I seminoma in the United States. Over the time period extending from 1990 to 2004, adjuvant RT recommendations declined. However, 75% of men received a recommendation for adjuvant RT in 2004, suggesting that providers in the United States are not adopting active surveillance despite evidence of a significant risk of radiation-induced second malignancy after adjuvant RT and evidence from large cohorts of patients that active surveillance provides survival rates equivalent to those achieved with adjuvant RT. During this time period, men younger than 30 years and men in less educated regions were less likely to receive a recommendation for adjuvant RT. For men younger than 30 years, who have the highest risk of radiation-induced malignancy,4 this trend may be appropriate. However, it is of concern that recommendations for RT varied according to sociodemographic strata.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Karen E. Hoffman, Anthony V. D'Amico
Data analysis and interpretation: Karen E. Hoffman, Ming-Hui Chen, Rinaa S. Punglia, Clair J. Beard, Anthony V. D'Amico
Manuscript writing: Karen E. Hoffman, Ming-Hui Chen, Rinaa S. Punglia, Clair J. Beard, Anthony V. D'Amico
Final approval of manuscript: Karen E. Hoffman, Ming-Hui Chen, Rinaa S. Punglia, Clair J. Beard, Anthony V. D'Amico
Acknowledgments
We thank and acknowledge, Barbara Silver, for the time and expertise she provided during the preparation of the revised manuscript.
Presented in part at the Genitourinary Cancer Symposium, cosponsored by the American Society of Clinical Oncology, the American Society for Therapeutic Radiology and Oncology, San Francisco, CA, February 14-16, 2008.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.National Comprehensive Cancer Network: The NCCN Testicular Cancer Clinical Practice Guidelines in Oncology (Version 1.2008). http://www.nccn.org
- 2.Travis LB, Curtis RE, Storm H, et al: Risk of second malignant neoplasms among long-term survivors of testicular cancer. J Natl Cancer Inst 89:1429-1439, 1997 [DOI] [PubMed] [Google Scholar]
- 3.van den Belt-Dusebout AW, de Wit R, Gietema JA, et al: Treatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol 25:4370-4378, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Travis LB, Fossa SD, Schonfeld SJ, et al: Second cancers among 40,576 testicular cancer patients: Focus on long-term survivors. J Natl Cancer Inst 97:1354-1365, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Bachaud JM, Berthier F, Soulie M, et al: Second non-germ cell malignancies in patients treated for stage I-II testicular seminoma. Radiother Oncol 50:191-197, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Chao CK, Lai PP, Michalski JM, et al: Secondary malignancy among seminoma patients treated with adjuvant radiation therapy. Int J Radiat Oncol Biol Phys 33:831-835, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Glanzmann C, Schultz G, Lutolf UM: Long-term morbidity of adjuvant infradiaphragmatic irradiation in patients with testicular cancer and implications for the treatment of stage I seminoma. Radiother Oncol 22:12-18, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Ruther U, Dieckmann KP, Bussar-Maatz R, et al: Second malignancies following pure seminoma. Oncology 58:75-82, 2000 [DOI] [PubMed] [Google Scholar]
- 9.van Leeuwen FE, Stiggelbout AM, van den Belt-Dusebout AW, et al: Second cancer risk following testicular cancer: A follow-up study of 1,909 patients. J Clin Oncol 11:415-424, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Huddart RA, Norman A, Shahidi M, et al: Cardiovascular disease as a long-term complication of treatment for testicular cancer. J Clin Oncol 21:1513-1523, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Zagars GK, Ballo MT, Lee AK, et al: Mortality after cure of testicular seminoma. J Clin Oncol 22:640-647, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Warde PR, Gospodarowicz MK, Goodman PJ, et al: Results of a policy of surveillance in stage I testicular seminoma. Int J Radiat Oncol Biol Phys 27:11-15, 1993 [DOI] [PubMed] [Google Scholar]
- 13.von der Maase H, Specht L, Jacobsen GK, et al: Surveillance following orchidectomy for stage I seminoma of the testis. Eur J Cancer 29A:1931-1934, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Horwich A, Alsanjari N, A'Hern R, et al: Surveillance following orchidectomy for stage I testicular seminoma. Br J Cancer 65:775-778, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duchesne GM, Horwich A, Dearnaley DP, et al: Orchidectomy alone for stage I seminoma of the testis. Cancer 65:1115-1118, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Warde P, Gospodarowicz MK, Panzarella T, et al: Stage I testicular seminoma: Results of adjuvant irradiation and surveillance. J Clin Oncol 13:2255-2262, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Oliver RT, Edmonds PM, Ong JY, et al: Pilot studies of 2 and 1 course carboplatin as adjuvant for stage I seminoma: Should it be tested in a randomized trial against radiotherapy? Int J Radiat Oncol Biol Phys 29:3-8, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Choo