Abstract
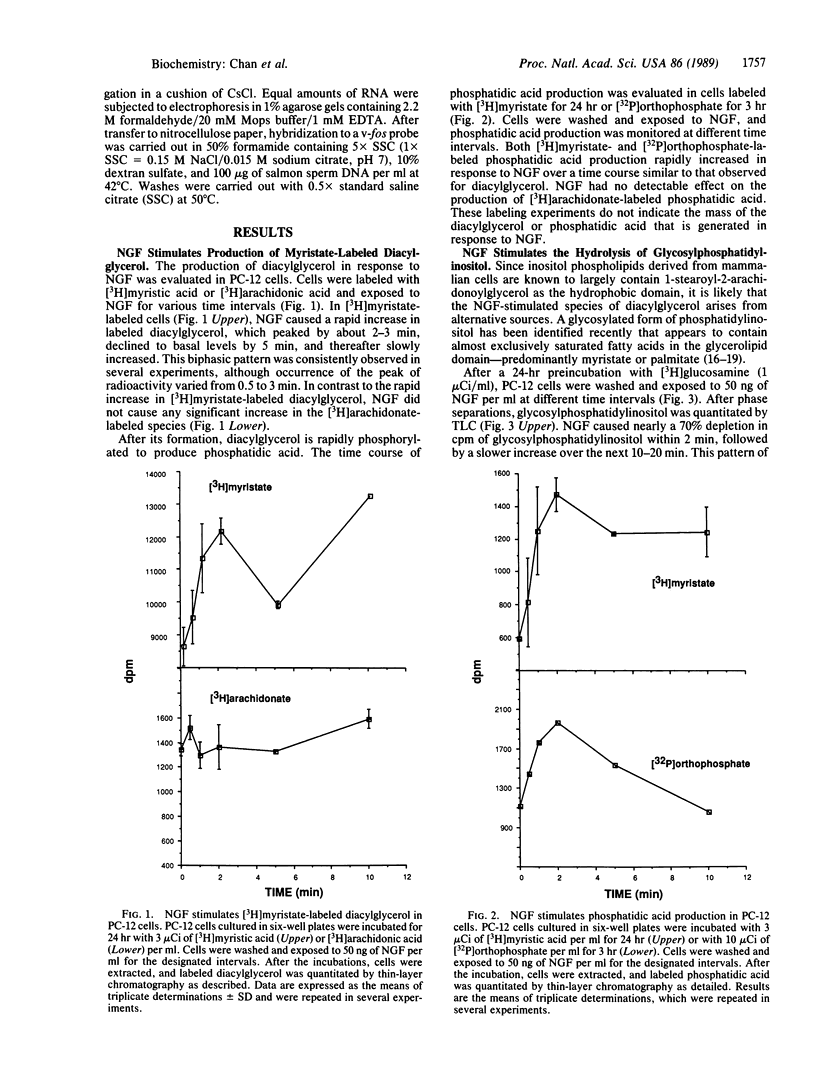
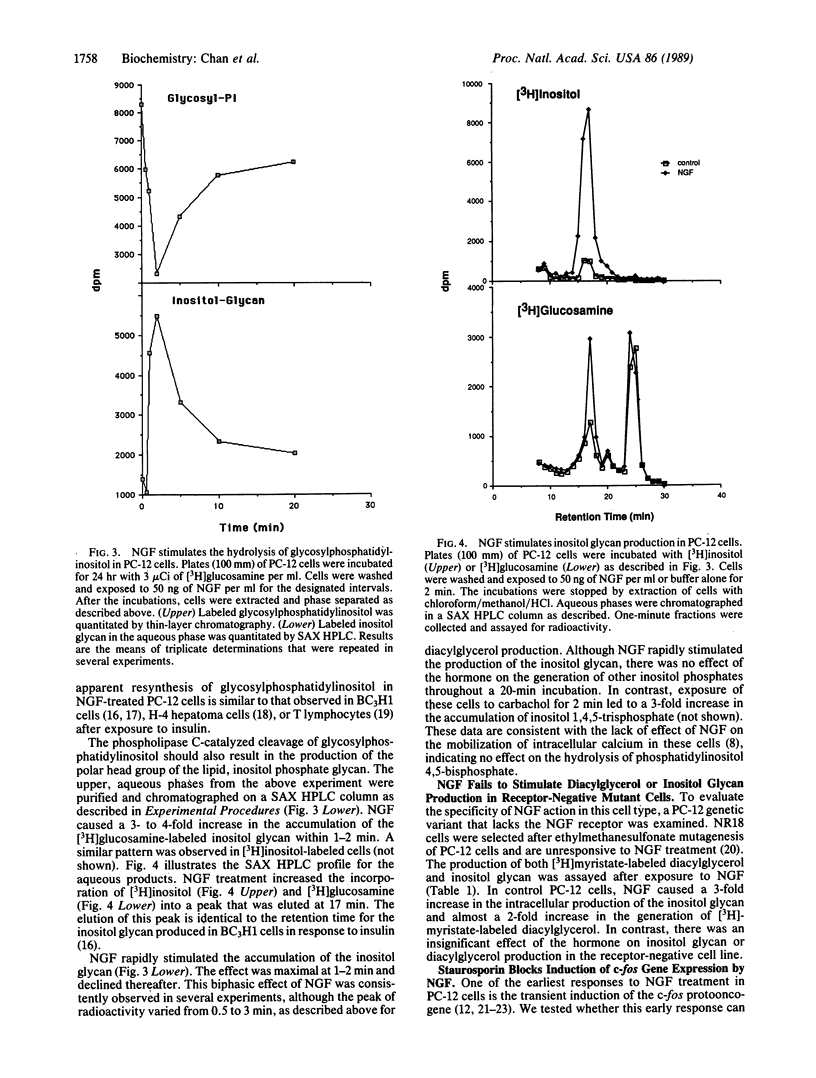
Treatment of PC-12 pheochromocytoma cells with nerve growth factor (NGF) results in the differentiation of these cells into a sympathetic neuron-like phenotype. Although the initial intracellular signals elicited by NGF remain unknown, some of the cellular effects of NGF are similar to those of other growth factors, such as insulin. We have investigated the involvement of a newly identified inositol-containing glycolipid in signal transduction for the actions of NGF. NGF stimulates the rapid generation of a species of diacylglycerol that is labeled with [3H]myristate but not with [3H]arachidonate. NGF stimulates [3H]myristate- or [32P]phosphate-labeled phosphatidic acid production over the same time course. Although NGF alone has no effect on the turnover of inositol phospholipids, it does stimulate the hydrolysis of glycosylphosphatidylinositol. The NGF-dependent cleavage of this lipid is accompanied by an increase in the accumulation of its polar head group, an inositol phosphate glycan, which is generated within 30-60 sec of NGF treatment. In an unresponsive PC-12 mutant cell line, neither the diacylglycerol nor inositol phosphate glycan response is detected. A possible role for the NGF-stimulated diacylglycerol is suggested by the inhibition of NGF-dependent c-fos induction by staurosporin, a potent inhibitor of protein kinase C. These results suggest that, like insulin, some of the cellular effects of NGF may be mediated by the phospholipase C-catalyzed hydrolysis of glycosylphosphatidylinositol.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert K. A., Helmer-Matyjek E., Nairn A. C., Müller T. H., Haycock J. W., Greene L. A., Goldstein M., Greengard P. Calcium/phospholipid-dependent protein kinase (protein kinase C) phosphorylates and activates tyrosine hydroxylase. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7713–7717. doi: 10.1073/pnas.81.24.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat N. R. Insulin dependent neurite outgrowth in cultured embryonic mouse brain cells. Brain Res. 1983 Dec;313(2):315–318. doi: 10.1016/0165-3806(83)90231-6. [DOI] [PubMed] [Google Scholar]
- Blenis J., Erikson R. L. Regulation of protein kinase activities in PC12 pheochromocytoma cells. EMBO J. 1986 Dec 20;5(13):3441–3447. doi: 10.1002/j.1460-2075.1986.tb04667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell M. A., Schechter A. L., Vaughn K. M. Clonal variants of PC12 pheochromocytoma cells with altered response to nerve growth factor. Cell. 1980 Oct;21(3):857–866. doi: 10.1016/0092-8674(80)90449-3. [DOI] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Contreras M. L., Guroff G. Calcium-dependent nerve growth factor-stimulated hydrolysis of phosphoinositides in PC12 cells. J Neurochem. 1987 May;48(5):1466–1472. doi: 10.1111/j.1471-4159.1987.tb05687.x. [DOI] [PubMed] [Google Scholar]
