Abstract
Neurogenesis in the hippocampus is characterized by the birth of thousand of cells that generate neurons throughout life. The fate of these adult newborn neurons depends on life experiences. In particular, spatial learning promotes the survival and death of new neurons. Whether learning influences the development of the dendritic tree of the surviving neurons (a key parameter for synaptic integration and signal processing) is unknown. Here we show that learning accelerates the maturation of their dendritic trees and their integration into the hippocampal network. We demonstrate that these learning effects on dendritic arbors are homeostatically regulated, persist for several months, and are specific to neurons born during adulthood. Finally, we show that this dendritic shaping depends on the cognitive demand and relies on the activation of NMDA receptors. In the search for the structural changes underlying long-term memory, these findings lead to the conclusion that shaping neo-networks is important in forming spatial memories.
Keywords: adult neurogenesis, memory, spatial learning, hippocampus, dendrite
In the search for the mechanisms underlying long-term memory formation, structural changes have been proposed to play a major role (1). Adult neurogenesis, a novel form of structural plasticity, occurs in the dentate gyrus (DG), a key structure in processing spatial relational memory (2). Adult neurogenesis in the DG is a complex, multistep process that starts with the proliferation of neural precursors residing in the dentate subgranular layer (2). At least 50% of the daughter cells die within a few days after their birth. The adult-born cells that survive this initial period of cell death differentiate mainly into granule neurons. These new mature neurons are synaptically integrated into the dentate network, where they receive functional inputs (3) and form functional synapses with their target cells (4).
Adult-born neurons contribute to the formation of memories, particularly spatial memory as measured in the water maze (5, 6). Reciprocally, spatial learning has been shown to influence adult neurogenesis (7). We have shown that during spatial learning and similar to the selective stabilization process observed during brain development, neuronal networks are sculpted by a tightly regulated selection and suppression of different populations of newly born neurons (8). This homeostatic regulation of the numbers of newly born neurons is important for spatial memory, because its alteration leads to memory deficits (8, 9).
One of the requirements for the new neurons to process information is the development of extensive dendritic arbors capable of receiving and integrating complex spatiotemporal patterns of synaptic inputs (4). Whether learning influences such a dendritic development is unknown. To address this issue, we used doublecortin (Dcx) and retroviral labeling to examine to what extent spatial learning in the water maze affects the dendritic morphology of adult-born neurons. Furthermore, we investigated whether the level of cognitive demand influences the development of new neurons and the mechanisms underlying these learning-induced changes in adult neurogenesis. We found that spatial learning sculpts newborn neurons not only by regulating their number, but also by shaping their dendritic arbor. Spatial learning increases the complexity of the dendritic arbor, an effect depending on the cognitive demand of the task and NMDA receptors.
Results
Spatial Learning Increases the Complexity of the Dendritic Arbor of Adult Newborn Neurons.
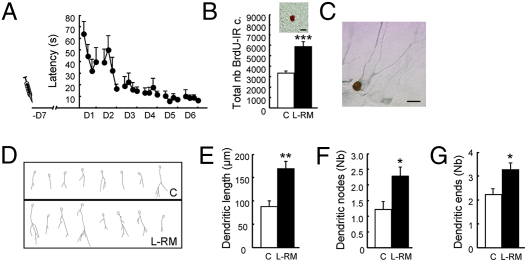
We and others have previously shown that spatial learning increases the survival of newborn neurons that were produced 1 week before the start of the training (7, 8). We thus analyzed the effects of learning on the development of their dendritic arbor, a key element in receiving and processing information. To do so, we injected BrdU 1 week before training and analyzed the expression of Dcx in the newly born neurons labeled with BrdU. Dcx is a cytoplasmic protein associated with neuronal differentiation and neurite elongation, which is exclusively expressed in immature neurons from 1 day to 4 weeks of age (10), thereby permitting the analysis of the dendritic arbor of the newly born cells. We used a classic water maze protocol of reference memory [learning reference memory (L-RM)]. Animals were trained for 6 days and killed on the next day (Fig. 1A and Table S1, experiment 1a). In this experiment and subsequent experiments, only control animals consisting of home-caged rats were included, because we have previously shown that the rate of adult neurogenesis (cell survival, cell proliferation, and cell death) in these animals does not differ from that measured in animals with a similar swimming experience or animals trained in the visible platform protocol (8, 9, 11). We first verified that spatial learning increased cell survival by analyzing the number of BrdU-immunoreactive (IR) cells in the DG. As expected, it was greater in the L-RM group compared with controls (Fig. 1B). A more detailed analysis indicated that learning increased BrdU cell numbers throughout the septotemporal axis of the DG (Fig. S1A). Because the morphological analysis of BrdU/Dcx-IR cells was performed on four septal sections (P: 2.8–4.16 mm from the bregma), we verified that within these sections, the number of BrdU-IR cells was higher in the L-RM group compared with controls (Fig. S1B). Morphological analysis of BrdU-Dcx-IR neurons (Fig. 1C) showed that spatial learning shapes their dendritic arbor (Fig. 1D). A quantitative analysis found greater dendritic lengths, numbers of dendritic nodes, and numbers of dendritic ends were higher in L-RM animals compared with controls (Fig. 1 E–G). Conversely, the size of the cell bodies remained unchanged (controls,: 55.59 ± 2.9 μm2; L-RM, 52.50 ± 7.9 μm2; t15 = 0.9; P = 0.3). By performing the concentric circle analysis of Sholl (12), we further examined dendritic morphological features, particularly the extent of dendritic growth away from the soma and the branching of dendrites at various distances from the soma. This analysis revealed that the dendritic length was greater in the L-RM group compared with controls, an effect due to an increased number of dendritic branches extending beyond 50–150 μm (Fig. S2A). A similar result was obtained for the number of dendrites that crossed the various radial distances from the soma (Fig. S2B).
