Abstract
Top-down mass spectrometry holds tremendous potential for the characterization and quantification of intact proteins, including individual protein isoforms and specific posttranslationally modified forms. This technique does not require antibody reagents and thus offers a rapid path for assay development with increased specificity based on the amino acid sequence. Top-down MS is efficient whereby intact protein mass measurement, purification by mass separation, dissociation, and measurement of product ions with ppm mass accuracy occurs on the seconds to minutes time scale. Moreover, as the analysis is based on the accurate measurement of an intact protein, top-down mass spectrometry opens a research paradigm to perform quantitative analysis of “unknown” proteins that differ in accurate mass. As a proof of concept, we have applied differential mass spectrometry (dMS) to the top-down analysis of apolipoproteins isolated from human HDL3. The protein species at 9415.45 Da demonstrates an average fold change of 4.7 (p-value 0.017) and was identified as an O-glycosylated form of apolipoprotein C-III [NANA-(2 → 3)-Gal-β(1 → 3)-GalNAc, +656.2037 Da], a protein associated with coronary artery disease. This work demonstrates the utility of top-down dMS for quantitative analysis of intact protein mixtures and holds potential for facilitating a better understanding of HDL biology and complex biological systems at the protein level.
Keywords: quantitative proteomics, Fourier-transfrom mass spectrometry, electron transfer dissociation, top-down proteomics, posttranslational modifications
In nature, mammalian proteins exist as multiple isoforms that are derived from variations in the genetic code, alternate splicing and processing events, and posttranslational protein modifications. The quantification of proteins and protein isoforms in biological systems is central to expanding the understanding of protein function. Antibody-based assays, such as the enzyme-linked immune-sorbent assay and the Western blot assay, are the primary tools used to quantify proteins in the laboratory. In many cases, these tools can provide exquisite sensitivity and selectivity due to the availability of well-characterized antibody reagents that have been carefully developed to selectively bind to specific proteins or protein isoforms. A limitation of antibody-based methods is the significant time and effort that is required to generate reagents for each protein studied. When large numbers of proteins or protein isoforms need to be quantified in a single experiment, methods that depend on the availability of selective antibody reagents become impractical.
Mass spectrometry based proteomics has led to the development of established platforms that can detect, identify, and quantitate thousands of proteins without the use of selective antibody reagents (1, 2). The majority of these techniques utilize proteolytic enzymes (e.g., trypsin, aspN, and Lys-C) to digest intact proteins into short peptides that are ideally suited for analysis by mass spectrometry. Collectively, these approaches have been described as “bottom-up” proteomic methods and have been shown to provide rich datasets comprised of thousands of peptide sequences that can be matched to sequences contained in protein databases (3). The routine analysis of mixtures of tryptic peptides by tandem mass spectrometry has resulted in the publication of a wide variety of bottom-up applications that include sequence analysis of complete genomes (4, 5) by multidimensional protein identification using strong cation exchange or isoelectric focusing, compositional analysis of the major components of high-density lipoprotein (HDL) by spectral counting (6), quantitative analysis of metastatic prostate cancer cell lines using stable isotopes (7), and quantitative analysis of plasma and cerebrospinal fluid by differential mass spectrometry (8). It is important to recognize that because bottom-up methods measure peptides, and not proteins, the data may sometimes be degenerate and cannot distinguish between multiple protein isoforms or distinct proteins that share a common peptide sequence.
Kelleher et al. have pioneered “top-down” proteomic techniques that characterize intact proteins without the use of enzymatic digestion (9, 10). Top-down methods provide a complementary and particularly promising approach for characterizing proteins that may exist in multiple forms (11). In a typical top-down experiment, proteins are introduced into the mass spectrometer directly, separated on the basis of their mass-to-charge ratio and then sequenced from acquired tandem mass spectra. Successful protein sequencing can be achieved only when cleavage occurs more or less randomly at the amide backbone and once per molecule so that a complete distribution of amide backbone cleavage is observed in the corresponding tandem MS spectrum. Classical threshold dissociation methods [collisionally assisted dissociation (CAD) and infrared multiphoton dissociation] often fail in this regard (12, 13). Newer low-energy electron-based dissociation methods electron capture dissociation (ECD) described by Zubarev and co-workers (14) and electron-transfer dissociation (ETD) described by Syka and co-workers (15) have opened opportunities for intact protein characterization as the cleavage of the N-Cα bond often occurs in a sequence independent manner and preserves posttranslational modification (PTMs) (12). Recently, with the introduction of commercial instrumentation that combines high-mass resolution and accurate mass measurement capabilities with electron-transfer dissociation, it is now possible for industrial laboratories to acquire high-resolution full-scan and ETD-MS/MS tandem mass spectra for intact proteins with molecular weights in the range of 5–50 kD (16).
