Abstract
Background
The human umbilical cord contains mucoid connective tissue and fibroblast-like cells. These cells named Wharton's jelly cells, (WJCs) display properties similar to mesenchymal stem cells therefore representing a rich source of primitive cells to be potentially used in regenerative medicine.
Results
To better understand their self-renewal and potential in vitro expansion capacity, a reference 2D map was constructed as a proteomic data set. 158 unique proteins were identified. More than 30% of these proteins belong to cytoskeleton compartment. We also found that several proteins including Shootin1, Adenylate kinase 5 isoenzyme and Plasminogen activator-inhibitor 2 are no longer expressed after the 2nd passage of in vitro replication. This indicates that the proliferative potency of these cells is reduced after the initial stage of in vitro growing. At the end of cellular culturing, new synthesized proteins, including, ERO1-like protein alpha, Aspartyl-tRNA synthetase and Prolyl-4-hydroxylase were identified. It is suggested that these new synthesized proteins are involved in the impairment of cellular surviving during replication and differentiation time.
Conclusions
Our work represents an essential step towards gaining knowledge of the molecular properties of WJCs so as to better understand their possible use in the field of cell therapy and regenerative medicine.
Background
Since the first identification of non-hematopoietic stem cells in the bone marrow as colony-forming unit-fibroblasts (CFU-Fs) and the detailed characterization and description of the tri-lineage potential of the mesenchymal stem cells (MSCs), our knowledge of the molecular properties of these cells has made great progress [1,2]. MSCs have a great appeal for tissue engineering and therapeutic applications because of their high in vitro expansion potential, self renewal capacity and multi-potentiality [3-5]. However, considering the invasive procedure related to their availability, there is an increasing interest in investigating the presence of MSCs in adult and fetal sources and especially their presence in fetal membranes such as umbilical cord matrix [6-9].
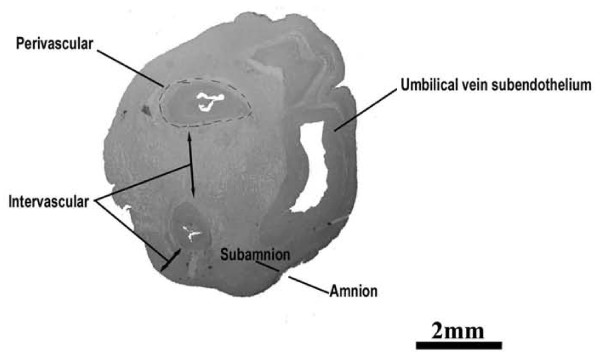
In the umbilical cord two arteries and one vein, surrounded by mucoid connective tissue, called Wharton's jelly are present (figure 1). It contains fibroblast-like cells (WJCs), which show properties similar to the MSCs and may represent a rich source of primitive cells [10,11]. Like bone marrow stromal cells, WJCs are plastic adherent, positively stained for markers of the mesenchymal cells and negatively stained for markers of the hematopoietic lineage [10-14].
Figure 1.
Umbilical Cord Compartment. Digital photo of human umbilical cord tissue. Traverse section through an umbilical cord after birth. Scale bar = 2 mm. Wharton's Jelly is the connective tissue included between the subamnion and the perivascular regions.
WJCs can be expanded longer than MSCs before its multeplicity is compromised [11]. For this reason they could be induced to form different cellular lineages i.e. adipose tissue, bone, cartilage, skeletal muscle cells, cardiomyocyte-like cells and neural cells enabling them to be used in biomedical engineering applications [15-17].
The aim of the present work was to build a 2D reference map as a proteomic dataset, useful to define the molecular characteristics of WJCs and also to discover a number of candidate biomarkers specifically expressed in these extra-embryonic stem cells. In addition we also studied the molecular change that occurs during the WJCs in vitro growth in order to find proteins possibly related to their long expansion ability and fast growth in vitro.
Methods
Isolation and cell culture
For cell culture institutional review board approval was obtained for all procedures. With the consent of the parents, fresh human umbilical cords were obtained from full-term births, aseptically stored in sterile saline and processed within 6 hours from partum to obtain the umbilical cord matrix mesenchymal stem cells. After removal of blood vessels, the abundant extracellular matrix of Wharton's jelly was scraped off with a scalpel, finely cut and centrifuged at 250 × g for 5 minutes at room temperature and the pellet was washed with serum-free Dulbecco's modified Eagle's medium (DMEM). Next, the cells were treated with collagenase (2 mg/ml) (Sigma) for 16 hours at 37°C, washed in PBS(1X), and treated with 2.5% trypsin for 30 minutes at 37°C under agitation (Wang, Sarugaser). Finally, the cells were washed in PBS(1X) and seeded in complete growth medium (HMSCGM) with growth supplements, all from Cambrex Bio Science (now Lonza, Walkersville, Inc., Walkersville, MD), in 5% CO2 in a 37°C incubator (ref Sarugaser, Weiss, Mitchell). The cells were cultured with the medium exchange every 3-4 days until reaching the confluence. The adherents cells were detached with 0,05% trypsin-EDTA, counted with Trypan Blue exclusion, and re-seeded at 3000 cells/cm2 to reach the 90% of confluence after 3-4 population doublings.
Immunophenotype
The WJCs from Wharton's jelly after the harvesting at the different experimental times (2nd, 4th, 8th and 12th culture passages) were immediately treated with 0.05% trypsin-EDTA and incubated with 1 μg/106 cells fluorescein isotiocynate (FITC)-conjugated or phycoerythryne (PE)-conjugated antibodies for 40 minutes at 4°C in the dark. The anti-CD73, anti-CD13, anti-CD90, anti-CD117, anti-CD14, anti-CD34, anti-CD105 and anti-CD45 (Becton Dickinson, San Jose, CA, USA), anti-CD29, anti-CD44 and anti-CD166 (Ancell, Bayport, MN, USA) antibodies were used. After washing, cells were analyzed on a flow cytometer (FACSCalibur, Becton Dickinson, San Jose, CA, USA) collecting 10,000 events and the data analyzed by Cell Quest Software (Becton Dickinson, San Jose, CA, USA).
