Abstract
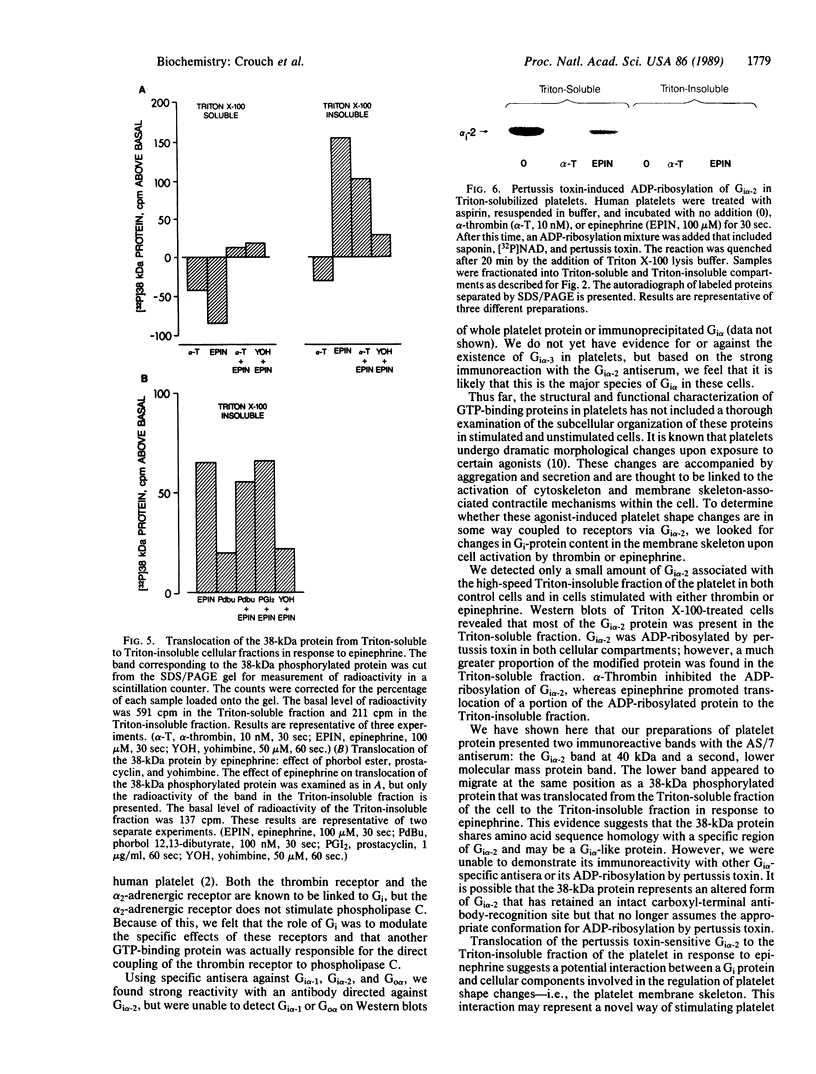
By using antibodies specific for alpha subunits of inhibitory GTP-binding proteins (Gi alpha polypeptides) to probe Western blots of whole platelet protein, we detected Gi alpha-2 as the predominant Gi alpha species present in platelets. The subcellular compartmentalization of distinct Gi alpha-2-immunoreactive polypeptides coupled to thrombin and alpha 2-adrenergic receptors was examined in Triton X-100 platelet lysates prepared by highspeed centrifugation. This treatment permitted separation of the Triton-insoluble membrane skeleton from Triton-soluble cell components. In cells treated with either alpha-thrombin or epinephrine, we observed that a greater proportion of Gi alpha-2 was localized in the Triton-soluble fraction than in the Triton-insoluble fraction. Pertussis toxin was found to catalyze ADP-ribosylation of Gi alpha-2 in whole platelets. In thrombin-stimulated cells, this activity was confined to the Triton-soluble fraction and was markedly lower than that of unstimulated cells. Epinephrine, on the other hand, promoted translocation of a portion of the pertussis toxin-sensitive Gi alpha-2 from the Triton-soluble fraction to the Triton-insoluble fraction. In addition, epinephrine stimulated translocation of a phosphorylated protein of approximately 38 kDa that was not ADP-ribosylated by pertussis toxin. This protein expressed immunoreactivity with the general Gi alpha antiserum AS/7 but not with the Gi alpha-2 antiserum LE/3. These findings suggest a role for specific localization of Gi alpha proteins in epinephrine-induced platelet responses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crouch M. F., Lapetina E. G. A role for Gi in control of thrombin receptor-phospholipase C coupling in human platelets. J Biol Chem. 1988 Mar 5;263(7):3363–3371. [PubMed] [Google Scholar]
- Goldsmith P., Gierschik P., Milligan G., Unson C. G., Vinitsky R., Malech H. L., Spiegel A. M. Antibodies directed against synthetic peptides distinguish between GTP-binding proteins in neutrophil and brain. J Biol Chem. 1987 Oct 25;262(30):14683–14688. [PubMed] [Google Scholar]
- Goldsmith P., Rossiter K., Carter A., Simonds W., Unson C. G., Vinitsky R., Spiegel A. M. Identification of the GTP-binding protein encoded by Gi3 complementary DNA. J Biol Chem. 1988 May 15;263(14):6476–6479. [PubMed] [Google Scholar]
- Katada T., Oinuma M., Kusakabe K., Ui M. A new GTP-binding protein in brain tissues serving as the specific substrate of islet-activating protein, pertussis toxin. FEBS Lett. 1987 Mar 23;213(2):353–358. doi: 10.1016/0014-5793(87)81521-1. [DOI] [PubMed] [Google Scholar]
- Kim S. Y., Ang S. L., Bloch D. B., Bloch K. D., Kawahara Y., Tolman C., Lee R., Seidman J. G., Neer E. J. Identification of cDNA encoding an additional alpha subunit of a human GTP-binding protein: expression of three alpha i subtypes in human tissues and cell lines. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4153–4157. doi: 10.1073/pnas.85.12.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Reep B., Chang K. J. Treatment of human platelets with trypsin, thrombin, or collagen inhibits the pertussis toxin-induced ADP-ribosylation of a 41-kDa protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5880–5883. doi: 10.1073/pnas.83.16.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess W., Weber P. C., Lapetina E. G. Activation of phospholipase C is dissociated from arachidonate metabolism during platelet shape change induced by thrombin or platelet-activating factor. Epinephrine does not induce phospholipase C activation or platelet shape change. J Biol Chem. 1984 Jul 10;259(13):8286–8292. [PubMed] [Google Scholar]
- Steen V. M., Tysnes O. B., Holmsen H. Synergism between thrombin and adrenaline (epinephrine) in human platelets. Marked potentiation of inositol phospholipid metabolism. Biochem J. 1988 Jul 15;253(2):581–586. doi: 10.1042/bj2530581. [DOI] [PMC free article] [PubMed] [Google Scholar]