Differential mass spectrometry (dMS) is a general proteomics workflow that provides relative quantitation from full-scan mass spectrometry data (17–19). Here, we set out to establish top-down dMS capabilities that combine the advantages of intact protein analysis with quantitation from full spectrum mass spectrometry data (Fig. 1). As a proof-of-concept, we applied dMS to the top-down analysis of HDL isolated from patients having high and low HDL-cholesterol (HDL-c) levels. HDL is one of five major classes of lipoprotein particles in humans. Elevated levels of HDL-c, aka “good cholesterol,” is associated with reduced risk of coronary artery disease and hypothesized to be cardioprotective in humans; however, the mechanisms are unknown (20–24). Many HDL-associated proteins have molecular weights below 30 kDa and thus are ideally suited for study by top-down mass spectrometry. Here we show that dMS reveals several proteins quantitatively different in the samples analyzed, one of which is a protein at 9,415.45 Da that exhibited an increased abundance in donors having low HDL-c. Protein identification and characterization of the intact protein was achieved via ETD liquid chromatography (LC)-MS/MS data generated on a commercially available high-resolution mass spectrometer and analysis by automated computer-based tools. The intact protein at 9,415.45 Da was identified by mass spectrometry as an O-glycosylated form of apolipoprotein C-III [NANA-(2 → 3)-Gal-β(1 → 3)-GalNAc, +656.2037 Da], a protein associated with coronary artery disease (25) and demonstrates a unique and potentially promising approach for studying the distribution and function of discrete protein isoforms. Collectively these results exhibit the utility of top-down dMS for quantitative analysis of intact protein mixtures and reveal the potential that these antibody-free methods have for expanding the current understanding of HDL biology and complex biological systems at the protein level.
Fig. 1.
Roadmap for top-down dMS of intact protein isoforms. Complex mixtures of intact protein isoforms are loaded on an LC column and analyzed by high-resolution FT-MS. The FTMS data are processed to detect mass spectral features that exhibit a statistically significant difference in abundance between the groups of samples. At this stage, the intact protein isoforms have been quantified and the accurate molecular weight is able to be determined. The samples are reanalyzed and the ETD capability of the Orbitrap-XL-ETD mass spectrometer is used to acquire high-resolution product ion spectrum for the selected protein feature. The resulting high-resolution ETD spectrum contains c and z type fragment ions that are specific to the amino acid sequence and posttranslational modifications of the intact protein. ProSightPC, a software suite tailored for the characterization of intact protein data, is used to interpret the ETD spectrum and return protein identification and posttranslational modification data.
Results and Discussion
Top-Down dMS.
