Abstract
Can the experience of an emotion persist once the memory for what induced the emotion has been forgotten? We capitalized on a rare opportunity to study this question directly using a select group of patients with severe amnesia following circumscribed bilateral damage to the hippocampus. The amnesic patients underwent a sadness induction procedure (using affectively-laden film clips) to ascertain whether their experience of sadness would persist beyond their memory for the sadness-inducing films. The experiment showed that the patients continued to experience elevated levels of sadness well beyond the point in time at which they had lost factual memory for the film clips. A second experiment using a happiness induction procedure yielded similar results, suggesting that both positive and negative emotional experiences can persist independent of explicit memory for the inducing event. These findings provide direct evidence that a feeling of emotion can endure beyond the conscious recollection for the events that initially triggered the emotion.
Keywords: declarative memory, emotional memory, memory erasure, implicit memory, hippocampus
A large body of work has investigated the psychological and neurobiological mechanisms underlying the influence of emotion on memory (1–9). Yet, very little is known about the opposite relationship, namely, how memory impacts emotion.†One especially intriguing question is whether the sustained experience of emotion is dependent upon, versus independent of, intact declarative memory for the events that initially caused the emotion. Consider the following real-life examples: the death of a close friend or family member, the fall of the twin towers, the end of a romantic relationship—these are all events capable of eliciting an intense and prolonged state of emotion such as sadness. In these previous examples, the experience of sadness and the memory for the sadness-inducing event are often inseparable, fused together within our stream of consciousness as we ruminate, regret, and repeatedly replay the event (10, 11). The tight fusion between emotion and memory is well known to those suffering from affective disorders. For example, individuals with depression or posttraumatic stress disorder show a striking tendency to ruminate about the causes of their negative affect, which in turn escalates and prolongs their emotional pain and suffering (12–14). Thus, there are compelling reasons to predict that the persistence of an emotional experience, such as sadness, is highly dependent on remembering the emotion-inducing event.
However, what would happen to the feeling of an emotion if we could no longer remember the emotion-inducing event? Would the feeling fade away in parallel with the vanquished memory? Alternatively, is it possible that the feeling could persist without the memory? To answer these questions, an experiment needs to be devised that can disconnect the experience of an emotion from the memory for what caused the emotion. In healthy people with normal memory, a reliable disconnection of this sort is very difficult to establish given the intertwined relationship between an emotional experience and its cause.‡ To overcome this obstacle we investigated a rare group of five patients who have severe anterograde amnesia following circumscribed bilateral hippocampal brain damage (Fig. 1, Table 1, and Tables S1 and S2). A central feature of these patients’ condition is a profound impairment in forming new conscious memories about events that unfold in their daily lives. This severe memory deficit confers a unique opportunity to investigate whether the experience of an emotion can outlast the memory for what caused it.
Fig. 1.
Prototypical example of the severe bilateral hippocampal atrophy present in this cohort of amnesic patients. (Right) a coronal MRI image of patient Am4 and (Left) the homologous image from a healthy brain. The white arrows point to the region of the hippocampus.
Table 1.
Memory test results and volumetric MRI data
| Participant | No. factual details recalled | Verbal recognition (% correct) | Picture recognition (% correct) | IQ–MQ | HC volume | HC % loss | Amygdala volume | Amygdala % loss |
| Am1 (3344) | 0 | NA | 40%*** | NA | 50%*** | NA | 45 | NA | NA | NA | NA |
| Am2 (2144) | 3 | 0 | 80% | 100% | 70%*** | 70%*** | 43 | −3.92** | 44% | −0.22 | 4% |
| Am3 (1846) | 2 | 3 | 60%*** | 20%*** | 90% | 90% | 27 | −4.23** | 47% | −0.9 | 14% |
| Am4 (1606) | 5 | 5 | 60%*** | 80% | 80%*** | 50%*** | 25 | −3.99** | 41% | −1.18 | 17% |
| Am5 (2363) | 15 | 9 | 60%*** | 100% | 90% | 90% | 25 | −2.64* | 28% | −0.6 | 8% |
| Group Data | ||||||||
| Severe Amnesics | ||||||||
| avg. | 5 | 4 | 60% | 75% | 76% | 75% | 33 | −3.70 | 40% | −0.73 | 11% |
| range | 0–15 | 20–100 | 50–90 | 25–45 | −2.6– −4.2 | 28–47 | −0.2– −1.2 | 4–17 |
| Normal Comparisons | ||||||||
| avg. | 29 | 35 | 88% | 100% | 100% | NA | NA | NA | NA | NA |
| range | 18–45 | 60–100 | 100–100 | |||||
Memory test results for the sadness induction are presented in the left side of each cell and results for the happiness induction are presented in the right side of each cell. IQ − MQ = spread between general intelligence and memory (Full Scale IQ − General Memory Index); HC volume = SDs below the hippocampal volume of 87 control subjects; HC % loss = estimated % loss of hippocampal tissue; Amygdala volume = SDs below the amygdala volume of 87 control subjects; Amygdala % loss = estimated % loss of amygdala tissue. For more details on the volumetric MRI analyses see ref. 38. *P < 0.05, **P < 0.01: significantly different than expected based on age and sex; ***performance was not significantly above chance based on the binomial test (P < 0.05).