Cell cycle
5 × 105 cells/sample for each passage were fixed by adding 500 μl of 70% cold ethanol and then stored at 4°C. After 24 h cells were washed and stained by adding 500 μl of a solution containing 50 μg/ml propidium iodide (PI) and 200 μg/ml RNase. Cells were incubated for 3 h at 4°C in the dark and acquired on a FACSCalibur flow cytometer using the CellQuest™ software (both from Becton Dickinson, USA). Debris was excluded from the analysis by gating a forward scatter versus side scatter plot. Cell aggregates were excluded by gating FL2 area versus FL2 width. The low flow rate mode (about 400-500 events/second) was used to record 10,000 non-debris events for each sample. PI fluorescence data were collected using linear amplification. DNA content was assessed, placing the G1 peak around the channel 400. Finally, data were analysed using ModFit LT™ software (Verity Software House, Toshan, ME, USA).
Telomere length assay
DNA extraction was performed using Wizard Genomic DNA Purification Kit (Promega) following the manufacturer's instructions. The length of telomere regions of genomic DNA was assessed on DNA from cells at different passages using the Telo TAGGG kit (Roche) according to the manufacturer's instructions. Appropriate controls, DNA extracted from cells with long or short telomere regions, were also provided with the kit.
Determination of cell senescence
The amount of senescent cells was evaluated at different conditions by the use of the Senescence β-Galactosidase Staining Kit (Abcam, Cambridge, UK) in accordance to the manufacturer's instructions. Thus, the cells at different experimental conditions (2nd, 4th, 8th and 12th passage) were plated a density of 10,000 cells/cm2 for 24 h before senescence-associated β-galactosidase staining. After completion of the staining procedures, 5 representative images were taken from diverse areas of each cell culture, using phase-contrast microscopy to asses the number of positive cells.
Adipogenic differentiation
To induce adipocyte differentiation, 20 × 103 cells/cm2 was cultured in DMEM high glucose (HG) (Sigma) supplemented with 10% FBS, 0.5 mM isobutyl-methylxantine (Sigma), 200 μM indomethacin (Sigma), 1 μM dexamethasone (Sigma) and 10 μg/ml insulin (Sigma). The cells were cultured, replacing the medium every 2-3 days. After 2-3 weeks of culture, the cells contained neutral lipids in fat vacuoles; they were fixed in 10% formalin and stained with fresh oil red-O solution (Sigma).
Osteogenic Differentiation
To induce osteogenic differentiation, 3 × 103 cells/cm2 was cultured in MEM (Sigma) supplemented with 10% FBS, 10 mM β-glycerophosphate (Sigma), 0.2 mM ascorbic acid (Sigma), and 10 nM dexamethasone (Sigma), and cultured for 3-4 weeks, replacing the medium every 2-3 days. To demonstrate osteogenic differentiation, the cultures were fixed and subjected to alkaline phosphatase.
Chondrogenic Differentiation
To induce chondrogenic differentiation, aliquots of 2.5 × 105 cells were pelleted in polypropylene conical tubes in 0.5 ml of DMEM HG containing 6.25 μg/ml insulin (Sigma), 6.25 g/ml transferrin (Sigma), 6.25 g/ml selenous acid (Sigma), 5.33 μg/ml linolenic acid (Sigma), 1.25 mg/ml BSA (Sigma), 0.35 mM proline (Sigma), 1 mM sodium pyruvate (Sigma), 100 nM dexamethasone (Sigma), 0.1 mM L-ascorbic acid-2-phosphate (Sigma), supplemented with 10 ng/ml transforming growth factor-beta 3 (TGF-β3) (R&D Systems, Minneapolis, MN). This medium was replaced every 3-4 days for 3-4 weeks. Pellets were formalin-fixed, embedded in paraffin, morphologically examined after toluidine blue staining.
Proteome characterization
To generate 2D map of WJCs and analyze their protein changes during culture condition we chose four passages (2nd, 4th, 8th and 12th) in vitro expansion that were processed for protein extraction. For all culture passages we considered three independent donors and we ran 3 gels for each biological replicate. 16 × 106 stem cells of each population were treated with lysis buffer (40 mM Tris pH 7.4, 8 M urea, 4% CHAPS) supplemented with protease inhibitor mixture and 2 mM TBP as reducing agent. Protein concentration was determined by using the Bradford assay with bovine serum albumin (BSA) as standard [18].
The lysated samples were loaded onto commercial 4-7 IPG strip and the second dimension was performed on a 9-16% acrylamide gel. Analytical gels were stained with ammoniacal silver nitrate, while gels used for MALDI-TOF MS protein identification were silver-stained without glutaraldehyde, according to the mass compatible method described by Shina et al. [19,20]. After staining, gels were scanned with a MagicScan scanner (GE Healthcare, formerly Amersham Biotech, Uppsala, Sweden) in transparency mode at 800 dpi and the images were stored as TIFF images. Once digitized, the gel images were analyzed with Image Master 2D platinum software, 6.0 versions (GE Healthcare, formerly Amersham Biotech, Uppsala, Sweden). A positional gel calibration was carried out by using a 2D calibration method included in the analysis package that calculates the position of protein spots in terms of their isoelectric point (pI) and Molecular Weight (MW) values. Several spots, Thioredoxin (pI 5.13-47.09 kDa), Endoplasmin (pI 4.60-90.13 Da), spectrin alpha chain (pI 5.6-118.17 kDa), 75 kDa glucose regulated protein (pI 6.2-74.02 kDa), Glycerol-3-phosphate dehydrogenase (pI6.1 -38.65 kDa), distributed throughout the reference gel and identified by mass spectrometry analysis were chosen as marker proteins. Then a protein list including these marker proteins was created and pI and MW were calibrated in all maps simultaneously by interpolating between the known pI and MW values of the markers. In order to construct a synthetic gel from all conditions included in the present study three different gel runs for each sample were performed and then subjected to image analysis with Image Master 2D evolution software (Ge Healthcare, formerly Amersham Biotech, Uppsala, Sweden). A spot was considered only if it was detected in three out of all experimental replicates for each sample and defined "common spot" (i.e. always present and in the same position on all gels). The quantity of each spot was normalized with respect to the total spot volume detected in the gel. Relative spot volumes were determined by modelling the optical density in individual spot segments using a two-dimensional Gaussian analysis.