Fig. 1.
Spatial learning increases the survival and the dendritic arbor complexity of adult-born neurons generated 1 week before learning. (A) Latency to find the escape platform. The syringe represents BrdU injection. (B) Total number of BrdU-IR cells (t15 = −3.85; P < 0.001). (Inset) Illustration of a BrdU-IR cell. (C) Illustration of a BrdU-IR cell expressing Dcx. (D) Examples of neuron tracings for one animal of each group. (E) Length of the dendritic arbor (t15 = −3.44; P < 0.01). (F) Number of nodes (t15 = −2.37; P < 0.05). (G) Number of endings (t15 = −2.35; P < 0.05). C, n = 6; L-RM, n = 11. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 compared with control. (Scale bar: 10 μm.)
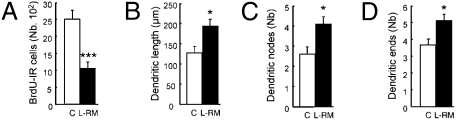
We next performed the same analysis on neurons born during the early phase of training (Table S1, experiment 1b). We had previously shown that spatial learning promotes the death of some of the immature neurons produced during either the early phase of training or 3 days before training (8). Thus, the spared adult-born neurons are selected by learning. We asked whether learning influences the development of the surviving new neurons that are younger than those examined in the previous experiment. We first analyzed the number of BrdU-IR cells on four sections and found a reduction in the number of newly born cells (Fig. 2A). We then analyzed the dendritic arbor of BrdU-Dcx-IR neurons. The results showed that the dendritic arbor was more developed in the group of animals that underwent training (Fig. 2 B–D). Indeed, the dendritic lengths, as well as the numbers of dendritic nodes and ends, were increased in L-RM animals compared with controls. Sholl analysis revealed greater dendritic lengths and numbers of crossing dendrites were higher in the L-RM group compared with controls, an effect more pronounced for the dendritic branches extending beyond 75–125 μm from the soma (Fig. S2 C and D).
Fig. 2.
Spatial learning increases the dendritic arbor complexity of adult-born neurons generated during the early phase of training. (A) Number of BrdU-IR cells in the septal region of the DG (t11 = 4.66; P < 0.001). (B) Length of the dendritic arbor (t11 = −2.77; P < 0.05). (C) Number of nodes (t11 = −2.84; P < 0.05). (D) Number of endings (t11 = −2.79; P < 0.05). C, n = 6; L-RM, n = 7. *P ≤ 0.05; ***P ≤ 0.001 compared with control.
Taken together, the results from these two experiments show that newborn neurons selected by spatial learning exhibit a more complex dendritic arbor than “naïve” newborn neurons.
Dendritic Arbor Is Regulated in a Homeostatic Manner.
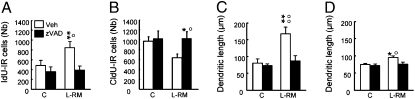
We recently showed that learning promotes the survival of relatively mature neurons at the expense of more immature cells that are removed from the dentate network, demonstrating a homeostatic regulation of the number of new neurons by learning (8). Indeed, in this study we used the pan-caspase inhibitor z-Val-Ala-Asp fluoromethylketone (zVAD) to block apoptosis. zVAD was infused during training. We have shown that the infusions block apoptosis of the youngest neurons (generated 3 days before training), as well as survival of the oldest new neurons (generated 7 days before training), and impair spatial learning (8). We hypothesized that these two neuronal populations are in competition for available space, and that the cells rescued are those that are successfully connected. For this reason, we investigated whether the dendritic arbor developments of different populations of adult-born neurons are interrelated events and thus subject to a homeostatic regulation. Animals were injected 7 days and 3 days before training with BrdU analogues IdU and CldU, respectively, and zVAD was injected at the end of the fourth through sixth days of training (see ref. 8) (Table S1, experiment 2). We first verified on four septal sections the blockage of a learning-induced increase in IdU-IR cells and a learning-induced decrease in CldU-IR cells in zVAD-infused animals (Fig. 3 A and B). We then analyzed the dendritic morphology of IdU-Dcx-IR cells and CldU-Dcx IR cells. Although the zVAD infusion did not influence development of the dendritic arbor of IdU-Dcx-IR cells or CldU-Dcx IR cells (Fig. S3 A and B), this treatment hindered learning-induced increases in dendritic lengths (Fig. 3 C and D) and the numbers of dendritic nodes and dendritic terminals (Fig. S3 C–F). Thus, the neurons that were present were less developed compared with those of vehicle animals subjected to training. Altogether, these results demonstrate that the dendritic arbor modifications of different populations of new neurons in response to learning are interrelated events.
Fig. 3.
Homeostatic regulation of the dendritic arbor of adult-born neurons by spatial learning. (A and B) Effects of zVAD and vehicle treatments on the number of IdU-IR cells (in the septal region of the DG) generated 7 days before exposure to the task (A) and the number of CldU-IR cells generated 3 days before exposure to the task (B). (C and D) Effects of zVAD and vehicle treatments on the length of dendrites of IdU-Dcx-IR cells (F3,25 = 8.2; P < 0.001) (E) and CldU-Dcx-IR cells (F3,25 = 5.18; P < 0.01) (F). C: Veh, n = 6, zVAD, n = 6; L-RM: Veh, n = 10, zVAD, n = 7. Veh, □; zVAD, ■. *P ≤ 0.05; **P ≤ 0.01 compared with control veh. °P ≤ 0.05; °°P ≤ 0.01 compared with zVad group.