The dissociation between emotion and memory in patients with amnesia harkens back to 1911, when the Swiss neurologist Claparède concealed a pin between his fingers while greeting one of his amnesic patients with a handshake (18). The sharp pin surprised the patient and elicited a small amount of pain that quickly dissipated. Within minutes, the patient had forgotten the encounter. Yet, when Claparède tried to reintroduce himself shortly thereafter, the amnesic patient adamantly refused to shake his hand. When pressed to explain her reaction, the patient retorted, “Is there perhaps a pin hidden in your hand?” Claparède claims, however, that even with repeated questioning the patient could never explicitly remember that she, herself, had been stuck in the hand with a pin. Despite her impoverished memory for the devious handshake, Claparède's patient continued to demonstrate preserved avoidance learning. Similar forms of “nonconscious” emotional learning in amnesic patients have been shown using a variety of different tasks, including preserved conditioned responses during a fear conditioning paradigm (19), preserved learning of the advantageous strategy on a gambling task (20; but see refs. 21, 22), and preserved affective associations for different people using variations of a “Good Guy-Bad Guy” paradigm (23–25). Analogous results have also been obtained in patients with Alzheimer's disease (26–28) and even in rats with amnesia (29). A common theme in all of these previous experiments is the finding of preserved behavioral changes (as measured by avoidance responses, autonomic responses, or forced-choice preference judgments) in the face of impaired memory for the learning conditions. Moreover, the behavioral changes were only evident when the amnesic patients were re-exposed to the stimulus that was conditioned during the original learning trials. Beyond these behavioral changes, little is known about how the amnesic patients actually felt. Again, Claparède's patient provides a perfect example—it is unknown whether she still felt upset or nervous following the handshake even though she could not explicitly remember being pricked by a sharp pin. To the best of our knowledge, there have been no studies that have carefully and systematically tracked an amnesic patient's emotional experience in conjunction with their memory for the emotion-inducing event. Here, we report such a study, in which we examined whether the conscious experience of an emotion can persist once the emotion-inducing event is forgotten.
In the experiment, each amnesic patient (Am) underwent a sadness induction procedure, which entailed watching a series of emotional film clips portraying themes of loss and death (Table S3). The patients returned to the laboratory on a separate day for a happiness induction procedure using highly amusing and humorous film clips (Table S4). Both emotion induction procedures followed the same timeline (Fig. 2). A detailed memory test for the film clips (probing both free recall and recognition memory) was administered 5–10 min following the end of the film clips. Immediately after the memory test, each patient's current state of emotion (i.e., how they feel “right now, at the present moment”) was measured using an in-depth questionnaire (see Methods). For comparison, emotion was also measured immediately before the induction (baseline), immediately after the induction (approximately 0–5 min postinduction), and at the end of the experiment (approximately 20–30 min postinduction). This entire procedure was also administered to a group of normal comparison (NC) participants without brain damage, matched pairwise to the amnesic patients on sex, age, and education. The normal comparison participants provide a reference group by which to garner a general sense of how long the emotion inductions last in individuals with intact memory for the film clips. By systematically tracking each amnesic patient's emotional experience before and after a memory test, the experiment opens up the possibility of uncovering a dissociation between the conscious awareness of an emotional state and the conscious recollection of that state's origin (30).
Fig. 2.
Timeline of the experiment. Both inductions (sadness and happiness) followed the same timeline. The entire procedure lasted approximately 1 hour depending on the pace of the individual participant. The numbers shown represent the approximate time (in minutes) relative to the end of the film clips. T0, T1, T2, and T3 signify the four emotion measurements. The critical emotion measure for determining whether the emotional experience persisted beyond memory is the T2 emotion measure.