Differential mass spectrometry has been successfully applied for the relative quantitation of complex peptide mixtures (17, 18). To adapt dMS to the analysis of intact protein mixtures, two primary requirements to retain are a reproducible biochemical sample processing method and a robust LC-MS analysis. Hence, we selected the analysis of HDL3 from human plasma for a proof of concept as the lipoprotein particles are readily isolated from human plasma via density gradient ultracentrifugation and entail only disulfide bond reduction and HPLC desalting before LC-MS analysis. In addition, LC-MS parameters were adjusted to accommodate intact proteins (i.e., C8 stationary phase instead of C18), and instrument acquisition parameters were optimized for higher charge state analytes (i.e., increased to 100,000 resolving power, tube lens voltage increased, etc.). These modifications allowed for a reproducible analysis of more than 40 technical replicate HDL3 samples with a median percent coefficient of variation of approximately 36% [details provided in SI Methods]. Shown in Fig. 2 is an example composite high-resolution electrospray ionization (ESI) mass spectrum generated by combining multiple mass spectra acquired over a short time window of the LC gradient between 33 and 34 min. The accurate mass of these ion species allows one to determine the molecular weight of the protein to within 0.1 Da and thus predict that the three primary analytes measured are apolipoprotein C-III and two posttranslationally modified forms of apolipoprotein C-III, O-glycosylation by the addition of NANA-(2 → 3)-Gal-β(1 → 3)-GalNAc (Δm = 656.2037 Da) and branched [NANA-(2 → 3)-Gal-β(1 → 3)]-[NANA-(2 → 6)]-GalNAc (Δm = 947.3580 Da). Tandem MS (MS/MS) experiments are required, however, for unambiguous identification. Because dMS is a semiquantitative technique based on the comparative analysis of integrated signal intensities for a particular analyte (aka feature, as defined by m/z and retention time) across multiple samples, we wanted to test instrument response across a range of analyte concentrations using apolipoprotein C-III as an example. As shown in Fig. 3, standard addition of purified apolipoprotein C-III into technical replicates of HDL3 isolated from human plasma was performed to ensure a linear instrument response from the 100 nM to 100 μM range.
Fig. 2.
Total ion current (TIC) chromatogram from LC-MS analysis of HDL3. High-resolution ESI-mass spectrum of eluting apolipoprotein C-III (33.0–33.8 min, 46 scans averaged) showing the unmodified (•), NANA-(2 → 3)-Gal-β(1 → 3)-GalNAc-Thr94 (▪), and branched [NANA-(2 → 3)-Gal-β(1 → 3)]-[NANA-(2 → 6)]-GalNAc-Thr94 (▴) protein forms. (Inset) Selected ion chromatogram (SIC) for the (M + 9H+)9+ ion of NANA-(2 → 3)-Gal-β(1 → 3)-GalNAc-Thr94 modified apolipoprotein C-III at m/z 1047.1678.
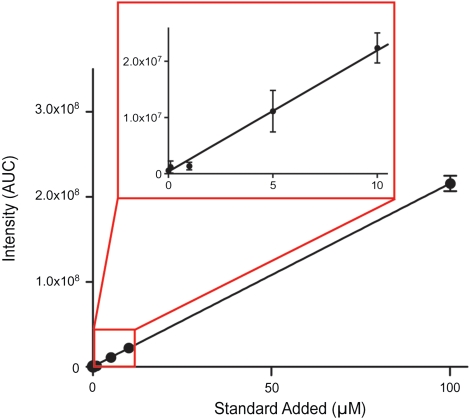
Fig. 3.
Instrument response following standard addition of purified apolipoprotein C-III into three technical replicates of HDL3 isolated from human plasma. Standards range in concentration from 100 nM to 100 μM. Data points correspond to signal from charge states (M + 11H)11+ to (M + 7H)7+ summed and plotted as area under curve (linear regression performed y = 2,152,772.4634x + 402,817.8176, R2 = 0.999946). Error bars represent standard error of the mean.
To demonstrate the utility of top-down dMS in finding relative changes in protein abundance, multiple human subjects having relatively low (avg: 44 mg/dL, N = 3) and high (avg: 74 mg/dL, N = 3) HDL-c measurements were chosen with the expectation that they would possess bona fide protein differences for dMS detection. All subjects were healthy, middle-aged donors with clinical characteristics as shown in Table 1. HDL3 (d = 1.13–1.21 g/mL) was isolated by density gradient ultracentrifugation of freshly acquired EDTA plasma, and samples were normalized for total protein concentration prior to LC-MS analysis. The HDL3 subfraction was chosen because of its atheroprotective potential and has been extensively characterized in many laboratories using proteomics, including stable isotope dilution mass spectrometry and bottom-up techniques (6, 26–28).
Table 1.
