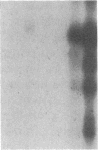
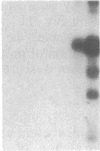
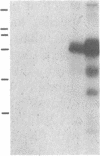
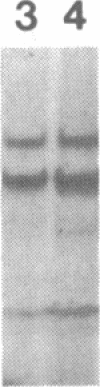
Abstract
The phosphorylation of histone by purified protein kinase C (PK-C) from rat brain is dependent on the presence of Ca2+ and lipids. Phosphorylation of a synthetic random polymer of arginine and serine (3:1) is only moderately enhanced by Ca2+ and lipids, but it is greatly enhanced in the absence of Ca2+ and lipids by a contaminant in crystalline bovine serum albumin or by heated cellular fractions. The phosphorylation ratio of histone to poly(arginine,serine) varies between different PK-C fractions from brains of rat, pig, or lamb. These variations are partly caused by a PK-C isozyme that prefers poly(arginine,serine) over histone as substrate. The kinase activator (KA) was partly purified from bovine serum albumin and from extracts of plasma membranes of human placenta. KA is also present in mitochondria, nuclei, and the cytosol. Sulfates and phosphates at 10 mM substitute for KA with poly(arginine,serine) as substrate. The phosphorylation of histone III in the presence of Ca2+ and lipids is moderately stimulated by KA, but the phosphorylation of lamin B and some other endogenous proteins is greatly enhanced by KA. With histones as substrates, inorganic anions do not stimulate phosphorylation. The phosphorylation of poly-(arginine,serine) is very sensitive to low concentrations of staurosporin and is inhibited by PK-C antibody, but, in contrast to histone phosphorylation, it is resistant to sphingosine and polymyxin B. The poly(arginine,serine) phosphorylating activity is more stable at 4 degrees C than the histone phosphorylating activity, but the latter is stabilized by 0.05% Triton X-100.
