The innate immune response to viruses involves type I IFN (i.e., IFN-α and -β)–modulated up-regulation of cellular genes that are key mediators of antiviral defenses. Cellular proteins such as RNA helicase retinoic acid–inducible gene I (RIG-I) and Toll-like receptors (TLRs) recognize pathogen-associated molecular patterns, which are the hallmark of viral infections (1, 2). Consequently, many viruses have developed strategies to evade the IFN system. Hepatitis C virus (HCV), a liver-tropic virus that chronically infects approximately 170 million people worldwide (3), is able to attenuate the IFN response at multiple levels within infected hepatocytes. For example, viral NS3/4A protease is able to cleave key intermediate proteins that are involved in TLR3- and RIG-I–mediated antiviral signaling (Fig. 1) in cultured cells (reviewed in ref. 4). Furthermore, viral NS5A protein is able to block activation of the double-stranded RNA–activated protein kinase PKR (reviewed in ref 4). Curiously, HCV infection induces a robust production of IFN-stimulated genes in the liver (5, 6). These observations present a conundrum: HCV inhibits production of IFN and IFN-stimulated genes (ISGs) in the cultured liver, yet induces IFN production in the infected organ. Either the virus cannot block IFN-mediated antiviral responses in the highly differentiated liver organ, or IFN is produced in the liver by cells other than HCV-infected hepatocytes.
Fig. 1.
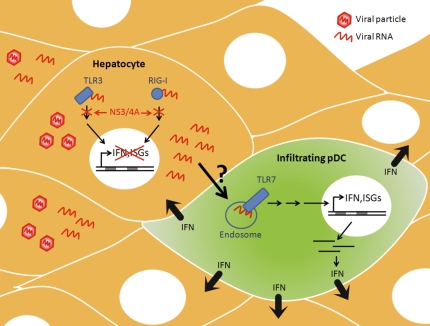
Secretion of IFN by pDCs after contact with HCV-infected hepatocytes. HCV-infected cells recognize viral RNA and signal through cellular sensors of foreign RNA, RIG-I, and TLR3 to induce synthesis of IFN and ISGs. In response, HCV evades the IFN response by virally encoded protease (NS3/4A) that cleaves key molecules involved in RIG-I and TLR3 signaling, thereby effectively turning off production of IFN and ISGs in infected hepatocytes. Through a cell contact–dependent mechanism, viral RNA is transferred from infected hepatocytes to infiltrating pDCs. pDCs recognize viral RNA through endosomal TLR7, resulting in the synthesis of ISGs and secretion of large amounts of IFN to surrounding hepatocytes.
Takahashi et al. (7) provide evidence that IFN is produced from a highly specialized class of dendritic cells known as plasmacytoid dendritic cells (pDCs) that are known to infiltrate the liver during HCV infection (Fig. 1). The authors showed that cocultivation of human pDCs purified from peripheral blood mononuclear cells (PBMCs) with HCV-infected hepatocytes resulted in marked IFN production that was proportional to the number of HCV-infected cells (7). Total PBMCs, but not pDC-depleted PBMCs, produced IFN when cocultured with HCV-infected hepatocytes, arguing that the pDCs themselves were the sources of IFN. Curiously, only approximately 10% of the pDCs in coculture produced IFN (7). This finding suggests that only a subpopulation of pDCs can be activated, or that activation of pDCs requires a limiting step that could be modulated by the growth rate or cell cycle position of pDCs.
One of the most striking observations was that IFN production by pDCs was dependent upon direct cell-to-cell contact with infected hepatocytes. Specifically, separation of pDCs and HCV-infected hepatocytes by a semipermeable membrane that allowed exchange of stimuli without direct cell-to-cell contact completely abrogated IFN production (7). In addition, neutralizing antibodies that prevented receptor-mediated entry of HCV particles did not inhibit IFN production, indicating that uptake of viral particles by the pDCs is not required for IFN production. However, IFN was not produced when hepatocytes were transfected with replication-defective viral RNAs. This finding argues that IFN production by neighboring pDCs requires a significant abundance of viral RNA in the infected hepatocytes or that viral replication complexes themselves trigger IFN production in pDCs (7).