Learning-Induced Changes in the Dendritic Arbor Are Long-Lasting.
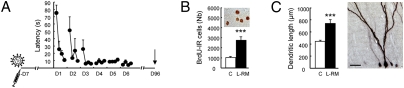
We next wished to determine whether learning-induced changes in the dendritic arbor persisted over a long period or was subjected to a selective regressive process as described during development (13). Because Dcx is expressed only transiently in newborn neurons (10), we labeled the newborn neurons by infecting the DG region with a retrovirus produced by the vector CAG-GFP (14). BrdU was injected on the same day. Four groups of animals were trained 7 days later in the L-RM and killed 1 day or 1, 2, or 3 months after training (Table S1, experiment 3). We first verified that the learning increase in BrdU-IR cell numbers was maintained over time. Then the morphological analysis of GFP-labeled neurons of animals revealed that the L-RM group had longer dendrites (Fig. 4 and Fig. S4) with more nodes and more ends (Table S2), indicating that the learning effect on dendrite morphological changes is maintained over a long period.
Fig. 4.
The effect of spatial learning on the dendritic arbor is long-lasting. (A) Latency to find the escape platform. The symbol represents retrovirus infusions; the syringe, BrdU injection; and the arrows, the time of sacrifice (3 months after training). (B) Total number of BrdU-IR cells. (C) Length of dendrites and 3-month-old adult-born neurons labeled with a GFP retrovirus. C, n = 7; L-RM, n = 8. ***P ≤ 0.001 compared with control.
Learning Effect on Dendritic Spines on Neurons Born During Adulthood.
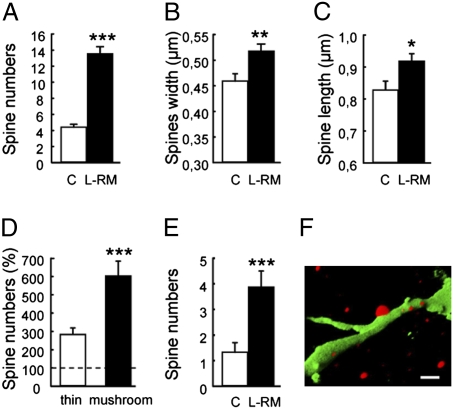
In the next step, we refined our analysis by determining whether learning affected the formation of dendritic spines that indirectly reflect the presence of inputs. We analyzed the spine density and shape of new neurons in naïve rats and rats trained in RM and killed 3 months after training. The morphological analysis of GFP-labeled neurons revealed that adult-born neurons of L-RM animals had more spines compared with controls (Fig. 5A). We analyzed the shape of each spine and found that L-RM animals had wider and longer spines compared with controls (Fig. 5 B and C). Spines were then divided into two categories, thin and mushrooms, based on head and neck size. The L-RM group had more thin spines and mushroom spines compared with controls (Fig. 5D). More interestingly, there were 3-fold more mushroom spines than thin spines, suggesting accelerated spine maturation. We confirmed this by examining the dendritic spine density of new neurons in naïve rats and rats trained in the RM and killed 1 week after training. At this developmental stage, spines were more numerous in adult-born neurons of L-RM animals compared with controls and already were receiving some dendritic input, as revealed by synaptophysin (Fig. 5 E and F).
Fig. 5.
Spatial learning increases the number of dendritic spines of adult-born neurons. (A) Number of spines (per 40 μm) (t103 = 9.25; P < 0.0001). (B) Spine width (t103 = 2.94; P < 0.01). (C) Spine length (t103 = 2.47; P < 0.01). (D) Number of thin spines and mushroom spines (% compared with control) (thin spines, t8 = -5.06, P < 0.001; mushroom spines, t8 = −5.75, P < 0.001). (E) Number of spines (per 40 μm) in neurons generated 1 week before the task (t15 = −3.53, P < 0.01). (F) Three-dimensional reconstruction of a confocal photomicrograph of a GFP-IR dendrite (green) contacted by synaptophysin (red) fibers. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 compared with control.
Learning-Induced Changes on Dendritic Arbor Are Specific to Neurons Born During Adulthood.
To investigate whether learning was also regulating the dendritic development of mature neurons, we used the lentiviral vector pTRIPΔU3-MND-Dsred2-WPRE, which labels all neurons. The lentivirus was injected 1 week before training (L-RM), and animals were killed 1 day after the end of the behavioral procedure (Table S1, experiment 4). Analysis of the morphological features of mature neurons of trained animals compared with home-cage rats (Fig. S5A) revealed no differences in dendritic arborization. The length of the dendrites and numbers of nodes and ends were the same in both groups (Fig. S5B–D). Likewise, Sholl analysis demonstrated no differences (Fig. S5 E and F). These results show that learning specifically affects the development of the dendritic arbor of adult-born neurons.
Learning-Induced Changes in the Dendritic Arbor Are Governed by the Cognitive Demand of the Task.