Full text
PDF
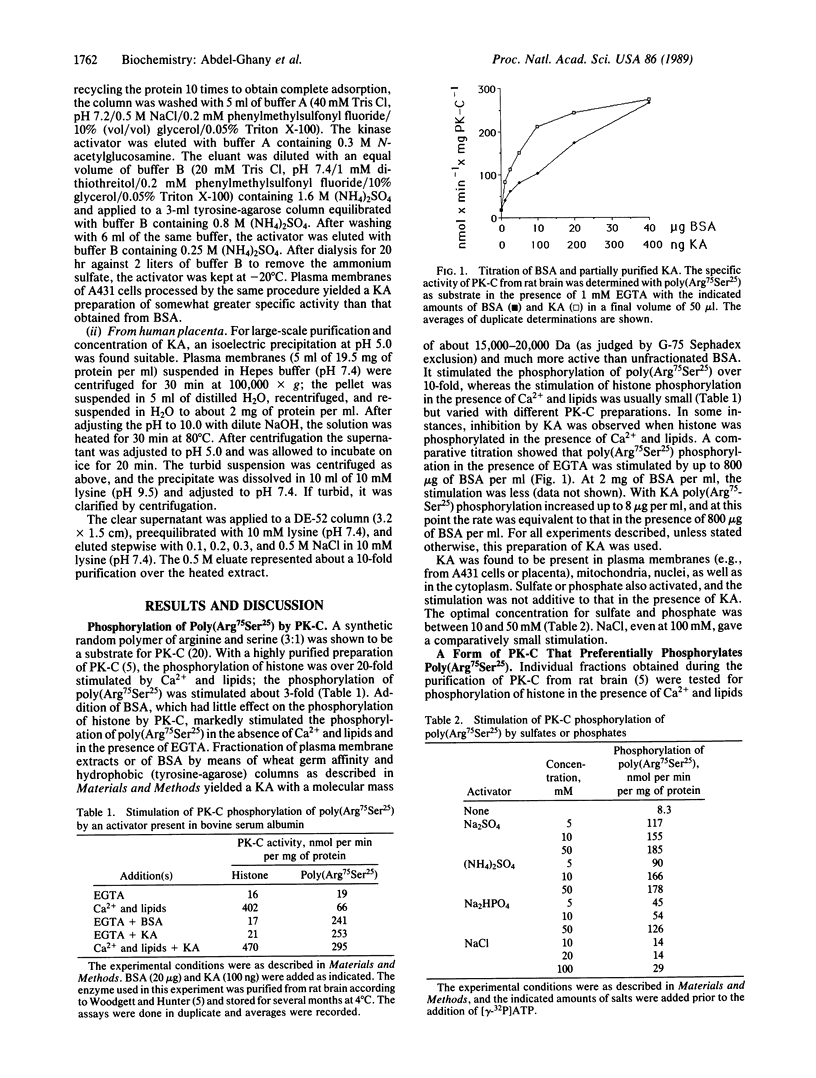
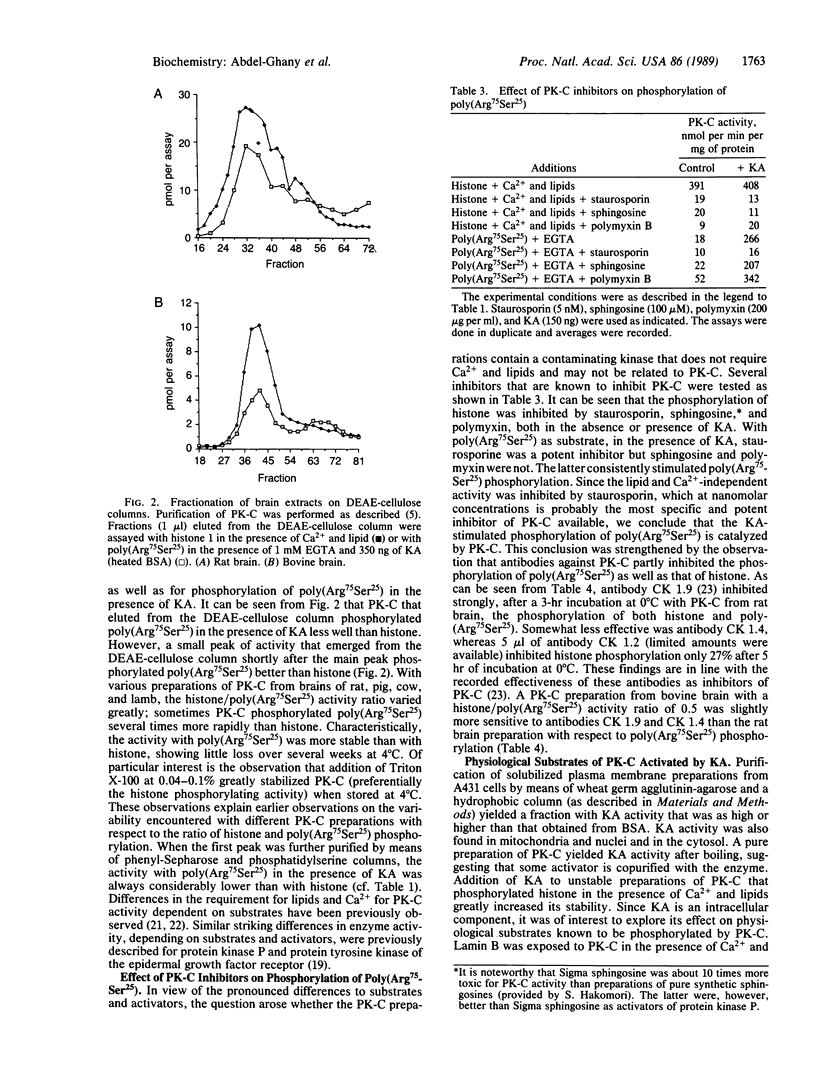
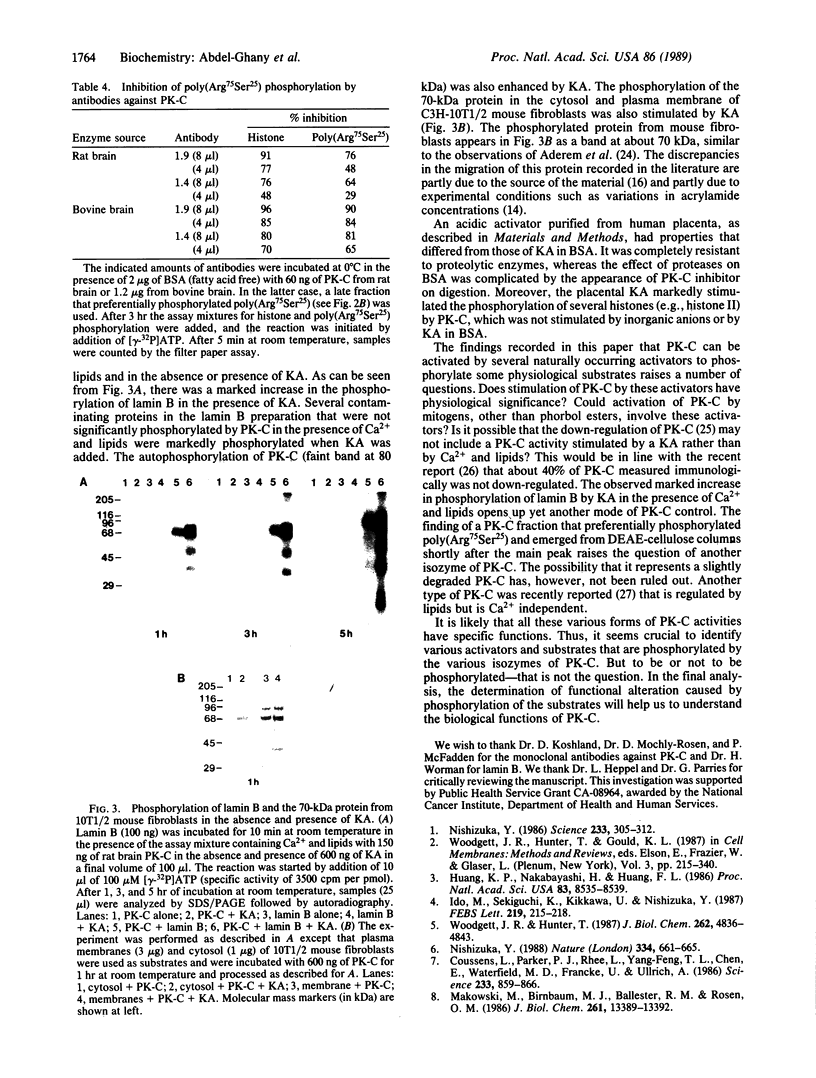

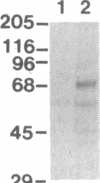
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Ghany M., Kole H. K., Racker E. Effect of protein kinase P on phosphorylations catalyzed by the epidermal growth factor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8888–8892. doi: 10.1073/pnas.84.24.8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Ghany M., Nakamura S., Navarro J., Racker E. A membrane-bound human placental protein kinase activated by endogenous polypeptides. Biosci Rep. 1983 Mar;3(3):275–282. doi: 10.1007/BF01122460. [DOI] [PubMed] [Google Scholar]
- Aderem A. A., Albert K. A., Keum M. M., Wang J. K., Greengard P., Cohn Z. A. Stimulus-dependent myristoylation of a major substrate for protein kinase C. Nature. 1988 Mar 24;332(6162):362–364. doi: 10.1038/332362a0. [DOI] [PubMed] [Google Scholar]
- Albert K. A., Nairn A. C., Greengard P. The 87-kDa protein, a major specific substrate for protein kinase C: purification from bovine brain and characterization. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7046–7050. doi: 10.1073/pnas.84.20.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Wen L., Glynn B. P., Witters L. A. Protein kinase C-stimulated phosphorylation in vitro of a Mr 80,000 protein phosphorylated in response to phorbol esters and growth factors in intact fibroblasts. Distinction from protein kinase C and prominence in brain. J Biol Chem. 1986 Jan 25;261(3):1459–1469. [PubMed] [Google Scholar]