Following gel analysis, protein spots on preparative WJCs 2-DE gels were excised and analyzed by the peptide mass finger printing (PMF) approach on a MALDI-TOF MS. Protein spots, once excised from the gel, were washed with 100% ethanol and 100 mM ammonium bicarbonate (NH4HCO3). The pieces of gel corresponding to a single protein were incubated for 60 min at 56°C in a volume of 50 mM NH4HCO3 supplemented with 10 mM DTT and then for 30 min in the dark in 55 mM iodoacetamide in 100 mM NH4HCO3 at room temperature. Finally, the gel was reswollen in 50 mM NH4HCO3 containing trypsin and incubated at 37°C overnight [21]. Peptide extract was applied to a C18ZipTip (Millipore, Bedford, MA, USA), rinsed with a 0.1% TFA and eluted directly on the MALDI target with 0.5 μl of a saturated α-cyano-4-hydroxycinnamic acid (1:1 = ACN: 0.1% TFA) solution.
For all MS analysis, PMF was obtained using REFLEX-IV MALDI-TOF (Bruker Daltonics, Germany) in the reflectron operation mode at a potential of 20 kV and a delayed extraction of 400 ns. The instrument was calibrated with external standards such as bradykinin (fragment 1-7) 757,39 m/z, angiotensin II 1046,54 m/z, ACTH (fragment 18-39) 2465,19 m/z, Glu Fibrinopeptide B 1571,57 m/z, and renin substrate tetradecapeptide porcine 1760,02 m/z. Each spectrum was produced by accumulating data from 100 laser shots over the m/z range of 700-3000 Da. All mass spectra were calibrated internally by trypsin (Promega Bioscience, CA) autolysis products at 842, 50 Da, 1.046,56 Da, 2.212,11 Da and 2.284,19 Da. Trypsin and keratin contamination peaks were excluded from the peak list used in the database searching.
The results from the PMF were employed to search the human NCBIn protein database by Mascot search engine, which compare the experimentally determined tryptic peptide masses with theoretical peptide masses calculated for protein from these databases. Search parameters are as follows: type of search, peptide mass fingerprint; enzyme, trypsin; fixed modification, carbamidomethylation (Cys); variable modifications, oxidation (Met); mass values, monoisotopic; peptide charge state, 1+; maximum missed cleavages, 1; and peptide mass tolerance, 100 ppm.
Confirmation of the proteins expressed by WJCs at the 2nd and 12th culture passages was made by Western blot analysis in the 1D and 2D mini system. WJCs samples at 2nd and 12th passages were extracted and centrifuged. An equal protein amount of 10 μg for 1D from each total cellular lysate was loaded into 9-16% SDS-PAGE and was electrophoretically transfererred into nitrocellulose membranes at 100 V overnight at 4°C. For 2D Western blot 20 μg of WJCs cells at 2nd and 12th passages were separated on 7 cm pH 4-7 IPG strip (GE Healthcare, formerly Amersham Biotech, Uppsala Sweden). IEF was performed by a 12 h in gel-rehydratation mode at 50V, followed by focusing at 5000 V for a total of 20 kVh. The strips were incubated in equilibration buffer (8 M urea, 2% SDS, 30%(v/v) glycerol, 50 mM Tris-HCl, pH8.8) reduced with 1% (w/v) DTT for 15 min and alkylated with 2.5% iodacetamide for 15 min in dark. The 2D running was made into 9-16% SDS-PAGE and the electrophoresed proteins were transferred to nitrocellulose at 200 V overnight at 4°C. Primary antibodies sources and dilutions were: rabbit polyclonal antibodies against ADK5 1:100 (Abcam, Cambridge Science Park, UK), PAI2 1:1000 (Abcam, Cambridge Science Park, UK), SHOT1 1:500 (Cell Signalling Technology inc., Denver, MA), ERO1A 1:1000 (Abcam, Cambridge Science Park, UK), P4HA1 1:1000 (Abcam, Cambridge Science Park, UK), P4HA2 1:1000 (Abcam, Cambridge Science Park, UK), VIME 1:1000 (Abcam, Cambridge Science Park, UK), chicken polyclonal antibody against EIF3I 1:2000 (Abcam, Cambridge Science Park, UK), and mouse monoclonal antibody against ACTB 1:1000 (Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies diluted 1:6000 were goat polyclonal to rabbit IgG (for ADK5, PAI2, SHOT1, ERO1A, P4HA1, P4HA2 and VIME) rabbit polyclonal to chicken IgY (for EIF3I) and goat polyclonal to mouse IgG (for ACTB), all conjugated with horseradish peroxidase (Abcam, Cambridge Science Park, UK). Signal detection was performed with an ECL plus kit (GE Healthcare formerly Amersham Biotech, Uppsala, Sweden) and visualized by autography on Biomax light film (Sigma Chemical, St. Louis, MO, USA). All blot experiments were performed at least three times.
Result and Discussion
Cellular isolation, culturing and characterization
As can be seen in figure 2A, the cells isolated from the human Wharton's jelly and used in the present work, display a consistent spindle-shaped elongated morphology similar to fibroblastoid cells. This feature appears to be maintained up to the end of culturing. A representative immunophenotype of the cells used in our experiment is reported in figure 2B. A positive response for CD105 (endoglin), CD73, CD90, CD29, CD166, CD13, CD44 (hyaluronan receptor) markers and a negative reactivity for CD34, CD45 and CD14 hematopoietic markers were detected at each time of culture passages. This high homogeneous expression of the above mentioned markers suggests that the samples used in the present study were not significantly contaminated by no-stem cell population (figure 2B). Figure 2C illustrates the ability of WJCs to differentiate in osteogenic, adipogenic and chondrogenic lineages further evidencing their pluripotency.
Figure 2.
WJCs characterization. A. Light microscopic micrographs of WJCs in monolayer culture. In monolayer culture, the cells assumed a polymorphic, fibrobroblast-like morphology, which was maintained throughout the passaging processes. 10 days (left panel), 15 days of culture (central panel) and cells at passage 1, prior to reach confluence (right panel). Original magnification ×100. B. Analyses of surface antigen marker by flow cytometry. Cell suspensions were stained with specific mouse anti-human monoclonal antibodies (Mabs) as indicated in filled histograms. The empty histogram is the respective IgG isotype control. C. Ability to differentiate into several lineages. Osteogenic differentiation (left panel) was indicated by the increase in alkaline phosphatase (magnification 200×). Adipogenic differentiation (central panel) is visually marked by accumulation of neutral lipid vacuoles in cultures (red oil staining) (magnification 200×). Chondrogenic differentiation (right panel) is visually marked by toluidine blue staining (magnification 100×). D. Flow cytometry of cells showing DNA stained with propidium iodide. G1 and G2/M indicate 2n and 4n cellular DNA content, respectively. S indicates cells undergoing DNA synthesis, intermediate in DNA content between 2n and 4n. In the phase contrast image, arrow indicates one mitotic event. E. Telomere length assay on WJCs at different culture passages. The maintenance of long telomeres is a key feature of stem cells, ensuring the capability to undergo several cell cycles of replication. Molecular weight marker (M), control DNA with short telomeres (S) and control DNA with long telomeres (L). p4, p8 and p12 DNA extracts from WJCs at different 4th 8th and 12th passages showing long telomeric ends. F, G. Growth Features of WJCs during expansion period. Proliferation (F) and doubling time (G) at different passages of WJCs in vitro culture calculated by Trypan blue exclusion test.