- Cremins J., Wagner J. A., Halegoua S. Nerve growth factor action is mediated by cyclic AMP- and Ca+2/phospholipid-dependent protein kinases. J Cell Biol. 1986 Sep;103(3):887–893. doi: 10.1083/jcb.103.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Morgan J. I. Superinduction of c-fos by nerve growth factor in the presence of peripherally active benzodiazepines. Science. 1985 Sep 20;229(4719):1265–1268. doi: 10.1126/science.4035354. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Frazier W. A., Angeletti R. H., Bradshaw R. A. Nerve growth factor and insulin. Science. 1972 May 5;176(4034):482–488. doi: 10.1126/science.176.4034.482. [DOI] [PubMed] [Google Scholar]
- Gaulton G. N., Kelly K. L., Pawlowski J., Mato J. M., Jarett L. Regulation and function of an insulin-sensitive glycosyl-phosphatidylinositol during T lymphocyte activation. Cell. 1988 Jun 17;53(6):963–970. doi: 10.1016/s0092-8674(88)90509-0. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Greene L. A., Ziff E. B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985 Nov 15;260(26):14101–14110. [PubMed] [Google Scholar]
- Greene L. A., Liem R. K., Shelanski M. L. Regulation of a high molecular weight microtubule-associated protein in PC12 cells by nerve growth factor. J Cell Biol. 1983 Jan;96(1):76–83. doi: 10.1083/jcb.96.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Shooter E. M. The nerve growth factor: biochemistry, synthesis, and mechanism of action. Annu Rev Neurosci. 1980;3:353–402. doi: 10.1146/annurev.ne.03.030180.002033. [DOI] [PubMed] [Google Scholar]
- Gunning P. W., Landreth G. E., Bothwell M. A., Shooter E. M. Differential and synergistic actions of nerve growth factor and cyclic AMP in PC12 cells. J Cell Biol. 1981 May;89(2):240–245. doi: 10.1083/jcb.89.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halegoua S., Patrick J. Nerve growth factor mediates phosphorylation of specific proteins. Cell. 1980 Nov;22(2 Pt 2):571–581. doi: 10.1016/0092-8674(80)90367-0. [DOI] [PubMed] [Google Scholar]
- Hall F. L., Fernyhough P., Ishii D. N., Vulliet P. R. Suppression of nerve growth factor-directed neurite outgrowth in PC12 cells by sphingosine, an inhibitor of protein kinase C. J Biol Chem. 1988 Mar 25;263(9):4460–4466. [PubMed] [Google Scholar]
- Hama T., Huang K. P., Guroff G. Protein kinase C as a component of a nerve growth factor-sensitive phosphorylation system in PC12 cells. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2353–2357. doi: 10.1073/pnas.83.8.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock J. W., Bennett W. F., George R. J., Waymire J. C. Multiple site phosphorylation of tyrosine hydroxylase. Differential regulation in situ by a 8-bromo-cAMP and acetylcholine. J Biol Chem. 1982 Nov 25;257(22):13699–13703. [PubMed] [Google Scholar]
- Ishii D. N., Recio-Pinto E., Spinelli W., Mill J. F., Sonnenfeld K. H. Neurite formation modulated by nerve growth factor, insulin, and tumor promoter receptors. Int J Neurosci. 1985 Apr;26(1-2):109–127. doi: 10.3109/00207458508985610. [DOI] [PubMed] [Google Scholar]