- Coussens L., Parker P. J., Rhee L., Yang-Feng T. L., Chen E., Waterfield M. D., Francke U., Ullrich A. Multiple, distinct forms of bovine and human protein kinase C suggest diversity in cellular signaling pathways. Science. 1986 Aug 22;233(4766):859–866. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- Fields A. P., Pettit G. R., May W. S. Phosphorylation of lamin B at the nuclear membrane by activated protein kinase C. J Biol Chem. 1988 Jun 15;263(17):8253–8260. [PubMed] [Google Scholar]
- Gould K. L., Hunter T. Platelet-derived growth factor induces multisite phosphorylation of pp60c-src and increases its protein-tyrosine kinase activity. Mol Cell Biol. 1988 Aug;8(8):3345–3356. doi: 10.1128/mcb.8.8.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck P., Huang K. P., Paul W. E. Lamin B is rapidly phosphorylated in lymphocytes after activation of protein kinase C. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2279–2283. doi: 10.1073/pnas.85.7.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. P., Nakabayashi H., Huang F. L. Isozymic forms of rat brain Ca2+-activated and phospholipid-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8535–8539. doi: 10.1073/pnas.83.22.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ido M., Sekiguchi K., Kikkawa U., Nishizuka Y. Phosphorylation of the EGF receptor from A431 epidermoid carcinoma cells by three distinct types of protein kinase C. FEBS Lett. 1987 Jul 13;219(1):215–218. doi: 10.1016/0014-5793(87)81219-x. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977 Nov 10;252(21):7610–7616. [PubMed] [Google Scholar]
- Jaken S., Kiley S. C. Purification and characterization of three types of protein kinase C from rabbit brain cytosol. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4418–4422. doi: 10.1073/pnas.84.13.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf J. L., Lee M. H., Sultzman L. A., Kriz R. W., Loomis C. R., Hewick R. M., Bell R. M. Cloning and expression of multiple protein kinase C cDNAs. Cell. 1986 Aug 15;46(4):491–502. doi: 10.1016/0092-8674(86)90874-3. [DOI] [PubMed] [Google Scholar]