As reported in figure 2D cells, after expansion, showed a homogeneous diploid content in the G1 cell-cycle phase. Furthermore, in figure 2D it can be also seen that the G1 and the G2 cell-cycle checkpoints appeared intact. This finding is consistent with cells actively cycling. The long telomeric end of DNA extracted from cells at all passages examined also confirms that these cells preserve their capability to undergo a high number of cellular divisions up to the 12th passage (figure 2E). Accordingly, a low frequency of β-galactosidase positive staining cells was found in all different passages (2nd, 4th, 8th and 12th) (Additional file 1). Overall, the data reported in figure 2 strongly indicate that the cells derived from Warthon's jelly belong to the mesenchimal stem-cell population.
In order to characterize the proteome profile of WJCs, the cells prepared at the 2nd, 4th, 8th and 12th culture passages were analyzed. Longer term in vitro culture passage led to an impairment of the cell expansion ability as accounted by the cell number count (figure 2F) and by the analysis of the doubling time (figure 2G). For these reasons they were not included in the present investigation.
2DE analysis and MS protein identification
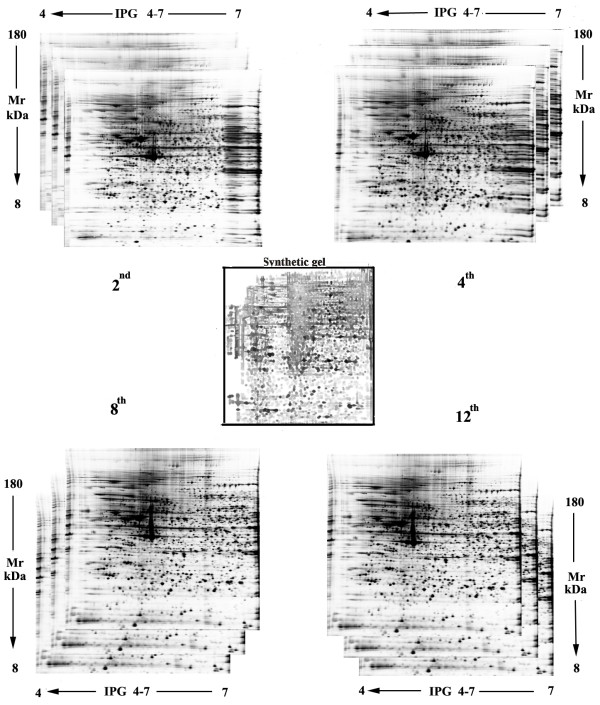
The master gels of the 2nd, 4th, 8th and 12th passage constructed for each step are reported in figure 3, by using cells prepared from three different human donors. On average 2450 ± 76, 2330 ± 91, 2200 ± 103 and 1750 ± 74 spots were detected from the 2nd to the 12th in vitro expansion passages respectively (Additional file 2).
Figure 3.
WJCs 2D map. Silver-stained 2D gels (pH4-7, 9-16% acrylamide, 200 μg protein loading) from the 2nd, 4th, 8th and 12th in vitro expansion passages. The synthetic gel is the result of the comparative analysis between the master gels obtained from 3 gels for each culture passage.
In order to evaluate the change of protein pattern expression occurring during in vitro cell expansion, the master gels, resulting from each culture passages, were compared with the synthetic gel constructed by using the gels resolved from all culture passages (figure 3).
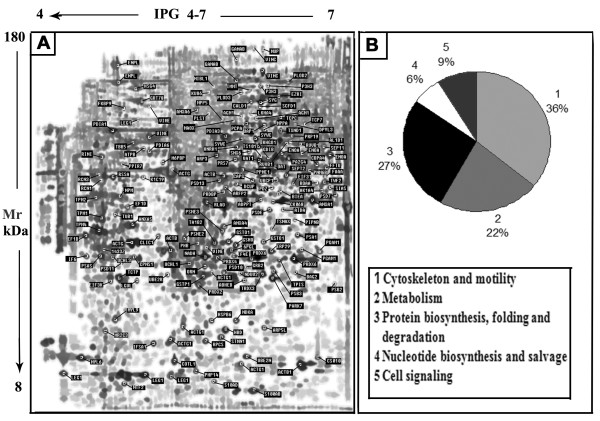
Image analysis of the synthetic gel (figure 4A) revealed the presence of 2150 spots. Since these spots are common to all passages they can therefore be considered housekeeping proteins. 251 out of 2150 spots were picked for mass spectrometry analysis. 183 spots, representing 158 unique proteins with a molecular mass ranging from 12 kDa to 180 kDa and with an isoelectric point in the 4-7 pH range were identified. The list of assigned proteins are indicated in the 2D map (figure 4A) and reported in Additional file 3. The identified proteins could be included into at least five functional categories (figure 4B), i.e. cytoskeleton and motility, metabolism, protein biosynthesis folding and degradation, nucleotide biosynthesis and cell signaling. Overall 60% of total proteome have been identified as proteins belonging to the cytoskeleton compartment and involved in protein biosynthesis, folding and degradation. The great number of proteins of these two groups may probably give to WJCs the proliferative capacity for the quickly change of their phenotype in response to adequate external stimuli.
Figure 4.
WJCs shyntetic gel (A) and the functional grouping of identified proteins (B). In the Figure 4B values represent the percent distribution of proteins classified by biological process of gene ontology terms into functional categories.
The large number of proteins associated with the energetic metabolism may confer to WJCs the necessary power for their intense proliferation. Antioxidant proteins such as thioredoxin, peroredoxin and gluthatione transferases, are also present in these cells. They may play a key role in preserving WJCs from oxidative injury during in vitro expansion.