How do pDCs sense the presence of HCV-infected cells? The ability of pDCs to secrete type I IFNs typically depends on cellular sensors that are able to detect the presence of DNA or RNA viruses (8). pDCs express a specialized subset of these cellular sensors, including TLR7 and TLR9, which sense endosomal RNA and DNA, respectively (8). When HCV RNA was introduced directly into the pDCs, they were able to produce IFN in a TLR7-dependent manner (Fig. 1). However, IFN was produced even when a replication-defective RNA was transfected into pDCs, indicating that IFN production was not triggered by viral replication within the pDCs themselves (7). This observation suggests that HCV RNA is transferred from infected hepatocytes to pDCs to trigger IFN production via TLR7 activation. The mechanism of RNA transfer is not yet known. One could envisage vesicle-mediated transfer by autophagosomes (9) or exosomes (10).
Does viral RNA transduction from hepatocytes to pDCs occur during natural infections? The number of pDCs in HCV-infected patients as well as their function has been reported to be dramatically reduced (11–13). Thus, Takahashi et al. (7) examined whether pDCs from HCV-infected patients were able to produce IFN after coculture with HCV-infected hepatocytes. Surprisingly, pDCs from HCV-infected patients were able to produce comparable levels of IFN as healthy donors, indicating that the pDCs themselves are not impaired in their ability to produce IFN. Because pDCs are prevalent in the HCV-infected liver, and HCV-infected cells in vitro activate pDCs from HCV-infected patients, pDC–hepatocyte interactions are likely to occur during natural HCV infections.
Intriguingly, this cell contact–dependent activation mechanism is not restricted to HCV RNA replicating cells. Coculturing of pDCs with cells that replicate a noncytopathic, subgenomic Venezuelan equine encephalitis virus replicon resulted in the production of IFN in a TLR7-dependent manner (7). However, pDCs did not produce IFN in response to Borna disease virus–infected cells, suggesting that some but not all RNA virus–infected cells are able to trigger this mechanism of activation (7).
The unique ability of pDCs to sense and become activated by contact with HCV-infected cells raises many questions. What is the mechanism by which viral RNA is transferred from infected hepatocyte to pDCs? Why are only a small proportion of pDCs able to secrete IFN in response to HCV-infected cells? If pDCs are really the IFN producers in the infected liver, why do they fail to control the infection in HCV-infected patients? A better understanding of the mechanisms involved in recognition and control of viral infection by pDCs in the infected liver will help shed light on some of these questions and will have an important impact on the design of cell-based therapies for HCV infection.
Footnotes
The authors declare no conflict of interest.
See companion article on page 7431 in issue 16 of volume 107.
References
- 1.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Wilkins C, Gale M., Jr Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol. 2010;22:41–47. doi: 10.1016/j.coi.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- 4.Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745–1754. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bigger CB, et al. Intrahepatic gene expression during chronic hepatitis C virus infection in chimpanzees. J Virol. 2004;78:13779–13792. doi: 10.1128/JVI.78.24.13779-13792.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su AI, et al. Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci USA. 2002;99:15669–15674. doi: 10.1073/pnas.202608199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi K, et al. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon and inhibit infection. Proc Natl Acad Sci USA. 2010;107:7431–7436. doi: 10.1073/pnas.1002301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 9.Wang RC, Levine B. Autophagy in cellular growth control. FEBS Lett. 2010;584:1417–1426. doi: 10.1016/j.febslet.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simons M, Raposo G. Exosomes—vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Kanto T, et al. Reduced numbers and impaired ability of myeloid and plasmacytoid dendritic cells to polarize T helper cells in chronic hepatitis C virus infection. J Infect Dis. 2004;190:1919–1926. doi: 10.1086/425425. [DOI] [PubMed] [Google Scholar]
- 12.Ulsenheimer A, et al. Plasmacytoid dendritic cells in acute and chronic hepatitis C virus infection. Hepatology. 2005;41:643–651. doi: 10.1002/hep.20592. [DOI] [PubMed] [Google Scholar]
- 13.Shiina M, Rehermann B. Cell culture-produced hepatitis C virus impairs plasmacytoid dendritic cell function. Hepatology. 2008;47:385–395. doi: 10.1002/hep.21996. [DOI] [PubMed] [Google Scholar]