- Makowske M., Birnbaum M. J., Ballester R., Rosen O. M. A cDNA encoding protein kinase C identifies two species of mRNA in brain and GH3 cells. J Biol Chem. 1986 Oct 15;261(29):13389–13392. [PubMed] [Google Scholar]
- Mochly-Rosen D., Koshland D. E., Jr Domain structure and phosphorylation of protein kinase C. J Biol Chem. 1987 Feb 15;262(5):2291–2297. [PubMed] [Google Scholar]
- Nakadate T., Jeng A. Y., Blumberg P. M. Effect of phospholipid on substrate phosphorylation by a catalytic fragment of protein kinase C. J Biol Chem. 1987 Aug 25;262(24):11507–11513. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Ohno S., Akita Y., Konno Y., Imajoh S., Suzuki K. A novel phorbol ester receptor/protein kinase, nPKC, distantly related to the protein kinase C family. Cell. 1988 Jun 3;53(5):731–741. doi: 10.1016/0092-8674(88)90091-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfman A., Wingrove T. G., Blackshear P. J., Macara I. G. Down-regulation of protein kinase C and of an endogenous 80-kDa substrate in transformed fibroblasts. J Biol Chem. 1987 Dec 5;262(34):16546–16552. [PubMed] [Google Scholar]
- Woodgett J. R., Hunter T. Isolation and characterization of two distinct forms of protein kinase C. J Biol Chem. 1987 Apr 5;262(10):4836–4843. [PubMed] [Google Scholar]
- Wu W. C., Walaas S. I., Nairn A. C., Greengard P. Calcium/phospholipid regulates phosphorylation of a Mr "87k" substrate protein in brain synaptosomes. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5249–5253. doi: 10.1073/pnas.79.17.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]