Proteins involved in nucleotide biosynthesis and signal transduction seem to be poorly represented in WJCs. It has to be noted that myocyte's characterizing proteins like caldesmon, α-actinin, tropomyosin, and vinculin are expressed up to the last culture passages [22]. Furthermore cardiomyogenic and hepatic putative markers such as tropomyosin and pyruvate kinase were also detected in these cells [23-26].
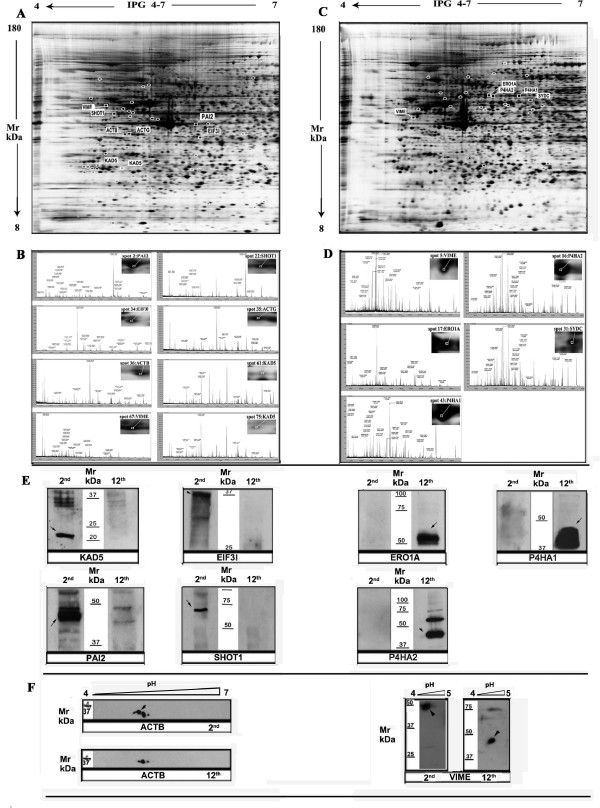
The presence of vimentin (VIME), which is the most ubiquituos intermediate filament protein and the first protein to be expressed during cell differentiation is common to other MSCs such as bone marrow and cord blood derived non-hematopoietic (mesenchymal, stromal) progenitor cells, fibroblasts and endothelial cells [27,28]. VIME is one of the most prominent phosphoprotein present in MSCs and its phosphorylation is significantly enhanced during cell division [29,30]. Six different isoforms of phosphorilated VIME were identified in WJCs. However, one of them i.e. Vimentin 4.9/47.4 (table 1; figure 5F) was no longer expressed after the 2nd passage, whereas another one i.e. Vimentin 5.1/45.7 (table 2; figure 5F) appeared only at the end of the last culture passage.
Table 1.
Proteins no longer expressed after the 2nd culture passage.
| Spot # | Abbr. Name | ACa | Protein Name | Scoreb | SCc % | pI | Mr(Da) Theo. | pI | Mr(Da) Exp. |
|---|---|---|---|---|---|---|---|---|---|
| 2 | PAI2 | P05120 | Plasminogen activator inhibitor 2 | 123 | 46 | 5,46 | 46596 | 5,44 | 30700 |
| 22 | SHOT1 | A0MZ66 | Shootin 1 | 56 | 39 | 5,27 | 72109 | 4,80 | 50629 |
| 34 | EIF3I | Q13347 | Eukaryotic translation initiation factor 3 subunit I | 214 | 61 | 5,38 | 36502 | 5,21 | 34955 |
| 35 | ACTG | P63261 | Gamma Actin | 68 | 32 | 5,31 | 41792 | 4,98 | 32910 |
| 36 | ACTB | P60709 | Beta Actin | 96 | 58 | 5,29 | 41736 | 5,04 | 33210 |
| 61 | KAD5 | Q9Y6K8 | Adenylate kinase isoenzyme 5 | 81 | 37 | 5,23 | 22420 | 4,80 | 28831 |
| 67 | VIME | P08670 | Vimentin | 100 | 35 | 5,06 | 53651 | 4,95 | 47492 |
| 75 | KAD5 | Q9Y6K8 | Adenylate kinase isoenzyme 5 | 79 | 54 | 5,23 | 22420 | 4,70 | 27829 |
A) Entry code number from SWISS PROT database.
B) Score is -10*Log(P), where P is the probability that the observed match is a random event, it is based on Swiss-Prot database using the MASCOT searching program as MALDI-TOF data.
C)Sequence coverage means the ratio of portion sequence covered by matched peptide to the full length of the protein sequence.
Figure 5.
Panel A and B indicate proteins no longer expressed after the 2nd passage. Protein spots detected by image analysis (A) are indicated with circles, whereas the proteins assigned by PMF analysis are annotated with the abbreviation name; the magnified views and peptide mass spectrum of spots 2, 22, 34, 35, 36, 61, 67 and 75 are indicated (B). Panel C and D indicate proteins spots expressed at the 12th passage. All detected spots are indicated with circles, whereas the identified protein spots are designed with the abbreviation name (C); the magnified views and peptide mass spectrum of spots 5, 16, 17,31 and 43 are indicated (D). Panel E and F indicate immunoblot analysis of the WJCs protein only expressed at the 2nd and at the 12th passages. 1-D gels probed with antibodies raised against KAD5, EIF31, PAI2, SHOT1, ERO1, PHA1 AND PHA2 (E). 2D blot analysis raised against ACTB and VIME at the 2nd and 12th passages (F).
Table 2.
New proteins expressed after the 12th culture passage.
| Spot # | Abbr. Name | ACa | Protein Name | Scoreb | SCc % | pI | Mr(Da) Theo. | pI | Mr(Da) Exp. |
|---|---|---|---|---|---|---|---|---|---|
| 5 | VIME | P08670 | Vimentin | 124 | 58 | 5,06 | 53651 | 5,09 | 45706 |
| 16 | P4HA2 | O15460 | Prolyl 4-hydroxylase subunit alpha-2 | 64 | 53 | 5,50 | 61623 | 5,50 | 61292 |
| 17 | ERO1A | Q96HE7 | Oxidoreductin-1-Lalpha | 98 | 71 | 5,43 | 55213 | 5,65 | 58342 |
| 31 | SYDC | P14868 | Aspartyl-tRNA synthetase, cytoplasmic | 89 | 67 | 6,10 | 57700 | 6,09 | 57478 |
| 43 | P4HA1 | P13674 | Prolyl 4-hydroxylase subunit alpha-1 | 101 | 45 | 5,70 | 61157 | 5,12 | 62401 |
A) Entry code number from SWISS PROT database.