Clinical characteristics of samples used for this study
| Subject ID | HDL fraction | Age, years | Sex | Total cholesterol, mg/dL | HDL-c, mg/dL | LDL-c, mg/dL | Triglycerides, mg/dL |
| 1 | HDL3 | 60 | M | 186 | 50 | 118 | 90 |
| 2 | HDL3 | 32 | M | 207 | 41 | 150 | 78 |
| 3 | HDL3 | 44 | M | 191 | 42 | 115 | 170 |
| 4 | HDL3 | 50 | F | 173 | 73 | 85 | 79 |
| 5 | HDL3 | 61 | F | 196 | 75 | 104 | 106 |
| 6 | HDL3 | 61 | M | 160 | 73 | 69 | 90 |
Each individual HDL3 sample was split into three technical replicates (total of 18 samples) to increase the statistical power of the analysis. In addition, all samples were prepared and profiled by LC-MS in a single block (i.e., all samples were analyzed nonstop by LC-MS, with interleaved quality assurance/quality control (QA/AC) samples every six HDL injections) to minimize instrument drift and variability; the time from sample preparation to profiling completion was < 1.5 days. Instrument stability was determined from peak area under curve (AUC) and retention times of interleaved QA/QC samples (i.e., three peptide mixture and trypsin-digested conalbumin samples). The measured peak AUCs of these standard samples are consistent with an instrument variability of < 5% CV with a maximum fold change of < 1.2 over the course of the entire experiment. Additionally, retention times of these same monitored standard samples are consistent with a maximum retention time shift of less than 0.61 min, which is then further corrected for by the alignment algorithm in the data analysis software. This instrument reproducibility, together with overall platform variability (estimated at 36% coefficient of variation), was acceptable for dMS analysis.
dMS analysis revealed multiple statistically significant differences in protein abundance. Of the 96,235 features detected (where a feature is defined as distinct m/z within a restricted retention time window, i.e., an observed isotope peak), approximately 380 demonstrated statistically significant changes in protein abundance between high HDL-c and low HDL-c subject groups at P < 0.02 and filtering as described in Materials and Methods (Table S1). Several features of ∼9 kDa were determined to show significant abundance differences across the HDL3 samples. As an example, shown in Fig. 4 is the dMS quantitation of three forms of apolipoprotein C-III measured in the six patient samples. Interestingly, the three major forms of this protein showed higher abundances, on average, in HDL3 samples taken from donors with low HDL-c measurements. Protein abundance measurements for each of the three major glycoforms detected can be found in Table S2.
Fig. 4.
Quantitation of the three forms of apolipoprotein C-III. Error bars represent standard error of the mean for multiple (n = 3) replicate LC-MS injections. Subjects 1–3 low HDL; subjects 4–6 high HDL.
Apolipoprotein C-III is a major protein component of both apolipoprotein B containing triglyceride-rich lipoproteins and HDL particles and has been extensively studied for function. Apolipoprotein C-III is mainly involved in modulating plasma triglyceride levels by the catabolism of triglyceride-rich particles, but plasma apolipoprotein C-III levels have been suggested to be linked to other conditions associated with metabolic syndrome (29). Apolipoprotein C-III-containing HDL may play a key role in the prevention of apolipoprotein C-III-induced hypertriglyceridemia (30). Recently, SELDI-TOF (surface-enhanced laser desorption ionization time of flight) analysis suggests a link between an ion species at m/z 8,697 of apolipoprotein C-III and papillary thyroid carcinoma (31). However, SELDI-TOF is a low-resolution technique, requires off-line LC purification, and has shown limited utility in biomarker development (32). Although it is believed there is a correlation between circulating apolipoprotein C-III levels and atherosclerosis (25), little is known of the specific functions of each known glycoform. Further top-down dMS experiments with a larger patient sample size may help to establish a relationship between quantitative changes in the amounts of posttranslationally modified apolipoproteins and cardiovascular physiology. For example, researchers have established cell-based ex vivo assays of macrophage reverse cholesterol transport, or macrophage efflux potential, that quantitates the ex vivo capacity of isolated HDL to efflux cholesterol from macrophage (33, 34). Linking top-down dMS with such functional assays may correlate quantities of specific protein isoforms to macrophage efflux potential. Those protein isoforms can be readily measured by top-down dMS in HDL particles or plasma from large numbers of human samples to establish a relationship with clinical outcome, atherosclerosis clinical imaging, or measured before and after therapeutic intervention.