Results
Sadness Induction.
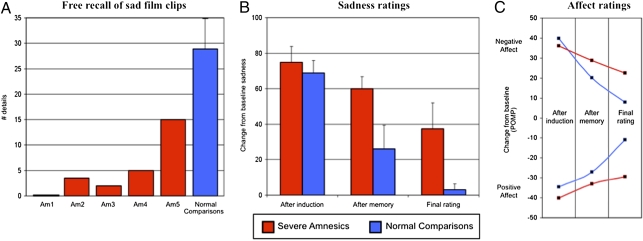
The sadness induction was highly effective at inducing the appropriate changes in emotion. Two raters (blind to all details about this study) viewed videotapes of the amnesic patients watching the film clips and found that they displayed changes in facial affect consistent with the experience of sadness (31), including crying, upturning of the inner eyebrows, downturning of the corners of the lips, and quivering of the chin or mouth. Immediately following the end of the film clips, all participants reported substantial increases in their level of sadness and negative affect, along with corresponding decreases in their level of happiness and positive affect (Figs. 3 B and C).
Fig. 3.
Recollection of film clips and postfilm emotion ratings following the sadness induction. (A) Total number of factual details recalled by each severe amnesic patient (Am1–Am5) along with the group mean for the normal comparison participants. (B) Average level of sadness reported by each group on a modified visual analog scale ranging from 0 (no emotion) to 100 (extreme emotion). Each score represents the change from baseline. All error bars represent the standard error of the mean. (C) Average negative and positive affect composite scores for each group. Each score represents the change from baseline using the percent of maximum possible (POMP).
The subsequent memory test revealed that the amnesic patients retained little or no factual memory for the sad film clips (Fig. 3A). Each amnesic patient's raw memory scores are presented in Table 1. Four of the amnesic patients recalled five or fewer details about the sad film clips, including one patient (Am1) who was unable to recollect anything about the films. Patient Am5 was the only amnesic who appeared to retain more than a minimal amount of information about the film clips. On the verbal recognition test, four of the patients failed to perform significantly above chance level, and on the picture recognition test, three of the patients failed to perform significantly above chance level, including one patient (Am1) who performed at chance level on both recognition tasks. By sharp contrast, the normal comparison participants had no difficulty remembering the film clips. They recalled many factual details about the films, and, on average, nearly six times as many details as the amnesic patients. One-tailed dependent t tests were computed between the two groups and a Bonferroni-corrected threshold was set at 0.0167. Significant differences were found for free recall [t (4) = 5.3, P = 0.0031, r = 0.94] and picture recognition [t(4) = 3.2, P = 0.0163, r = 0.85]. Verbal recognition was slightly above the corrected significance threshold [t(4) = 2.8, P = 0.0258, r = 0.81]. All of these contrasts had large effect sizes, as indicated by the Pearson r values.
Because the memory test revealed, as predicted, that the amnesic patients’ recollection of the sad films was impaired, the critical question then becomes whether the patients’ sadness would be comparably suspended or would persist beyond the time of the memory test. The results clearly show that the amnesic patients remained sad even though they had little to no memory for the film clips. In fact, when compared with the normal comparison participants (whose memory for the film clips was intact), the patients’ sadness and affect change persisted at a higher level of intensity and for a longer period of time (Figs. 3 B and C). Moreover, this effect was robust, occurring in all of the amnesic patients, especially patients Am1 and Am2 (who had among the most impaired memory scores). Due to the statistical limitations inherent when working with small sample sizes combined with the high amount of variability in each participant's emotion ratings, we decided to present each participant's individual emotion ratings in Fig. 4. Notably, every single amnesic patient reported experiencing increased levels of sadness that persisted even in the context of severely impaired declarative memory for the sad film clips.
Fig. 4.
Each participant's emotion ratings following the sadness induction for (A) level of sadness (VAS) and (B) total affect composite score. Each severe amnesic patient (Am1–Am5) is graphed alongside their matched normal comparison participant (NC1–NC5). Each score represents the change from baseline in POMP units. T1 is after induction, T2 is after memory, and T3 is the final rating.
Happiness Induction.