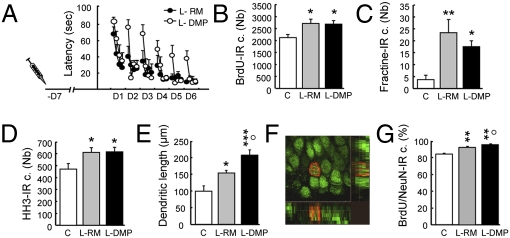
We next explored whether the level of cognitive demand influences the morphological development of new neurons. To do so, we used the delayed matching-to-place (DMP) task, which has greater cognitive demands than the L-RM task (15). In this procedure, animals are trained to escape onto a hidden platform, but, in contrast to the RM version, the position of the platform is changed daily. Its position is kept constant within each daily session. This task has been shown to be more demanding, because the animal not only has to acquire the spatial environment to find the platform, but must also update its memory to perform the task. Thus, the cognitive load on the hippocampus is greater, because this latter task supports incremental learning, as the RM model does, as well as fast one-trial learning. We first examined how the learning associated with both RM (L-RM) and DMP (L-DMP) affects newborn neuron survival, cell death, and cell proliferation to ensure that both versions of the water maze regulate neurogenesis in a homeostatic manner (Table S1, experiment 5; Fig. 6 A–D). We then examined the dendritic development of adult-born neurons and labeled them with BrdU 1 week before training. Our results demonstrate that spatial learning increased dendritic arbor complexity, as shown by the significant increase in the dendritic length (Fig. 6E) and the number of dendritic nodes and ends (Fig. S6 A–C). In these conditions, the size of the cell body remained unchanged (control, 62.64 ± 1.61 μm2; L-RM, 63.79 ± 1.47 μm2; L-DMP, 62.50 ± 2.26 μm2; F2,21 = 0.16; P = 0.8). More importantly, the effect of learning on dendritic growth was more pronounced in the L-DMP group, in which the cognitive task was more demanding. These results reveal that increasing the cognitive demand further increases dendritic growth. We then explored whether learning accelerates neuronal maturation by quantifying the BrdU-IR cells expressing NeuN, a marker of mature neurons (Fig. 6F). Spatial learning increased the percentage of BrdU-NeuN-IR cells, an effect that was more pronounced in the L-DMP group (Fig. 6G). These results show that learning accelerated the development of the adult-born neurons (dendritic arbor and neuronal maturation) as a function of the cognitive demand.
Fig. 6.
The learning effect on the dendritic arbor is governed by the cognitive demand of the task. (A) Latency to find the escape platform. (B) Total number of BrdU-IR cells (F2,21 = 4.58; P = 0.02). (C) Total number of apoptotic cells measured by the number of fractine-IR cells (F2,21 = 6.45; P = 0.06). (D) Total number of proliferating cells measured by the number of HH3-IR cells (F2,21 = 3.7; P = 0.04). (E) Length of the dendritic arbor (F2,21 = 9.85; P < 0.001). (F) BrdU-IR cell (red) stained with NeuN (green), a typical marker for mature newborn neurons. (G) Percentage of BrdU-IR cells expressing NeuN (F2,21 = 44.22; P < 0.001). C, n = 8; L-RM, n = 8; L-DMP, n = 8. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 compared with control. °P ≤ 0.05, L-RM compared with L-DMP.
Learning-Induced Changes in the Dendritic Arbor Depend on NMDA Receptors.
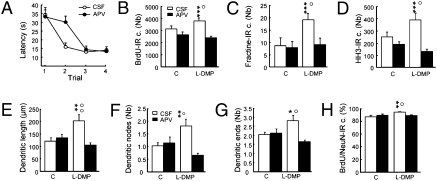
Because NMDA receptors (NMDARs) play a crucial role in spatial memory (15), regulate dendritic development (16–18) and are present on adult-born neurons (19, 20), we next investigated their possible involvement in learning-induced changes in neurogenesis (Table S1, experiment 6). We used the DMP protocol because we had shown that this protocol induced the most pronounced morphological changes. To investigate the role of NMDARs, we used a selective antagonist of these receptors, 2-amino-5-phosphonovaleric acid (APV), which was infused into the lateral ventricle for the entire training period. As reported previously (15), APV infusion impaired DMP learning (Fig. 7A). Interestingly, although AVP did not modify basal neurogenesis, it did abolish learning-induced changes in neurogenesis (Fig. 7 B–D). Indeed, the increases in cell survival, cell death, and cell proliferation occurring during the learning process were all inhibited after blockage of NMDAR. The increased dendritic arbor complexity induced by learning was also disrupted by APV infusions (Fig. 7 E–G); dendritic lengths and the numbers of nodes and ends were all reduced in APV-treated DMP rats compared with nontreated rats. Finally, APV treatment also blocked the effect of learning on the maturation of new neurons (Fig. 7H). To confirm the effect of NMDAR blockade on the learning effect on neurogenesis, we used another NMDA antagonist, MK801. As was found with APV, MK801 disrupted DMP learning, learning-induced increases in cell survival, and learning-induced increases in dendritic arbor complexity. Indeed, dendritic lengths and the numbers of nodes and ends were all reduced in MK801-treated DMP rats compared with nontreated rats (Fig. S7). Taken together, these findings demonstrate that the learning-induced changes in adult neurogenesis depend on NMDARs.
Fig. 7.