R, Thomas G, Woo T, et al: Long-term outcome of postorchiectomy surveillance for stage I testicular seminoma. Int J Radiat Oncol Biol Phys 61:736-740, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Oliver RT, Mason MD, Mead GM, et al: Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: A randomised trial. Lancet 366:293-300, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Oliver RT, Ong J, Shamash J, et al: Long-term follow-up of Anglian Germ Cell Cancer Group surveillance versus patients with stage 1 nonseminoma treated with adjuvant chemotherapy. Urology 63:556-561, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Warde P, Specht L, Horwich A, et al: Prognostic factors for relapse in stage I seminoma managed by surveillance: A pooled analysis. J Clin Oncol 20:4448-4452, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Martin JM, Panzarella T, Zwahlen DR, et al: Evidence-based guidelines for following stage 1 seminoma. Cancer 109:2248-2256, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Zippin C, Lum D, Hankey BF: Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer 76:2343-2350, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Nattinger AB, McAuliffe TL, Schapira MM: Generalizability of the surveillance, epidemiology, and end results registry population: Factors relevant to epidemiologic and health care research. J Clin Epidemiol 50:939-945, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Surveillance, Epidemiology, and End Results (SEER) Program: SEER*Stat Database: Incidence— SEER 17 Regs Limited-Use, Nov 2006 Sub (1973-2004 varying), Linked To County Attributes, Total U.S., 1969-2004 Counties. Bethesda, MD, National Cancer Institute, 2007
- 26.Krieger N, Williams DR, Moss NE: Measuring social class in US public health research: Concepts, methodologies, and guidelines. Annu Rev Public Health 18:341-378, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Paasche-Orlow MK, Parker RM, Gazmararian JA, et al: The prevalence of limited health literacy. J Gen Intern Med 20:175-184, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh G, Miller B, Hankey B, et al: Area Socioeconomic Variations in U.S. Cancer Incidence, Mortality, Stage, Treatment, and Survival, 1975-1999. NCI Cancer Surveillance Monograph Series, Number 4. Bethesda, MD: National Cancer Institute, 2003. NIH Publication No. 03-0000
- 29.Gordon W Jr, Siegmund K, Stanisic TH, et al: A study of reproductive function in patients with seminoma treated with radiotherapy and orchidectomy: (SWOG-8711): Southwest Oncology Group. Int J Radiat Oncol Biol Phys 38:83-94, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Jonker-Pool G, van Basten JP, Hoekstra HJ, et al: Sexual functioning after treatment for testicular cancer: Comparison of treatment modalities. Cancer 80:454-464, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Steele GS, Richie JP, Stewart AK, et al: The National Cancer Data Base report on patterns of care for testicular carcinoma, 1985-1996. Cancer 86:2171-2183, 1999 [PubMed] [Google Scholar]
- 32.Mettlin CJ, Menck HR, Winchester DP, et al: A comparison of breast, colorectal, lung, and prostate cancers reported to the National Cancer Data Base and the Surveillance, Epidemiology, and End Results Program. Cancer 79:2052-2061, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Tyldesley S, Voduc D, McKenzie M, et al: Surveillance of stage I testicular seminoma: British Columbia Cancer Agency Experience 1992 to 2002. Urology 67:594-598, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Bieri S, Rouzaud M, Miralbell R: Seminoma of the testis: Is scrotal shielding necessary when radiotherapy is limited to the para-aortic nodes? Radiother Oncol 50:349-353, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Dosoretz DE, Shipley WU, Blitzer PH, et al: Megavoltage irradiation for pure testicular seminoma: Results and patterns of failure. Cancer 48:2184-2190, 1981 [DOI] [PubMed] [Google Scholar]
- 36.Fossa SD, Horwich A, Russell JM, et al: Optimal planning target volume for stage I testicular seminoma: A Medical Research Council randomized trial: Medical Research Council Testicular Tumor Working Group. J Clin Oncol 17:1146, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Choo R, Sandler H, Warde P, et al: Survey of radiation oncologists: Practice patterns of the management of stage I seminoma of testis in Canada and a selected group in the United States. Can J Urol 9:1479-1485, 2002 [PubMed] [Google Scholar]
- 38.Woods LM, Rachet B, Coleman MP: Origins of socio-economic inequalities in cancer survival: A review. Ann Oncol 17:5-19, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Ayanian JZ, Guadagnoli E: Variations in breast cancer treatment by patient and provider characteristics. Breast Cancer Res Treat 40:65-74, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Elston Lafata J, Cole Johnson C, Ben-Menachem T, et al: Sociodemographic differences in the receipt of colorectal cancer surveillance care following treatment with curative intent. Med Care 39:361-372, 2001 [DOI] [PubMed] [Google Scholar]