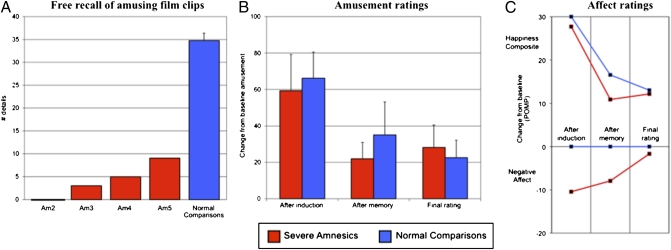
Four of the amnesic patients underwent a happiness induction using highly amusing film clips. The happiness induction was effective at inducing the appropriate changes in emotion. Two raters (blind to all details about this study) viewed videotapes of the amnesic patients watching the amusing film clips and found that they displayed changes in facial affect consistent with the experience of happiness (31), including broad open-mouthed smiles, raising of the cheeks, wrinkles around the eyes, and intense laughter. Immediately following the end of the film clips, all participants reported increases in their level of happiness and amusement (Figs. 5 B and C).
Fig. 5.
Recollection of film clips and postfilm emotion ratings following the happiness induction. (A) Total number of factual details recalled by each severe amnesic patient (Am2-Am5) along with the group mean for the normal comparison participants. (B) Average level of amusement reported by each group on a modified visual analog scale ranging from 0 (no emotion) to 100 (extreme emotion). Each score represents the change from baseline. All error bars represent the standard error of the mean. (C) Average happiness and negative affect composite scores for each group. Each score represents the change from baseline using the percent of maximum possible (POMP).
The subsequent memory test revealed that the amnesic patients retained little or no factual memory for the amusing film clips (Fig. 5A). Each amnesic patient's raw memory scores are presented in Table 1. Three of the amnesic patients recalled five or fewer details about the amusing film clips, including one patient (Am2) who was unable to recollect anything about the films. Patient Am5 was the only amnesic who appeared to retain more than a minimal amount of information about the film clips (incidentally, he also showed the smallest increase in happiness postinduction). On the verbal recognition test, patient Am3 performed at chance level, and on the picture recognition test, two of the patients failed to perform significantly above chance level, including one patient (Am4) who performed at chance level. By sharp contrast, all normal comparison participants scored at ceiling (i.e., 100% correct) on both recognition tests. They also recalled many factual details about the films, and on average, over eight times as many details as the amnesic patients. One-tailed dependent t tests were computed between the two groups and a Bonferroni-corrected threshold was set at 0.0167. A significant between-group difference was found on the free recall test [t(3) = 14.5, P = 0.0004, r = 0.99]. Verbal recognition [t(3) = 1.3, P = 0.1392, r = 0.60] and picture recognition [t(3) = 2.6, P = 0.0398, r = 0.83] were above the corrected significance threshold and were likely underpowered given the small size of the sample. Nevertheless, all of the contrasts had large effect sizes, as indicated by the Pearson r values.
Because the memory test revealed that the amnesic patient's recollection of the amusing films was impaired, the critical question then becomes whether the patient's experience of happiness would be comparably suspended or would persist beyond the time of the memory test. The results demonstrate that the amnesic patients continued to experience elevated levels of happiness and amusement beyond the memory test. When compared with the normal comparison participants (whose memory for the film clips was intact), the patients’ happiness and amusement had a similar level of intensity and followed a similar time course and trajectory (Figs. 5 B and C). Each participant's individual emotion ratings are presented in Fig. S1 (as before, formal statistical tests are not practical here because of the small sample size and large variability in emotion ratings). Notably, all amnesic patients reported experiencing elevated levels of happiness and amusement that persisted despite severely impaired memory for the amusing film clips.
Discussion
The results of this investigation reveal a striking dissociation between the sustained experience of emotion in the face of impaired declarative memory for that emotion's origin. Moreover, the dissociation was found for both happiness and sadness, supporting the conclusion that feelings of different valence can persist independent of explicit memory for the inducing event. It is important to emphasize that it would be difficult (if not impossible) to obtain a dissociation of this magnitude without the benefit of studying patients who have severe amnesia. For healthy individuals with normal memory, such a dissociation might even seem implausible, especially when considering situations that elicit intense states of emotion (such as traumatic events, the death of a loved one, being diagnosed with cancer, going through a divorce, the birth of a child, or winning a lottery). It is quite remarkable to imagine that someone can completely forget about the triggering event and still experience an intense state of emotion. Yet, the results of this study reveal that amnesic patients with damage circumscribed to their hippocampus are capable of experiencing emotions well after their memories for the emotion-inducing events have faded away. Patient Am1 provides a salient example of this phenomenon. While watching the sad film clips, she exhibited extreme facial displays of sadness, including tearful crying that lasted for several minutes. Shortly after the induction was over, Am1 was unable to recall even a single detail about the preceding film clips and her recognition performance was at chance level. Yet, her sadness lingered for over 30 minutes, lasting much longer than any other participant, including normal comparison participants with fully intact memory for the film clips. Thus, patient Am1 demonstrates a complete dissociation between emotion and memory and, even beyond that, a sustained feeling of sadness that persisted at an abnormally high level.