Influence of NMDA receptors on spatial learning and learning-induced changes in the number of adult-born neurons and their dendritic arbor complexity. (A) Latency to find the escape platform. (B) Total number of BrdU-IR cells (F3,33 = 8.32; P < 0.001). (C) Total number of apoptotic cell measured by the number of fractine-IR cells (F3,33 = 9.66; P < 0.001). (D) Total number of proliferating cells measured by the number of HH3-IR cells (F3,33 = 3.21; P < 0.05). (E) Length of the dendritic arbor (F3,31 = 6.47, P < 0.001). (F) Number of nodes (F3,31 = 5.80, P < 0.01). (G) Number of endings (F3,31 = 5.74, P < 0.01). (H) Percentage of BrdU-IR cells expressing NeuN (F3,31 = 3.70, P < 0.05). C: CSF, n = 7, APV, n = 9; L-DMP: CSF, n = 10, APV, n = 9. Vehicle, □; AVP, ■. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 compared with control CSF. °P ≤ 0.05; °°P ≤ 0.01 compared with APV group.
Discussion
Our results indicate that spatial learning regulates adult hippocampal neurogenesis not only by regulating the number of surviving adult-born neurons, but also by accelerating their development. We found that spatial learning increases the complexity of the dendritic arbor and accelerates the differentiation of newborn neurons toward a mature neuronal phenotype. Using retrovirus labeling, we also found that learning-induced changes in new neurons are long-lasting and are specific to adult-born neurons. Finally, we also report that this learning-induced shaping is a function of the cognitive demand of the task and depends on NMDARs.
Analyses of the developmental pattern of newborn neurons in “normal conditions” have shown that by 6 weeks after birth, the dendritic arbors of adult-born neurons resemble those of mature neurons (3, 14, 21). The few studies that have examined the long-term development of adult-born neurons have reported an evolution in their morphology (e.g., spine numbers) over a period of several months (3, 4, 22). Here we show that the dendritic arbors of newborn neurons can continue to develop over 3 months, a time at which they have still not reached maturity. Indeed, these dendritic arbors were still not as developed as those of mature neurons, reinforcing the idea that the development of adult-born neurons is a long-lasting process, with maturation completed even later than was originally demonstrated.
We also demonstrated that spatial learning in the water maze (L-RM) increased dendritic complexity, as shown by the increased numbers of nodes and endings and increased dendritic lengths. These results suggest that learning might accelerate the development of newborn neurons reaching a mature stage. Indeed, the percentage of BrdU-NeuN neurons was increased in animals subjected to spatial training. Furthermore, spines appeared earlier in animals that learned the task, and 3-month-old adult-born neurons in the learning group exhibited a higher proportion of mature spines (mushroom) than immature spines (thin). A previous study found no learning-induced changes in dendritic development (21), most likely due to the nontimed nature of the study (in which a heterogeneous neuronal population was examined) and/or a lack of survival-promoting effects of learning (23). Surprisingly, the effects that we describe here are in the range of (and even exceed), those obtained after 4 weeks of treatment with fluoxetine (24), indicating that the activity generated during learning is a powerful stimulus for regulating neo-dendritic maturation. Interestingly, the effect of learning on the shaping of the dendritic arbor was specific to adult-born neurons. Indeed, our analysis of the morphological changes of mature neurons revealed no effect of learning. Even if learning influences the structural remodeling of mature networks at the level of spines or synapses (25, 26), our results emphasize the robust role of neurogenesis in structural changes underlying memory processes.
During development, regressive events have been described at two different stages: (i) After an initial phase of cell proliferation, a fraction of neurons die, and (ii) at a later stage, after an initial overproduction of contacts, dendritic branches are eliminated without significant changes in cell numbers (13). This selective elimination of intercell “contacts” subserves an increased specificity in connections. For this reason, we examined the long-term development of dendritic arbors using a GFP recombinant retrovirus specifically incorporated into dividing cells. We found that the learning-induced dendritic growth was maintained for at least 3 months, indicating a lack of competitive rearrangements. Thus, contrary to what is seen during development, there does not appear to be an overproduction of dendritic branches followed by the elimination of some of these branches during maturation. Nonetheless, we cannot exclude the possibility that regressive events occur later, a hypothesis that remains to be tested.
Learning-induced dendritic shaping involved two populations of new neurons that were born at different times relative to training. The oldest population comprised new neurons generated 1 week before the start of the training and whose survival was promoted by learning, and the youngest population comprised newly born neurons generated 3 days before training or during the early phase of training that were spared by the apoptotic wave (8). We have previously shown that these two events, adding and removing new neurons, are interrelated; thus, blocking of learning-induced apoptosis by zVAD blocks the increased survival of older neurons (8). Here we show that zVAD also blocked the learning-induced increase in dendritic arbor complexity of these oldest newly born neurons (the survival of which is altered by zVAD), indicating competition between different neuronal populations for the supply of afferent-derived trophic factors and/or input influences that may stabilize neurons by activity generated in the course of learning. Taken together, these results indicate that learning regulates, in a homeostatic manner, the development of new neurons to maintain a precise quantitative relationship between neurons.