- Johnson D., Lanahan A., Buck C. R., Sehgal A., Morgan C., Mercer E., Bothwell M., Chao M. Expression and structure of the human NGF receptor. Cell. 1986 Nov 21;47(4):545–554. doi: 10.1016/0092-8674(86)90619-7. [DOI] [PubMed] [Google Scholar]
- Koizumi S., Contreras M. L., Matsuda Y., Hama T., Lazarovici P., Guroff G. K-252a: a specific inhibitor of the action of nerve growth factor on PC 12 cells. J Neurosci. 1988 Feb;8(2):715–721. doi: 10.1523/JNEUROSCI.08-02-00715.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Schubert D., Verma I. M. Induction of the proto-oncogene fos by nerve growth factor. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7330–7334. doi: 10.1073/pnas.82.21.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan J. Post-synaptic PI-effect of nerve growth factor in rat superior cervical ganglia. J Neurochem. 1979 May;32(5):1599–1601. doi: 10.1111/j.1471-4159.1979.tb11107.x. [DOI] [PubMed] [Google Scholar]
- Landreth G., Cohen P., Shooter E. M. Ca2+ transmembrane fluxes and nerve growth factor action on a clonal cell line of rat phaeochromocytoma. Nature. 1980 Jan 10;283(5743):202–204. doi: 10.1038/283202a0. [DOI] [PubMed] [Google Scholar]
- Maher P. A. Nerve growth factor induces protein-tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6788–6791. doi: 10.1073/pnas.85.18.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato J. M., Kelly K. L., Abler A., Jarett L. Identification of a novel insulin-sensitive glycophospholipid from H35 hepatoma cells. J Biol Chem. 1987 Feb 15;262(5):2131–2137. [PubMed] [Google Scholar]
- McTigue M., Cremins J., Halegoua S. Nerve growth factor and other agents mediate phosphorylation and activation of tyrosine hydroxylase. A convergence of multiple kinase activities. J Biol Chem. 1985 Jul 25;260(15):9047–9056. [PubMed] [Google Scholar]
- Milbrandt J. Nerve growth factor rapidly induces c-fos mRNA in PC12 rat pheochromocytoma cells. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4789–4793. doi: 10.1073/pnas.83.13.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn A. C., Nichols R. A., Brady M. J., Palfrey H. C. Nerve growth factor treatment or cAMP elevation reduces Ca2+/calmodulin-dependent protein kinase III activity in PC12 cells. J Biol Chem. 1987 Oct 15;262(29):14265–14272. [PubMed] [Google Scholar]
- Nairn A. C., Palfrey H. C. Identification of the major Mr 100,000 substrate for calmodulin-dependent protein kinase III in mammalian cells as elongation factor-2. J Biol Chem. 1987 Dec 25;262(36):17299–17303. [PubMed] [Google Scholar]
- Race H. M., Wagner J. A. Nerve growth factor affects cyclic AMP metabolism, but not by directly stimulating adenylate cyclase activity. J Neurochem. 1985 May;44(5):1588–1592. doi: 10.1111/j.1471-4159.1985.tb08799.x. [DOI] [PubMed] [Google Scholar]