An intriguing question that arises from this work concerns the specific signal(s) that amnesic patients are using to determine their emotional state. There are many possibilities, ranging from emotional signals arising from the body, to nonverbal emotional images resonating in the mind, all of the way to emotion-congruent thoughts. Another possibility is that patients are using whatever remaining knowledge they have regarding the film clips to guide their emotion ratings. This latter possibility might be plausible for some patients, such as patient Am5, who had somewhat higher levels of recall and recognition (albeit still well below normal). For other patients, however, it appears much less likely that any residual memory for the film clips is influencing their emotional state. The most compelling example is patient Am1, who appeared to have essentially no memory trace for the film clips to rely on when filling out the postmemory emotion measures. Other patients had slightly more memory. For example, patient Am3 remembered seeing a movie with “Meryl Streep”. Her normal comparison, on the other hand, remembered seeing the specific scene in Sophie's Choice where Meryl Streep's daughter is ripped away from her by a Nazi soldier and sent to die. Despite the profound differences between the recollections of these two individuals, both reported experiencing a similar magnitude of sadness. This argues against the likelihood that declarative memory is playing a prominent role in guiding the amnesic patients’ subjective assessment of how they feel. It will be important for future work to try and decipher the precise mechanism by which amnesic patients are determining their emotional state.
Several of the amnesic patients, despite their impoverished memory for the film clips, showed a slow decay of sadness, sometimes even slower than the normal comparison participants. In fact, some of the patients were still experiencing high levels of sadness and negative affect during a time when all such emotion had dissipated in the comparisons. Such a finding was most evident in patients Am1 and Am2 (Fig. 4). After the experiment was over, Am2 provided some insightful comments upon debriefing. She claimed that in everyday life, she frequently experiences different emotions and has no idea of what caused them. Without a clear understanding of the emotion's source, Am2 reports feeling compelled to search for a cause. Interestingly, she claims to only feel this urge while experiencing negative emotions, not positive ones. In her words, “It's not so much with the happy or the good feelings. You just kind of accept them. You don't worry about why. It's more for what I would call negative feelings… like when I'm feeling really sad, then I have to find out why. [Experimenter: “And do these sorts of feelings stay even though you don't know why?”] Yeah… and they don't go away.” Thus, one potential explanation for the sluggish decay of sadness evident in some of the amnesic patients is that without a clear understanding of why they are feeling sad, the emotion persists (32). On the other hand, the amnesics’ experience of happiness appears to decay at a relatively normal rate (Figs. 5 B and C). Altogether, these findings suggest that remembering the origin of an emotional state may help expedite the recovery of negative emotions while having less of an effect on the recovery of positive emotions, an asymmetry that may have some adaptive value.
The results of this study have direct implications for how society treats individuals with memory disorders (such as patients with Alzheimer's disease), as events that have long been forgotten could continue to induce suffering or well-being. For example, a simple visit or telephone call from family members might have a lingering positive influence on a patient's affective state even though the patient may quickly forget the visit or phone call. Likewise, a funny joke might boost a patient's level of happiness long after the punch line is forgotten. On the other hand, routine neglect from staff at nursing homes may leave the patient feeling sad, frustrated, and lonely (even though the patient can't remember why). As the number of individuals suffering from Alzheimer's disease and other forms of dementia reaches epidemic proportions, it will be imperative for society to follow a scientifically-informed standard of care for patients with memory impairments. Here we provide clear evidence showing that the reasons for treating amnesic patients with respect and dignity go beyond simple human morals.
Finally, the findings in our amnesic patients could be taken to suggest that the brain is organized in such a way that the feeling of emotion can persist without any explicit memory for its cause (33–35). Such a conclusion runs counter to the popular notion that by simply erasing our painful memories we can also erase the psychological suffering; a concept vividly illustrated in the movie Eternal Sunshine of a Spotless Mind. As scientific advances take us ever closer to the possibility of selectively removing painful memories, we must tread cautiously. To be certain, there are a host of contentious legal and ethical issues surrounding the debate on selective memory erasure, especially with regard to treating victims of trauma (36). The results of our study further complicate the debate by highlighting the possibility that erasing negative memories may have the paradoxical effect of actually prolonging (rather than alleviating) feelings of distress. Indeed, it appears that the experience of emotion can operate largely independently from memory, even though the two processes are often perceived as being fused together within our stream of consciousness.