Dendritic arbor and spine densities represent mirror images of the available postsynaptic space and indirectly reflect the presence of inputs. The relationship between synaptic input and dendritic development has been extensively investigated; each neuron is innervated by an appropriate number of inputs, and each input innervates an appropriate number of neurons (27). Dendritic development has been associated with the arrival of synaptic inputs acting like a trophic factor governing selective postsynaptic stabilization (13, 28). Through enhanced network activity, inputs sculpt dendritic development by turning on the necessary dendritic differentiation programs (29). Our results suggest that newborn neurons that survive in response to learning might be those that are successfully connected, with the development of their neo-dendrites modulated during learning by afferent-dependent activation. Thus, an increased complexity of the dendritic arbor, together with an increase in dendritic spines, could reflect increased connectivity of adult-born neurons in the dentate networks. Analysis of the normal developmental pattern of newborn neurons has shown that adult-born neurons receive functional glutamatergic depolarizing afferents within 1 month after their birth (22, 30, 31). Interestingly, during epileptic crises, accelerated development of the dendritic arbor of adult-born neurons is associated with their accelerated electrophysiologic maturation (32). Thus, the more developed dendritic organization observed in rats subjected to learning is most likely associated with better functional integration of adult-born neurons in the networks, a hypothesis that remains to be tested. However, other authors have suggested that the arrival of a presynaptic input could act as a stop-growth or stabilization signal for the postsynaptic dendritic arbor. This sprouting would allow the brain to compensate for low network activity (33) and might be an attempt by the brain to adapt (recovering or sparing) to function loss (34). If this were the case, then a more complex dendritic organization should be associated with a loss of memory, and a less developed dendritic organization should be associated with a gain of function. But in fact the opposite was observed; alteration of reference memory was associated with low branching, whereas complex memory processing (L-DMP) was linked to high branching.
Compared with learning in the RM task, learning in the DMP task produced a major increase in dendritic arbor complexity in newborn neurons. In contrast, the increase in cognitive demand had no beneficial effect on newborn neuron survival. This finding reinforces the hypothesis that the neo-dendrites constitute an essential component for receiving and integrating incoming information. The marked effects of DMP compared with RM on dendritic arbor complexity are integrally linked to the fact that the animals were required to rapidly incorporate novel information into spatial representations of the environment. An alternative explanation could be that the effect on dendritic arborization in the DMP animals was due to the extra swimming during trial 1, meaning that the difference would be the result of physical exercise rather than learning per se. But we ruled out this hypothesis in a previous study by showing that swimming in the water maze without learning (yoked animals) or training (aged impaired rats) by itself left neurogenesis unchanged (8, 9). Thus, rapid hippocampal encoding of novel information for rapid learning of a one-time experience is acting on the development of dendrites of newborn neurons. Moreover, this rapid one-trial learning is dependent on NMDARs, because animals that received APV (or MK801) showed a specific impairment on the second trial but could locate the new platform position within four trials in the same manner that controls animals did. In other words, they could build the cognitive map of the environment and use it, but they were impaired in learning new information as fast as controls did. Thus, it can be suggested that the increased complexity of the dendritic arbor in newborn neurons reflects a process aimed at rapidly elaborating an adaptative behavior.
Our results demonstrate that the blockade of NMDARs disrupts learning-induced changes in neurogenesis by impairing the homeostatic regulation of newborn cell numbers. Interestingly, these treatments did not disrupt basal neurogenesis, a finding consistent with some (35), but not all, previous studies (36, 37). The discrepancy between these studies might be related to differences in drug regimens, species, strains, and sex, all of which are known to influence neurogenesis (2, 38). The lack of effect on basal neurogenesis in the present study indicates specific impairment of learning-driven activity mediated by NMDAR activity.
One question that remains to be answered is how glutamate acts on new neurons, the survival of which is promoted by learning. NMDA antagonists might act directly on newborn neurons. Although the presence of NMDARs on dividing cells is controversial (19, 37), these receptors have been shown to respond to changes in glutamate levels (39). In vitro application of NMDA on hippocampal precursor cells increased neuronal differentiation, whereas treatment with the antagonist APV decreased neuronal differentiation. At a later developmental stage, adult-born neurons express NMDARs (19, 37) and functional glutamatergic afferents that have been described to occur in mice toward the end of the second week after birth (30, 31). However, it has been recently shown that adult-born neuron maturation and functional recruitment into the dentate network is faster in rats compared with mice (38) and that glutamatergic afferents arrive earlier in rats, when newborn neurons are 10 days old (40), a time window consistent with our data. Thus, learning may accelerate the maturation of adult-born neurons by inducing proneural genes and/or by accelerating the arrival of glutamatergic inputs and their functional integration into the network, as has been described during epileptic crises (32). Alternatively, NMDA antagonists could act indirectly on preexisting dentate neurons. On the one hand, reduced glutamate release from mature dentate glutamatergic neurons or other glutamatergic neurons impinging within the DG could be responsible for the observed effects on adult-born neurons. On the other hand, because GABAergic hilar interneurons are under glutamatergic control (41), a reduction in GABAergic signaling could be involved as well. Indeed, adult-born neurons first receive depolarizing GABA inputs that are known to promote the maturation of the new neurons (30, 31, 42, 43). Further investigation is needed to elucidate the mechanisms through which glutamate regulates learning-induced changes in neurogenesis.
Our results illustrate environmental regulation of dendritic development in the adult DG. To the best of our knowledge, very few studies have examined the influence of spatial learning on structural plasticity in this brain area. An increase in spine density within the molecular layer (44) and a sprouting of the mossy fiber sprouting within the CA3 subfield (45, 46) are consistent with the effects reported here.
Given that the majority of synaptic input is received and integrated by dendritic arbors, and that the size and structure of these arbors determine their computational capabilities (47, 48), our results suggest a strong link between the learning-dependent dendritic structural plasticity of adult-born neurons and memory. But adult-born neurons generated 1 week before spatial learning are not involved in ongoing learning, suggesting that they do not participate in memory encoding and consolidation (49). A recent study demonstrated that young neurons are involved in memory retrieval (50); however, how immature neurons that do not participate in encoding and stabilizing a memory trace after the initial acquisition can sustain memory retrieval remains unknown. Here we hypothesize that, given that the effect of learning on the sculpting of dendritic arbors lasts for several months, selected adult-born neurons may be required for subsequent learning.