High-Resolution Orbitrap-ETD Analysis of Intact Proteins.
In the dMS workflow, quantitative changes in protein abundances are first established followed by structural characterization by tandem mass spectrometry (MS/MS) to determine the amino acid sequence of the protein and to identify and to locate posttranslational modifications. ETD is a recently developed fragmentation technique that is highly efficient, compatible with the chromatography time scale, and allows for extensive fragmentation of protein species while preserving labile posttranslational modifications, including phosphorylation and glycosylation, O-GlcNac, and nitrosylation (12, 35). ETD has recently become available on a commercial high-resolution instrument (Orbitrap-XL with ETD capabilities), thus opening up the opportunity to characterize intact proteins ions by LC-MS and enabling charge state determination and accurate mass assignment for product ions from LC-MS/MS spectra.
High-resolution Orbitrap-ETD experiments confirmed the identity and posttranslational modifications of the apolipoprotein C-III. Shown in Fig. 5B is the high-resolution ETD-MS/MS spectrum of the (M + 9H)9+ ions at m/z = 1,047.7253, which allows the definitive identification O-glycosylated form of apolipoprotein C-III (Δm = 656.2037 Da). ProsightPC v1.0 provided a single protein match (apolipoprotein C-III, accession no. P02656), with an expectation score of 2 × 10-49. A total of 35 and 17 matching c and z• ions, respectively, were assigned with an average mass accuracy of 3 ppm. These accurate mass fragment ions localized the O-glycosylation modification to the C-terminal end of this protein between the c58 and c77 ions (Asp59-Val77) as shown in Fig. 5A, consistent with other findings showing Thr74 to be the site of modification (36, 37). Additionally, high-resolution threshold dissociation methods (i.e., CAD) revealed seven and three matching b and y fragment ions, respectively, with the predominant fragmentation being the selective removal of the sugar side chain (-291.09 Da, -656.20 Da), supporting the presence of an O-glycosylation modification (Fig. S1).
Fig. 5.
The amino acid sequence of apolipoprotein C-III with the matching c and z• ions labeled (A). High-resolution electron-transfer dissociation spectrum of (M + 9H+)9+ ions at m/z = 1,047.7253 (B). The NANA-(2 → 3)-Gal-β(1 → 3)-GalNAc- glycosylation, believed to be located at residue Thr74, is localized to a portion of the protein between Asp59-Val77. Ions colored red represent fragment ions containing sugar moiety.
High-resolution Orbitrap ETD performed on other intact proteins within these samples demonstrates a clear advantage over other fragmentation technologies. As an example, the protein apolipoprotein C-I (6626.506 Da, accession no. P02654) produced extensive fragments by ETD, producing 35 and 31 matching c and z• ions, respectively. This represents a sequence coverage of ∼73% (40 of 55 possible bonds broken, 61 total matching ions). In comparison, electron capture dissociation [ECD, on LTQ-FT (Fourier-transform) Ultra instrument] and CAD (on LTQ-Orbitrap instrument) produced 55 (69% coverage) and 23 (38% coverage) total matching fragment ions, respectively (Fig. S2). The targeted ETD of other HDL-associated proteins revealed improved tandem mass spectra relative to CAD. The information content obtained from these dissociation techniques clearly favors ETD for the analysis of intact proteins.
Conclusion
We have demonstrated the utility of top-down dMS for the detection of quantitative differences in abundance of protein and protein isoforms from human HDL samples. These differences were determined in the presence of a complex background of unchanging proteins and prior to any protein identification efforts. Determining quantitative information for all detectable proteins in these samples allows one to focus on only those proteins that are determined to have statistically significant changes in abundance and thus alleviates one major bottleneck of top-down proteomics, which is the identification and characterization of proteins by tandem mass spectrometry. Moreover, electron-transfer dissociation ETD promises to be a more general fragmentation method for the detailed characterizations of small proteins < 30 kDa and associated PTMs. The small sample set used in this experiment was chosen to demonstrate the ability of top-down dMS to detect quantitative differences in intact proteins and thus opens up the paradigm whereby patterns of modifications on a protein or protein isoforms can be correlated with specific activities or functions using a broad profiling platform.