Methods
Participants.
All participants gave their informed written consent before participating in the study. The University of Iowa Institutional Review Board approved all study procedures. Each amnesic patient was matched pairwise with a healthy, nonbrain-damaged comparison participant based on sex, age, and education. The average age of the severe amnesic patients was 46.8 years (range 25–59) and the average education was 13.2 years (range 12–16). The average age of the normal comparisons was 47.8 years (range 27–60) and the average education was 13.2 years (range 12–16).
Amnesic participants were selected from the Iowa Patient Registry in the Division of Behavioral Neurology and Cognitive Neuroscience at the University of Iowa. On average, the severe amnesic patients had a 33-point spread between general intelligence and memory (range 25–45), a 40% reduction in hippocampal volume (range 28–47%), and no significant reduction in amygdala volume (Table 1). Reductions in hippocampal volume in the range of 40% typically signify a complete loss of hippocampal neurons (37). All volumetric comparisons were made to a normative dataset of 87 healthy participants, controlling for both age and sex. Further details on the volumetric MRI analyses in these patients have been previously published (38).
Emotion Induction Procedure.
Each emotion induction entailed watching a series of eight film clips aimed at inducing either sadness (Table S3) or happiness (Table S4). The sad films all portrayed themes of loss and death, whereas the happy films were all aimed at being highly amusing and humorous. All participants first completed the sadness induction, and then returned to the laboratory at a later date to complete the happiness induction. Before viewing the film clips, participants were blinded to the specific valence of the films they would be watching. Many of the film clips were chosen from sets of previously validated films shown to be highly effective at inducing emotion across a large sample of healthy participants (39–42). We purposefully selected films that have a short duration (average clip duration approximately 2 min) to mitigate any problems arising from the amnesic patients forgetting what is happening during each scene. During each induction, participants viewed the film clips, one at a time, with a brief rest period (10–20 seconds) between each film. In total, the sadness induction lasted approximately 19 min and the happiness induction lasted approximately 17 min. A copy of the sadness and happiness inductions can be obtained for research purposes by contacting the first author (J.S.F.).
Memory Test.
A detailed memory test for the film clips was administered 5–10 min following the end of each induction. On average, the memory testing commenced approximately 6 min after the end of the last film clip. The memory test was comprised of three different sections: free recall, verbal recognition, and picture recognition (SI Text).
Emotion Measurement.
Each participant's “state” emotional experience was measured at four different time points (Fig. 2). At each time point, participants are asked to rate how they feel “right now, at the present moment” on a number of different scales and questionnaires. There were three modified visual analog scales (VAS), on which participants rated their current level of sadness, happiness, and amusement using a 100-point scale going from “no emotion” to “extreme emotion.” There was an 11-point bipolar valence scale where participants rated how they currently feel from “bad/unpleasant” to “good/pleasant.” Participants also completed the Positive and Negative Affect Schedule (PANAS) using the “at the present moment” instructions (43). The PANAS consists of two 10-item scales used to extract measures of positive affect and negative affect. Eleven additional items were added from the PANAS-X (44) to compute the specific affect scales of joviality and sadness. All PANAS items were rated on a five-point Likert-type scale.
At each measurement time point, eight summary scores were computed: sadness (VAS), happiness (VAS), amusement (VAS), valence, negative affect, positive affect, joviality, and sadness (PANAS-X). Each of these summary scores was converted into a percent of maximum possible (POMP) score (standardized units representing the “percent of maximum possible” for each scale, ranging from 0–100%) (45). To assess for changes in emotional experience induced by the film clips, POMP difference scores were computed by comparing all postinduction ratings with baseline ratings, for each participant. Composite scores representing global changes in negative affect, positive affect, total affect, and happiness were calculated based on each participant's POMP difference scores (SI Text).
Supplementary Material
Acknowledgments
We thank Brooke Feinstein and Natalie Denburg for technical assistance and Antonio Damasio and David Watson for valuable comments. This research was supported by grants from the National Institutes of Health (National Institute of Neurological Disorders and Stroke P50 NS19632, National Institute on Drug Abuse R01 DA022549), a National Science Foundation Graduate Research Fellowship, and awards from the Kiwanis Foundation and the Fraternal Order of Eagles.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914054107/DCSupplemental.