In conclusion, our results demonstrate that the development of the dendritic arborization—and its key receptive unit, the spines—of adult-born neurons is influenced by spatial learning and is dependent on the cognitive load on the hippocampus. This postnatal specification of networks by learning provides a potent modulator of plasticity for the adult brain to adapt to incoming information.
Experimental Procedures
Animals.
Male Sprague-Dawley rats were individually housed and maintained on a 12 h on/12 h off light/dark cycle, and experiments were carried out during the light cycle. To label newly born cells, rats were injected with BrdU (1 × 100 mg/kg i.p.).
Retrovirus-Mediated Labeling of New Neurons.
The murine Moloney leukemia virus–based retroviral vector CAG-GFP has been described in detail (14) and is a gift from Drs. Gerald Pao and Inder Verma (Salk Institute).
Lentivirus-Mediated Labeling of Neurons.
The lentivirus-based lentiviral vector, pTRIPΔU3-MND-Dsred2-WPRE (Dsred2-LV), was produced by transient transfection of 293T cells according to standard protocols (51) (SI Experimental Procedures).
Surgery.
Rats were anesthetized with ketamine (60 mg/kg) and xylazine (7.5 mg/kg), and minipumps were implanted as described previously (15) (SI Experimental Procedures).
Behavioral Procedures.
Reference Memory (L-RM) Procedure.
Rats were trained in a water maze as described previously (8). In brief, animals were required to locate the submerged platform in a fixed location using the spatial cues available within the testing room. They were all tested in four trials per day (90 s, with an intertrial interval of 30 s and beginning from three different starting points that varied randomly each day). The time to reach the platform was measured (Videotrack; Viewpoint).
Delay-Matching to Place (DMP) Procedure.
The protocol was based on a previous study (15). Animals were trained to escape onto a hidden platform, the position of which was changed daily. Within a daily session, the platform's position was kept constant. The control group was composed of animals that were kept in the housing room for the duration of the entire experiment. The third DMP experiment (CSF/MK801 animals) used the Atlantis platform. The platform's position was changed daily as previously, but was made available only after 60 s on the first trial to keep the animals from finding the new position by chance.
Immunohistochemistry.
Animals were perfused 1 day after the completion of the behavioral procedure. Free-floating sections (50 μm) were processed in a standard immunohistochemical procedure as described previously (8).
Statistical Analyses.
All data (mean ± SEM) were analyzed by the Student t test or by ANOVA followed by the Duncan test for individual group differences or the Fischer test for ANOVA with repeated measures.
Supplementary Material
Acknowledgments
We thank Dr. M. Koehl (Inserm U862), Dr. S. J. Sara (CNRS UMR7152), and Dr. P. Roullet (CNRS 5169) for useful discussion and comments and M. L. Gage for editorial comments. We acknowledge Dr. M. F. Montaron, C. Dupuy, J. M. Claustrat, and S. Rey for their technical help. We thank Dr. S. Sans and colleagues for help with the Volocity software. The microscopy was done at the Bordeaux Imaging Center. The help of P. Legros, L. Malicieux, S. Marais, and C. Poujol is acknowledged. This work was supported by the Institut de la Santé et de la Recherche Médicale, University of Bordeaux 2, Région Aquitaine (to D.A.) and Agence Nationale pour la Recherche (to D.A. and S.O.). A.F. was a recipient of a La Fondation pour la Recherche Medicale Aquitaine fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914613107/DCSupplemental.
References
- 1.Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- 2.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: From precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 3.Toni N, et al. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- 4.Toni N, et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dupret D, et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 8.Dupret D, et al. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 2007;5:e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drapeau E, Montaron MF, Aguerre S, Abrous DN. Learning-induced survival of new neurons depends on the cognitive status of aged rats. J Neurosci. 2007;27:6037–6044. doi: 10.1523/JNEUROSCI.1031-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Drapeau E, et al. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci USA. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- 13.Changeux JP, Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264:705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- 14.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steele RJ, Morris RG. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9:118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nat Neurosci. 2004;7:254–260. doi: 10.1038/nn1191. [DOI] [PubMed] [Google Scholar]
- 17.Prithviraj R, Inglis FM. Expression of the N-methyl-D-aspartate receptor subunit NR3B regulates dendrite morphogenesis in spinal motor neurons. Neuroscience. 2008;155:145–153. doi: 10.1016/j.neuroscience.2008.03.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sin WC, Haas K, Ruthazer ES, Cline HT. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature. 2002;419:475–480. doi: 10.1038/nature00987. [DOI] [PubMed] [Google Scholar]