B) Score is -10*Log(P), where P is the probability that the observed match is a random event, it is based on Swiss-Prot database using the MASCOT searching program as MALDI-TOF data.
C) Sequence coverage means the ratio of portion sequence covered by matched peptide to the full length of the protein sequence.
It is interesting to note that proteins reported to be markers of MSCs such as Annexin 1, Annexin 2, or heat shock protein 27 b (HSP27 b) and proteins considered markers for hESCs such as elongation factor Tu (TUFM), isocitrate dehydrogenase (IDH1), peroxiredoxins 1, 2, and 6 (PRDX1, PRDX2, PRDX6) are simultaneously expressed in WJCs [31]. Furthermore, WJCs contain nuclesphosmin, a nucleolus protein, which is highly expressed in proliferating cell as well as in undifferentiated hESCs [32-35]. Two signal transduction proteins, i.e. 14-3-3 protein zeta/delta and Twinfillin-like protein 2, not yet found in any of the stem cell lines so far investigated, are present in WJCs. The possibility that they may be WJCs specific proteins needs to be further investigated. The gel matching between the master gels and the synthetic gel has allowed to detect 47 proteins that were no longer expressed after the initial passages (figure 5A).
8 out of 47 were assigned by PMF (figure 5B) and six of them were validated by Western blotting experiments (table 1 and figure 5E, F). These proteins are involved in important cellular physiological functions including protein biosynthesis, cellular division, internal cellular motility and neuronal differentiation.
Shootin 1 (SHOT1) and two Adenylate Kinase 5 isoenzymes (KAD5) have been identified as brain specific proteins that may play a key role in neurogenesis [36-38]. The eukaryotic translation initiation factor 3 subunit 1 (EIF3I) has an essential role in the rate-limiting initiation phase of translation and is required for several steps in the initiation of protein synthesis [39]. Although the physiological role of Plasminogen activator inhibitor 2 (PAI2) has not yet been well defined, several intracellular functions, including capacity to alter genes expression, ability to influence the cellular proliferation and differentiation or inhibition of apoptosis, have been attributed to this protein [40,41].
The inability of WJCs to express, after the initial stage of replication, specific proteins involved in neuronal differentiation, may indicate that WJCs are progressively reducing their differentiation capacity during in vitro expansion.
Gel matching analysis has also allowed to identify 27 new proteins that appear only at the end of culturing (figure 5C).
5 out of 27 (table 2) were assigned by PMF (figure 5D) including two isoforms of Prolyl-4-hydroxylase i.e. the subunit alpha-1(4PHA1) and the subunit alpha-2 (4PHA2). Western blotting analysis was carried out on them and on ERO1-like protein alpha; the data obtained are shown in figure 5F.
4PHAs promote the post-translational formation of 4-hydroxyproline in hypoxia-inducible factor (HIF) alpha proteins which physiologically regulates a broad range of relevant cellular functions including apoptosis, cellular survival and energy metabolism [42]. 4PHAs are considered cellular oxygen sensors able to modulate the cellular response towards hypoxia [43-45].
In addition, these enzymes are involved in the intracellular collagen maturation by catalyzing the hydroxylation of proline residues of the procollagen. Their presence may probably be a consequence of the impairment of the cellular expansion ability that occurs to WJCs at the end of cellular in vitro expansion (figure 2F, G).
Accordingly, Miyaishi et al. have found that human fibroblasts at low population doubling rate increase the level of 4PHA1 and 4PHA2 expression [46].
At the end of culturing we have also found ERO1-like protein alpha (ERO1A). This enzyme is responsible for ROS cellular production during oxidative stress [47-49]. It is well known that ROS are considered the major contributors to cellular aging [50]. This protein, induced by hypoxia and regulated via HIF-pathway may play a key role in the maintenance of cellular redox homeostasis.
An interesting protein we have found to be expressed only at the end of culture expansion is the Aspartyl-tRNA synthetase. This enzyme interacting with differentiation-related gene 1 protein has a growth inhibitory role on cellular proliferation [51].
Taking into account the functional roles attributed to these proteins, it cannot be excluded that they are related to the molecular mechanism of WJCs in vitro self-renewal and multipotency.
Conclusion
In this report we describe an extensive study of human WJCs proteome profiling. Several important proteins, including shootin 1, adenylate kinases 5 isoenzyme and plasminogen activator inhibitor 2, are no longer expressed after the early stage of cellular in vitro replication. This is probably correlated with the gradual reduction of their staminal multipotency. In addition, at the end of the cellular expansion, new proteins probably involved in the impairment of cellular survival during replication and differentiation time were identified. These proteins could be related to the biological cellular mechanism occurring in the cellular WJCs in vitro senescence.
Further investigation will be needed to elucidate biological mechanisms involved in maintaining active proliferation and maximal cellular plasticity in order to optimize in vitro culturing procedure.
WJCs obtained from the Wharton jelly umbilical cord therefore appear to be an inexpensive biological source for the isolation of stem cells thus circumventing the ethical constraint that arises from the use of the embryonic tissue.
In this context, this study carried out to increase the knowledge about the molecular features of WJCs, may give a great contribution to the field of regenerative medicine.
Abbreviations
WJCs: Wharton's jelly cells; CFU-Fs: colony-forming unit-fibroblasts; MSCs: mesenchymal stem cells; hESCs: human embryonic stem cells; ANX1: annexin 1; ANX2: annexin 2; HSP27 b: heat shock protein 27 b; TUFM: elongation factor Tu; IDH1: isocitrate dehydrogenase; PRDX1: peroxiredoxin 1; PRDX2: peroxiredoxin 2; PRDX6: peroxiredoxin 6; SHOT1: shootin 1; KAD5: adenylate kinase 5; EIF3I: eukaryotic translation initiation factor 3 subunit I; PAI2: plasminogen activator inhibitor type-2; P4HA1: prolyl-4-hydroxylase subunit 1; P4HA2: prolyl-4-hydroxylase subunit 2; HIF: hypoxia-inducible factor; ERO1A: ERO1-like protein alpha; VIME: vimentin; ACTB: β-actin.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SA: Conception and design, data analysis and interpretation, manuscript writing; MM: Provision of study material, cellular characterization, manuscript writing; FDG: 2DE sample preparation, proteomics experiments, image analysis, collection and/or assembly of data; LP: cellular isolation and culturing; SM: statistical evaluation, data analysis and interpretation; EE: statistical analysis; PL: Flow Cytometry immunophenotype characterization; GS: Provision of study material; SM: Final approval of manuscript; CDI: Financial support, data analysis and interpretation, manuscript writing, final approval of manuscript; All authors have read and approved the final manuscript.