- Recio-Pinto E., Rechler M. M., Ishii D. N. Effects of insulin, insulin-like growth factor-II, and nerve growth factor on neurite formation and survival in cultured sympathetic and sensory neurons. J Neurosci. 1986 May;6(5):1211–1219. doi: 10.1523/JNEUROSCI.06-05-01211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Landsberg C., Jastorff B. The role of cAMP in nerve growth factor-promoted neurite outgrowth in PC12 cells. J Cell Biol. 1986 Mar;102(3):821–829. doi: 10.1083/jcb.102.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel A. R., Cuatrecasas P. In search of a second messenger for insulin. Am J Physiol. 1988 Jul;255(1 Pt 1):C1–11. doi: 10.1152/ajpcell.1988.255.1.C1. [DOI] [PubMed] [Google Scholar]
- Saltiel A. R., Fox J. A., Sherline P., Cuatrecasas P. Insulin-stimulated hydrolysis of a novel glycolipid generates modulators of cAMP phosphodiesterase. Science. 1986 Aug 29;233(4767):967–972. doi: 10.1126/science.3016898. [DOI] [PubMed] [Google Scholar]
- Saltiel A. R., Sherline P., Fox J. A. Insulin-stimulated diacylglycerol production results from the hydrolysis of a novel phosphatidylinositol glycan. J Biol Chem. 1987 Jan 25;262(3):1116–1121. [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M., Whitlock C., Stallcup W. Alterations in the surface properties of cells responsive to nerve growth factor. Nature. 1978 Jun 29;273(5665):718–723. doi: 10.1038/273718a0. [DOI] [PubMed] [Google Scholar]
- Sheng M., Dougan S. T., McFadden G., Greenberg M. E. Calcium and growth factor pathways of c-fos transcriptional activation require distinct upstream regulatory sequences. Mol Cell Biol. 1988 Jul;8(7):2787–2796. doi: 10.1128/mcb.8.7.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Angeletti P. U., Levi-Montalcini R., Kettler R. Selective induction by nerve growth factor of tyrosine hydroxylase and dopamine- -hydroxylase in the rat superior cervical ganglia. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1598–1602. doi: 10.1073/pnas.68.7.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Calker D., Heumann R. Nerve growth factor potentiates the agonist-stimulated accumulation of inositol phosphates in PC-12 pheochromocytoma cells. Eur J Pharmacol. 1987 Mar 17;135(2):259–260. doi: 10.1016/0014-2999(87)90623-6. [DOI] [PubMed] [Google Scholar]
- Volonté C., Parries G. S., Racker E. Stimulation of inositol incorporation into lipids of PC12 cells by nerve growth factor and bradykinin. J Neurochem. 1988 Oct;51(4):1156–1162. doi: 10.1111/j.1471-4159.1988.tb03081.x. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Shooter E. M. The biology and mechanism of action of nerve growth factor. Annu Rev Biochem. 1982;51:845–868. doi: 10.1146/annurev.bi.51.070182.004213. [DOI] [PubMed] [Google Scholar]
- Yu M. W., Tolson N. W., Guroff G. Increased phosphorylation of specific nuclear proteins in superior cervical ganglia and PC12 cells in response to nerve growth factor. J Biol Chem. 1980 Nov 10;255(21):10481–10492. [PubMed] [Google Scholar]