- 19.Nácher J, et al. N-methyl-d-aspartate receptor expression during adult neurogenesis in the rat dentate gyrus. Neuroscience. 2007;144:855–864. doi: 10.1016/j.neuroscience.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA receptor–mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 21.Plümpe T, et al. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006;7:77–91. doi: 10.1186/1471-2202-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehninger D, Kempermann G. Paradoxical effects of learning the Morris water maze on adult hippocampal neurogenesis in mice may be explained by a combination of stress and physical activity. Genes Brain Behav. 2006;5:29–39. doi: 10.1111/j.1601-183X.2005.00129.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Roo M, Klauser P, Garcia PM, Poglia L, Muller D. Spine dynamics and synapse remodeling during LTP and memory processes. Prog Brain Res. 2008;169:199–207. doi: 10.1016/S0079-6123(07)00011-8. [DOI] [PubMed] [Google Scholar]
- 26.Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cline HT. Dendritic arbor development and synaptogenesis. Curr Opin Neurobiol. 2001;11:118–126. doi: 10.1016/s0959-4388(00)00182-3. [DOI] [PubMed] [Google Scholar]
- 28.Rajan I, Cline HT. Glutamate receptor activity is required for normal development of tectal cell dendrites in vivo. J Neurosci. 1998;18:7836–7846. doi: 10.1523/JNEUROSCI.18-19-07836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Libersat F, Duch C. Mechanisms of dendritic maturation. Mol Neurobiol. 2004;29:303–320. doi: 10.1385/MN:29:3:303. [DOI] [PubMed] [Google Scholar]
- 30.Espósito MS, et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Overstreet-Wadiche LS, Bromberg DA, Bensen AL, Westbrook GL. Seizures accelerate functional integration of adult-generated granule cells. J Neurosci. 2006;26:4095–4103. doi: 10.1523/JNEUROSCI.5508-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tripodi M, Evers JF, Mauss A, Bate M, Landgraf M. Structural homeostasis: Compensatory adjustments of dendritic arbor geometry in response to variations of synaptic input. PLoS Biol. 2008;6:e260. doi: 10.1371/journal.pbio.0060260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finger S, Hart T, Jones E. Recovery time and sensorimotor cortex lesion effects. Physiol Behav. 1982;29:73–78. doi: 10.1016/0031-9384(82)90368-7. [DOI] [PubMed] [Google Scholar]
- 35.Arvidsson A, Kokaia Z, Lindvall O. N-methyl-D-aspartate receptor–mediated increase of neurogenesis in adult rat dentate gyrus following stroke. Eur J Neurosci. 2001;14:10–18. doi: 10.1046/j.0953-816x.2001.01611.x. [DOI] [PubMed] [Google Scholar]
- 36.Nacher J, Rosell DR, Alonso-Llosa G, McEwen BS. NMDA receptor antagonist treatment induces a long-lasting increase in the number of proliferating cells, PSA-NCAM-immunoreactive granule neurons and radial glia in the adult rat dentate gyrus. Eur J Neurosci. 2001;13:512–520. doi: 10.1046/j.0953-816x.2000.01424.x. [DOI] [PubMed] [Google Scholar]
- 37.Petrus DS, et al. NMDA and benzodiazepine receptors have synergistic and antagonistic effects on precursor cells in adult hippocampal neurogenesis. Eur J Neurosci. 2009;29:244–252. doi: 10.1111/j.1460-9568.2008.06579.x. [DOI] [PubMed] [Google Scholar]
- 38.Snyder JS, et al. Adult-born hippocampal neurons are more numerous, faster- maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deisseroth K, et al. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 40.Ambrogini P, et al. Hippocampus. 2009. Synaptogenesis in adult-generated hippocampal granule cells is affected by behavioral experiences. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 41.Jonas P, Bischofberger J, Fricker D, Miles R. Interneuron diversity series: Fast in, fast out—temporal and spatial signal processing in hippocampal interneurons. Trends Neurosci. 2004;27:30–40. doi: 10.1016/j.tins.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 42.Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 43.Wang LP, Kempermann G, Kettenmann H. A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol Cell Neurosci. 2005;29:181–189. doi: 10.1016/j.mcn.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 44.O'Malley A, O'Connell C, Murphy KJ, Regan CM. Transient spine density increases in the mid-molecular layer of hippocampal dentate gyrus accompany consolidation of a spatial learning task in the rodent. Neuroscience. 2000;99:229–232. doi: 10.1016/s0306-4522(00)00182-2. [DOI] [PubMed] [Google Scholar]
- 45.Ramírez-Amaya V, Escobar ML, Chao V, Bermúdez-Rattoni F. Synaptogenesis of mossy fibers induced by spatial water maze overtraining. Hippocampus. 1999;9:631–636. doi: 10.1002/(SICI)1098-1063(1999)9:6<631::AID-HIPO3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 46.Rekart JL, Sandoval CJ, Bermudez-Rattoni F, Routtenberg A. Remodeling of hippocampal mossy fibers is selectively induced seven days after the acquisition of a spatial but not a cued reference memory task. Learn Mem. 2007;14:416–421. doi: 10.1101/lm.516507. [DOI] [PubMed] [Google Scholar]
- 47.Häusser M, Mel B. Dendrites: Bug or feature? Curr Opin Neurobiol. 2003;13:372–383. doi: 10.1016/s0959-4388(03)00075-8. [DOI] [PubMed] [Google Scholar]
- 48.London M, Häusser M. Dendritic computation. Annu Rev Neurosci. 2005;28:503–532. doi: 10.1146/annurev.neuro.28.061604.135703. [DOI] [PubMed] [Google Scholar]
- 49.Kee N, Teixeira CM, Wang AH, Frankland PW. Imaging activation of adult-generated granule cells in spatial memory. Nat Protoc. 2007;2:3033–3044. doi: 10.1038/nprot.2007.415. [DOI] [PubMed] [Google Scholar]
- 50.Trouche S, Bontempi B, Roullet P, Rampon C. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proc Natl Acad Sci USA. 2009;106:5919–5924. doi: 10.1073/pnas.0811054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sena-Esteves M, Tebbets JC, Steffens S, Crombleholme T, Flake AW. Optimized large-scale production of high-titer lentivirus vector pseudotypes. J Virol Methods. 2004;122:131–139. doi: 10.1016/j.jviromet.2004.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.