Supplementary Material
β-galactosidase staining at different passages in vitro expansion. β-galactosidase staining at the 2nd (A), 4th (B), 8th (C), 12th (D) passage in vitro expansion. Examples of positive cells are indicated by arrows (magnification 100×).
Scatter graphs of the % volume of about 2150 proteins between any of four WJCs lines at 2nd, 4th, 8th and 12th culture passages. The correlation coefficient CC among any four lines has been reported.
Housekeeping proteins from WJCs.
Contributor Information
Stefania Angelucci, Email: s.angelucci@unich.it.
Marco Marchisio, Email: mmarchisio@unich.it.
Fabrizio Di Giuseppe, Email: fabrizio_samp@yahoo.it.
Laura Pierdomenico, Email: l.pierdomenico@unich.it.
Marilisa Sulpizio, Email: marilisa.sulpizio@istruzione.it.
Enrica Eleuterio, Email: enricaeleuterio@yahoo.it.
Paola Lanuti, Email: paolalanuti@yahoo.it.
Giuseppe Sabatino, Email: sabatino@unich.it.
Sebastiano Miscia, Email: s.miscia@unich.it.
Carmine Di Ilio, Email: c.diilio@unich.it.
Acknowledgements
Authors would like to thank Mr Mario Scurti (University "G. d'Annunzio", Chieti, Italy) for his helpful technical assistance in informatic support. This work was supported by "Fondazione Carichieti", Chieti, Italy, by Italian Ministry of Education, University research (MIUR), Co-funding research projects of national interest (COFIN) 2007 Grant.
References
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204–213. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus AJ, Woodbury D. Fetal stem cells from extra-embryonic tissues: do not discard. J Cell Mol Med. 2008;12:730–742. doi: 10.1111/j.1582-4934.2008.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, Miki T, Marongiu F, Nakajima H, Nikaido T, Portmann-Lanz CB, Sankar V, Soncini M, Stadler G, Surbek D, Takahashi TA, Redl H, Sakuragawa N, Wolbank S, Zeisberger S, Zisch A, Strom SC. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cell. 2008;26:300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cell. 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human Umbilical Cord Perivascular (HUCPV) Cells: A Source of Mesenchymal Progenitors. Stem Cells. 2005;23:220–229. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- Troyer DL, Weiss ML. Wharton's Jelly-Derived Cells Are a Primitive Stromal Cell Population. Stem Cells. 2008;26:591–599. doi: 10.1634/stemcells.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Barry FP, Boynton RE, Haynesworth S, Murphy JM, Zaia J. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105) Biochem Biophys Res Commun. 1999;265:134–139. doi: 10.1006/bbrc.1999.1620. [DOI] [PubMed] [Google Scholar]
- Barry F, Boynton R, Murphy M, Haynesworth S, Zaia J. The SH-3 and SH-4 antibodies recognize distinct epitopes on CD73 from human mesenchymal stem cells. Biochem Biophys Res Commun. 2001;289:519–524. doi: 10.1006/bbrc.2001.6013. [DOI] [PubMed] [Google Scholar]
- De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, Troyer D, Medicetty S. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- Gang EJ, Jeong JA, Han S, Yan Q, Jeon CJ, Kim H. In vitro endothelial potential of human UC blood-derived mesenchymal stem cells. Cytotherapy. 2006;8:215–227. doi: 10.1080/14653240600735933. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;76:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Oakley BR, Kirsch DR, Morris NR. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980;105:361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Sinha P, Poland J, Schnölzer M, Rabilloud T. A new silver staining apparatus and procedure for matrix-assisted laser desorption/ionizationtime of flight analysis of proteins after two-dimensional electrophoresis. Proteomics. 2001;1:835–840. doi: 10.1002/1615-9861(200107)1:7<835::AID-PROT835>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Gharahdaghi F, Weinberg CR, Meagher DA, Imai BS, Mische SM. Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: a method for the removal of silver ions to enhance sensitivity. Electrophoresis. 1999;20:601–605. doi: 10.1002/(SICI)1522-2683(19990301)20:3<601::AID-ELPS601>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Shim WS, Jiang S, Wong P, Tan J, Chua YL, Tan YS, Sin YK, Lim CH, Chua T, Teh M, Liu TC, Sim E. Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells. Biochem Biophys Res Commun. 2004;324:481–488. doi: 10.1016/j.bbrc.2004.09.087. [DOI] [PubMed] [Google Scholar]
- Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275–1284. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- Cuenllas E, Gaitan S, Bueren JA, Tejero C. Kinetic studies of pyruvate kinase during in vitro differentiation of GM-CFC haemopoietic precursor and bone marrow cells in mice. Biosci Rep. 1990;10:141–154. doi: 10.1007/BF01116573. [DOI] [PubMed] [Google Scholar]
- Hong H, Chen JZ, Zhou F, Xue L, Zhao GQ. Influence of serum from liver-damaged rats on differentiation tendency of bone marrow-derived stem cells. World J Gastroenterol. 2004;10:2250–2253. doi: 10.3748/wjg.v10.i15.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey CJ, De la Hall PM. Hepatic stem cells: a review. Pathology. 2001;33:130–141. doi: 10.1080/00313020120038719. [DOI] [PubMed] [Google Scholar]
- Rosada C, Justesen J, Melsvik D, Ebbesen P, Kassem M. The human umbilical cord blood: a potential source for osteoblast progenitor cells. Calcif Tissue Int. 2003;72:135–142. doi: 10.1007/s00223-002-2002-9. [DOI] [PubMed] [Google Scholar]
- Li J, Sensebé L, Hervé P, Charbord P. Nontransformed colony-derived stromal cell lines from normal human marrows. II. Phenotypic characterization and differentiation pathway. Exp Hematol. 1995;23:133–141. [PubMed] [Google Scholar]