†Emotion is typically parsed into two components: emotional expression and emotional experience. The expression of emotion includes a myriad of responses (physiological, behavioral, and cognitive) that are induced by a triggering stimulus. The experience of emotion represents the conscious subjective “feeling” state associated with these responses. Throughout this paper, references to emotion mainly refer to this latter component (i.e., the feeling of emotion).
‡We may, on occasion, have an ephemeral whisper of such a disconnection, for example, when we feel a burst of emotion and, for a brief second, do not remember the cause. Our declarative memory, however, rapidly supplies knowledge of the trigger, and we are back to the state of feeling the emotion and knowing what caused it. In fact, most definitions of emotion explicitly assume that emotional experiences are intentional states triggered by an identifiable source or object (15–17). Moods, on the other hand, do not necessarily have a clear cause and can persist independent of our awareness for their source (16, 17). The present study specifically focuses on emotions that are triggered by a clearly identifiable source.
References
- 1.Cahill L, Prins B, Weber M, McGaugh JL. β-Adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- 2.Hamann S. Cognitive and neural mechanisms of emotional memory. Trends Cogn Sci. 2001;5:394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- 3.LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan TW. Retrieval of emotional memories. Psychol Bull. 2007;133:761–779. doi: 10.1037/0033-2909.133.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bower GH. Mood and memory. Am Psychol. 1981;36:129–148. doi: 10.1037//0003-066x.36.2.129. [DOI] [PubMed] [Google Scholar]
- 6.Kensinger EA, Corkin S. Two routes to emotional memory: Distinct neural processes for valence and arousal. Proc Natl Acad Sci USA. 2004;101:3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phelps EA. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Dolcos F, LaBar KS, Cabeza R. Remembering one year later: Role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci USA. 2005;102:2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proc Natl Acad Sci USA. 2002;99:4115–4120. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JM, Alloy LB. A roadmap to rumination: A review of the definition, assessment, and conceptualization of this multifaceted construct. Clin Psychol Rev. 2009;29:116–128. doi: 10.1016/j.cpr.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomsen DK. The association between rumination and negative affect: A review. Cogn Emotion. 2006;20:1216–1235. [Google Scholar]
- 12.Ehlers A, Mayou RA, Bryant B. Psychological predictors of chronic posttraumatic stress disorder after motor vehicle accidents. J Abnorm Psychol. 1998;107:508–519. doi: 10.1037//0021-843x.107.3.508. [DOI] [PubMed] [Google Scholar]
- 13.Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. J Abnorm Psychol. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 15.Reisenzein R, Doring SA. Ten perspectives on emotional experience: Introduction to the special issue. Emotion Review. 2009;1:195–205. [Google Scholar]
- 16.Ekman P, Davidson RJ. The Nature of Emotion: Fundamental Questions. New York: Oxford University Press; 1994. [Google Scholar]
- 17.Neumann R, Seibt B, Strack F. The influence of mood on the intensity of emotional responses: Disentangling feeling and knowing. Cogn Emotion. 2001;15:725–747. [Google Scholar]
- 18.Claparède E. Recognition et moïté. Arch Psychol. 1911;11:79–90. [Google Scholar]
- 19.Bechara A, et al. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- 20.Turnbull OH, Evans CEY. Preserved complex emotion-based learning in amnesia. Neuropsychologia. 2006;44:300–306. doi: 10.1016/j.neuropsychologia.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Gupta R, et al. Declarative memory is critical for sustained advantageous complex decision-making. Neuropsychologia. 2009;47:1686–1693. doi: 10.1016/j.neuropsychologia.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutbrod K, et al. Decision-making in amnesia: Do advantageous decisions require conscious knowledge of previous behavioural choices? Neuropsychologia. 2006;44:1315–1324. doi: 10.1016/j.neuropsychologia.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Johnson MK, Kim JK, Risse G. Do alcoholic Korsakoff's syndrome patients acquire affective reactions? J Exp Psychol Learn Mem Cogn. 1985;11:22–36. doi: 10.1037//0278-7393.11.1.22. [DOI] [PubMed] [Google Scholar]
