Abstract
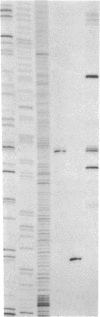
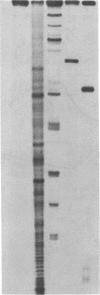
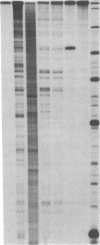
A hybrid enzyme consisting of an oligodeoxyribonucleotide fused to a unique site on staphylococcal nuclease site-selectively cleaves a number of natural RNAs including Escherichia coli M1 RNA (377 bases), 16S rRNA (1542 bases), and yeast tRNA(Phe). The oligonucleotide directs the nuclease activity of the enzyme to the nucleotides directly adjacent to the complementary target sequence on the substrate RNA. In the case of M1 RNA, hydrolysis occurs primarily at one phosphodiester bond, converting 50% of the starting material to product. Furthermore, the reaction products can be enzymatically manipulated: tRNA(Phe) was cleaved in the anticodon region and was religated to form the full-length tRNA in high yield. Because the specificity of these hybrid enzymes can be easily altered, they should prove to be useful tools for probing RNA structure and function.
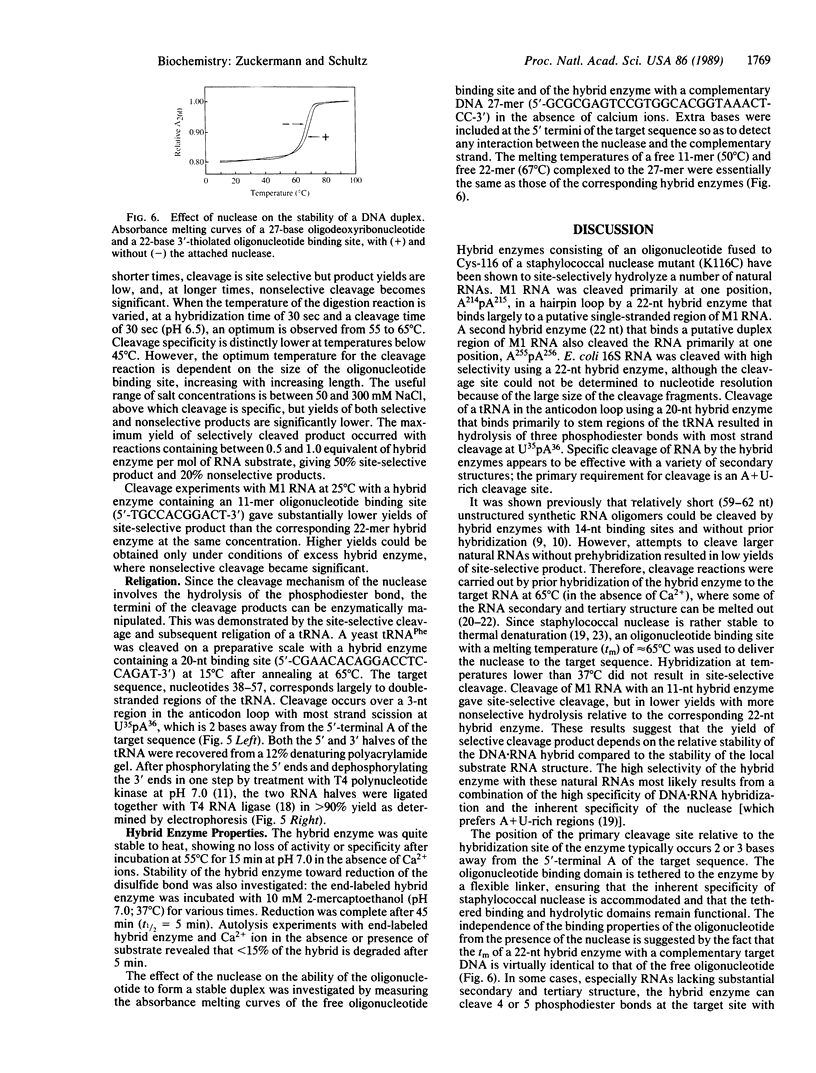
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruce A. G., Uhlenbeck O. C. Enzymatic replacement of the anticodon of yeast phenylalanine transfer ribonucleic acid. Biochemistry. 1982 Mar 2;21(5):855–861. doi: 10.1021/bi00534a007. [DOI] [PubMed] [Google Scholar]
- Calderon R. O., Stolowich N. J., Gerlt J. A., Sturtevant J. M. Thermal denaturation of staphylococcal nuclease. Biochemistry. 1985 Oct 22;24(22):6044–6049. doi: 10.1021/bi00343a004. [DOI] [PubMed] [Google Scholar]
- Cameron V., Uhlenbeck O. C. 3'-Phosphatase activity in T4 polynucleotide kinase. Biochemistry. 1977 Nov 15;16(23):5120–5126. doi: 10.1021/bi00642a027. [DOI] [PubMed] [Google Scholar]
- Cech T. R. The chemistry of self-splicing RNA and RNA enzymes. Science. 1987 Jun 19;236(4808):1532–1539. doi: 10.1126/science.2438771. [DOI] [PubMed] [Google Scholar]
- Cedergren R., Grosjean H. RNA design by in vitro RNA recombination and synthesis. Biochem Cell Biol. 1987 Aug;65(8):677–692. doi: 10.1139/o87-090. [DOI] [PubMed] [Google Scholar]
- Chu B. C., Orgel L. E. Nonenzymatic sequence-specific cleavage of single-stranded DNA. Proc Natl Acad Sci U S A. 1985 Feb;82(4):963–967. doi: 10.1073/pnas.82.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey D. R., Schultz P. G. Generation of a hybrid sequence-specific single-stranded deoxyribonuclease. Science. 1987 Dec 4;238(4832):1401–1403. doi: 10.1126/science.3685986. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Cole P. E., Hilbers C. W., Shulman R. G. The molecular mechanism of thermal unfolding of Escherichia coli formylmethionine transfer RNA. J Mol Biol. 1974 Jul 25;87(1):63–88. doi: 10.1016/0022-2836(74)90560-9. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Fuchs S., Anfinsen C. B. Catalytic properties and specificity of the extracellular nuclease of Staphylococcus aureus. J Biol Chem. 1967 Apr 10;242(7):1541–1547. [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H. Site specific enzymatic cleavage of RNA. Nucleic Acids Res. 1979 Sep 11;7(1):179–192. doi: 10.1093/nar/7.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer G. B., Dervan P. B. Sequence-specific cleavage of single-stranded DNA: oligodeoxynucleotide-EDTA X Fe(II). Proc Natl Acad Sci U S A. 1985 Feb;82(4):968–972. doi: 10.1073/pnas.82.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Gamper H. B., Cimino G. D., Hearst J. E. Solution hybridization of crosslinkable DNA oligonucleotides to bacteriophage M13 DNA. Effect of secondary structure on hybridization kinetics and equilibria. J Mol Biol. 1987 Sep 20;197(2):349–362. doi: 10.1016/0022-2836(87)90128-8. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- James B. D., Olsen G. J., Liu J. S., Pace N. R. The secondary structure of ribonuclease P RNA, the catalytic element of a ribonucleoprotein enzyme. Cell. 1988 Jan 15;52(1):19–26. doi: 10.1016/0092-8674(88)90527-2. [DOI] [PubMed] [Google Scholar]
- Modrich P. Studies on sequence recognition by type II restriction and modification enzymes. CRC Crit Rev Biochem. 1982;13(3):287–323. doi: 10.3109/10409238209114231. [DOI] [PubMed] [Google Scholar]
- Ogden R. C., Adams D. A. Electrophoresis in agarose and acrylamide gels. Methods Enzymol. 1987;152:61–87. doi: 10.1016/0076-6879(87)52011-0. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Filimonov V. V. Thermodynamic analysis of transfer RNA unfolding. J Mol Biol. 1978 Jul 15;122(4):447–464. doi: 10.1016/0022-2836(78)90421-7. [DOI] [PubMed] [Google Scholar]
- Sampson J. R., Uhlenbeck O. C. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1033–1037. doi: 10.1073/pnas.85.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara S., Mukai S., Nishihara T., Inoue H., Ohtsuka E., Morisawa H. Site-directed cleavage of RNA. Nucleic Acids Res. 1987 Jun 11;15(11):4403–4415. doi: 10.1093/nar/15.11.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassov V. V., Zarytova V. F., Kutiavin I. V., Mamaev S. V., Podyminogin M. A. Complementary addressed modification and cleavage of a single stranded DNA fragment with alkylating oligonucleotide derivatives. Nucleic Acids Res. 1986 May 27;14(10):4065–4076. doi: 10.1093/nar/14.10.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann R., Corey D., Schultz P. Efficient methods for attachment of thiol specific probes to the 3'-ends of synthetic oligodeoxyribonucleotides. Nucleic Acids Res. 1987 Jul 10;15(13):5305–5321. doi: 10.1093/nar/15.13.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]