- Ogawara M, Inagaki N, Tsujimura K, Takai Y, Sekimata M, Ha MH, Imajoh-Ohmi S, Hirai S, Ohno S, Sugiura H, Yamauchi T, Inagaki M. Differential targeting of protein kinase C and CaM kinase II signalings to vimentin. J Cell Biol. 1995;131:1055–1066. doi: 10.1083/jcb.131.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson JE, He T, Trejo-Skalli AV, Härmälä-Braskén AS, Hellman J, Chou YH, Goldman RD. Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J Cell Sci. 2004;117:919–932. doi: 10.1242/jcs.00906. [DOI] [PubMed] [Google Scholar]
- Roche S, Delorme B, Oostendorp RA, Barbet R, Caton D, Noel D, Boumediene K, Papadaki HA, Cousin B, Crozet C, Milhavet O, Casteilla L, Hatzfeld J, Jorgensen C, Charbord P, Lehmann S. Comparative proteomic analysis of human mesenchymal and embryonic stem cells: towards the definition of a mesenchymal stem cell proteomic signature. Proteomics. 2009;9:223–232. doi: 10.1002/pmic.200800035. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Olson MO, Busch H. Phosphorylation of acid-soluble proteins in isolated nucleoli of Novikoff hepatoma ascites cells. Effects of divalent cations. J Biol Chem. 1974;249:5580–5585. [PubMed] [Google Scholar]
- Chan WY, Liu QR, Borjigin J, Busch H, Rennert OM, Tease LA, Chan PK. Characterization of the cDNA encoding human nucleophosmin and studies of its role in normal and abnormal growth. Biochemistry. 1989;28:1033–1039. doi: 10.1021/bi00429a017. [DOI] [PubMed] [Google Scholar]
- Baharvand H, Hajheidari M, Ashtiani SK, Salekdeh GH. Proteomic signature of human embryonic stem cells. Proteomics. 2006;6:3544–3549. doi: 10.1002/pmic.200500844. [DOI] [PubMed] [Google Scholar]
- Wang BB, Lu R, Wang WC, Jin Y. Inducible and reversible suppression of Npm1 gene expression using stably integrated small interfering RNA vector in mouse embryonic stem cells. Biochem Biophys Res Commun. 2006;347:1129–1137. doi: 10.1016/j.bbrc.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Toriyama M, Shimada T, Kim KB, Mitsuba M, Nomura E, Katsuta K, Sakumura Y, Roepstorff P, Inagaki N. Shootin1: A protein involved in the organization of an asymmetric signal for neuronal polarization. J Cell Biol. 2006;175:147–157. doi: 10.1083/jcb.200604160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rompay AR, Johansson M, Karlsson A. Identification of a novel human adenylate kinase. cDNA cloning, expression analysis, chromosome localization and characterization of the recombinant protein. Eur J Biochem. 1999;261:509–517. doi: 10.1046/j.1432-1327.1999.00294.x. [DOI] [PubMed] [Google Scholar]
- Tüzün E, Rossi JE, Karner SF, Centurion AF, Dalmau J. Adenylate kinase 5 autoimmunity in treatment refractory limbic encephalitis. J Neuroimmunol. 2007;186:177–180. doi: 10.1016/j.jneuroim.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Pan X, Hershey JW. Individual overexpression of five subunits of human translation initiation factor eIF3 promotes malignant transformation of immortal fibroblast cells. J Biol Chem. 2007;282:5790–5800. doi: 10.1074/jbc.M606284200. [DOI] [PubMed] [Google Scholar]
- Croucher DR, Saunders DN, Lobov S, Ranson M. Revisiting the biological roles of PAI2 (SERPIN2) in cancer. Nat Rev Cancer. 2008;8:535–545. doi: 10.1038/nrc2400. [DOI] [PubMed] [Google Scholar]
- Medcalf RL, Stasinopoulos SJ. The undecided serpin. The ins and outs of plasminogen activator inhibitor type 2. Febs J. 2005;272:4858–4867. doi: 10.1111/j.1742-4658.2005.04879.x. [DOI] [PubMed] [Google Scholar]
- Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl- 4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/S0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Stockmann C, Fandrey J. Hypoxia-induced erythropoietin production: a paradigm for oxygen-regulated gene expression. Clin Exp Pharmacol Physiol. 2006;33:968–979. doi: 10.1111/j.1440-1681.2006.04474.x. [DOI] [PubMed] [Google Scholar]
- Miyaishi O, Kozaki K, Saga S, Sato T, Hashizume Y. Age-related alteration of proline hydroxylase and collagen-binding heat shock protein (HSP47) expression in human fibroblasts. Mech Ageing Dev. 1995;85:25–36. doi: 10.1016/0047-6374(95)01660-0. [DOI] [PubMed] [Google Scholar]
- Gess B, Hofbauer KH, Wenger RH, Lohaus C, Meyer HE, Kurtz A. The cellular oxygen tension regulates expression of the endoplasmic oxidoreductase ERO1-Lalpha. Eur J Biochem. 2003;270:2228–2235. doi: 10.1046/j.1432-1033.2003.03590.x. [DOI] [PubMed] [Google Scholar]
- Cabibbo A, Pagani M, Fabbri M, Rocchi M, Farmery MR, Bulleid NJ, Sitia R. ERO1-L, a human protein that favors disulfide bond formation in the endoplasmic reticulum. J Biol Chem. 2000;275:4827–4833. doi: 10.1074/jbc.275.7.4827. [DOI] [PubMed] [Google Scholar]
- Sevier CS, Kaiser CA. Ero 1 and redox homeostasis in the endoplasmic reticulum. Biochim Biophys Acta. 2008;1783:549–556. doi: 10.1016/j.bbamcr.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Lu T, Finkel T. Free radicals and senescence. Exp Cell Res. 2008;314:1918–1922. doi: 10.1016/j.yexcr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano K, Taoka M, Yamauchi Y, Itagaki C, Shinkawa T, Nunomura K, Okamura N, Takahashi N, Izumi T, Isobe T. Large-scale identification of proteins expressed in mouse embryonic stem cells. Proteomics. 2005;5:1346–1361. doi: 10.1002/pmic.200400990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
β-galactosidase staining at different passages in vitro expansion. β-galactosidase staining at the 2nd (A), 4th (B), 8th (C), 12th (D) passage in vitro expansion. Examples of positive cells are indicated by arrows (magnification 100×).
Scatter graphs of the % volume of about 2150 proteins between any of four WJCs lines at 2nd, 4th, 8th and 12th culture passages. The correlation coefficient CC among any four lines has been reported.
Housekeeping proteins from WJCs.