- 24.Todorov A, Olson IR. Robust learning of affective trait associations with faces when the hippocampus is damaged, but not when the amygdala and temporal pole are damaged. Soc Cogn Affect Neurosci. 2008;3:195–203. doi: 10.1093/scan/nsn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tranel D, Damasio AR. The covert learning of affective valence does not require structures in hippocampal system or amygdala. J Cogn Neurosci. 1993;5:79–88. doi: 10.1162/jocn.1993.5.1.79. [DOI] [PubMed] [Google Scholar]
- 26.Blessing A, Keil A, Linden DEJ, Heim S, Ray WJ. Acquisition of affective dispositions in dementia patients. Neuropsychologia. 2006;44:2366–2373. doi: 10.1016/j.neuropsychologia.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Evans-Roberts CEY. Turnbull OH Remembering relationships: Preserved emotion-based learning in Alzheimer's disease. Exp Aging Res. doi: 10.1080/0361073X.2011.536750. in press. [DOI] [PubMed] [Google Scholar]
- 28.Quoniam N, et al. Implicit and explicit emotional memory for melodies in Alzheimer's disease and depression. Ann N Y Acad Sci. 2003;999:381–384. doi: 10.1196/annals.1284.047. [DOI] [PubMed] [Google Scholar]
- 29.Hine B, Paolino RM. Retrograde amnesia: Production of skeletal but not cardiac response gradient by electroconvulsive shock. Science. 1970;169:1224–1226. doi: 10.1126/science.169.3951.1224. [DOI] [PubMed] [Google Scholar]
- 30.Kihlstrom JF, Mulvaney S, Tobias BA, Tobis IP. The emotional unconscious. In: Eich E, et al., editors. Cognition and Emotion. New York: Oxford University Press; 2000. pp. 30–86. [Google Scholar]
- 31.Ekman P, Friesen WV. Unmasking the Face. Cambridge, MA: Malor Books; 2003. [Google Scholar]
- 32.Maslach C. Negative emotional biasing of unexplained arousal. J Pers Soc Psychol. 1979;37:953–969. [Google Scholar]
- 33.Chartrand TL, van Baaren RB, Bargh JA. Linking automatic evaluation to mood and information processing style: Consequences for experienced affect, impression formation, and stereotyping. J Exp Psychol Gen. 2006;135:70–77. doi: 10.1037/0096-3445.135.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruys KI, Stapel DA. The secret life of emotions. Psychol Sci. 2008;19:385–391. doi: 10.1111/j.1467-9280.2008.02097.x. [DOI] [PubMed] [Google Scholar]
- 35.Winkielman P, Berridge KC. Unconscious emotion. Curr Dir Psychol Sci. 2004;13:120–123. [Google Scholar]
- 36.Kolber AJ. Therapeutic forgetting: The legal and ethical implications of memory dampening. Vanderbilt Law Rev. 2006;59:1561–1626. [Google Scholar]
- 37.Gold JJ, Squire LR. Quantifying medial temporal lobe damage in memory-impaired patients. Hippocampus. 2005;15:79–85. doi: 10.1002/hipo.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen JS, Tranel D, Bruss J, Damasio H. Correlations between regional brain volumes and memory performance in anoxia. J Clin Exp Neuropsychol. 2006;28:457–476. doi: 10.1080/13803390590949287. [DOI] [PubMed] [Google Scholar]
- 39.Rottenberg J, Ray R, Gross JJ. Emotion elicitation using films. In: Coan JA, Allen JJ, editors. Handbook of Emotion Elicitation and Assessment. New York, NY: Oxford University Press; 2007. pp. 9–28. [Google Scholar]
- 40.Gross JJ, Levenson RW. Emotion elicitation using films. Cogn Emotion. 1995;9:87–108. [Google Scholar]
- 41.Philippot P. Inducing and assessing differentiated emotion-feeling states in the laboratory. Cogn Emotion. 1993;7:171–193. doi: 10.1080/02699939308409183. [DOI] [PubMed] [Google Scholar]
- 42.Schaefer A, Nils F, Sanchez X, Philippot P. Assessing the effectiveness of a large database of emotion-eliciting films: A new tool for emotion researchers. Cogn Emotion. in press. [Google Scholar]
- 43.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 44.Watson D, Clark LA,, University of Iowa (1994) The PANAS-X: Manual for the positive and negative affect schedule-expanded form. Available at www.psychology.uiowa.edu/Faculty/Watson/PANAS-X.pdf. Accessed November 19, 2009. [Google Scholar]
- 45.Cohen P, Cohen J, Aiken LS, West SG. The problem of units and the circumstance for POMP. Multivariate Behav Res. 1999;34:315–346. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.