Materials and Methods
Preparation of HDL Samples.
Density gradient ultracentrifugation (d = 1.13–1.21 g/mL) was used to isolate HDL3 from fresh EDTA plasma essentially as described (38). Plasma from six individual human subjects was used. A single phlebotomy procedure was performed on each subject. HDL protein concentrations were determined by bicinchoninic acid assay (39). All samples were prepared for LC-MS analysis by desalting on an HPLC column (octylsilane trap column, Michrom Resources), lyophilized to dryness, and then resuspended in 0.1-M acetic acid (solvent A) containing 10 mM tris(2-carboxyethyl)phosphine (to prevent protein disulfide bond formation). All samples were reconstituted to a total protein concentration of 0.2 mg/mL.
Apolipoprotein C-III Standard Addition Samples.
Purified human apolipoprotein C-III purchased (Academy Biomedical) was first analyzed by direct-infusion Fourier-transform mass spectrometry and then used for standard addition experiments without further preparation. Six known concentrations (0, 0.1, 1, 5, 10, and 100 μmol) of this apolipoprotein C-III sample were spiked into aliquots of prepared HDL3 samples and processed for mass spectrometric analysis as described below. Three replicates of each spike-in concentration were prepared and analyzed. The peak area under curve for apolipoprotein C-III charge states (M + 11H)11+ through (M + 7H)7+ were summed and used for establishing a linear curve of signal intensity vs. protein concentration.
Mass Spectrometric Profiling.
HDL3 samples were analyzed by a reverse-phase nano-HPLC coupled to a LTQ-FT hybrid mass spectrometer (ThermoFisher). A 1-μL aliquot of each sample was loop-injected (Agilent Series 1200) onto a Biobasic C8 trap column (1 cm × 75 μm, New Objective) and eluted from a nano-LC column packed with POROS R1 media (6 cm × 75 μm capillary) using a binary gradient increasing from 10% solvent B (0.1 M acetic acid in acetonitrile) to 70% solvent B at a rate of 2%/min (Agilent 1100 series). HDL3 samples (N = 6) were analyzed in triplicate for a total of 18 LC-MS injections. In addition, 7 QA/QC samples consisting of three peptides (bradykinin, angiotensin, and neurotensin at 100 fmol/μl in 0.1 M acetic acid) were interspersed between the HDL3 samples to measure instrument performance throughout the experiment. An interwoven block design was used to eliminate systemic bias due to run order. Intact proteins eluting from the LC column were introduced into the mass spectrometer by electrospray ionization using a 3-kV needle voltage, heated metal capillary temperature of 270 °C, and tube lens voltage 80 V. Ion injection times into linear ion trap were adjusted by the instrument automatic gain control (AGC, 1 × 107 a.u. setting) with a maximum accumulation time not to exceed 1 sec. Ions were passed to the Fourier-transfrom mass spectrometry (FTMS) cell, and full-scan spectra were acquired approximately every 3 sec. An instrument-resolving power of 100,000 was used for the collection of all full-scan spectra. The .RAW LC-MS data files are posted in the public TRANCHE repository https://proteomecommons.org/tranche/. High-resolution Obritrap ETD-MS/MS spectra were acquired either by data-dependent acquisition or by targeting a specific m/z as described in the SI Text.
Differential Mass Spectrometry Analysis of HDL Samples.
To identify proteins of interest (as defined by m/z ratio, intensity and retention time) we used dMS, a label-free LC-MS method for finding ions that exhibit a statistically significant difference in abundance in complex mixtures of peptides or proteins as previously described (8, 17, 18). Expression profiles generated from the high-resolution LC-MS data above were analyzed using the Rosetta Elucidator™ system (version 3.2) (40). The Peak Teller algorithm was used to align, measure, and extract m/z, retention time, and intensity data for the features contained in the dataset. We selected features readily measured by the mass spectrometer, AUC greater than zero value in all 18 measurements and then exported the data from Elucidator. The statistical analysis was performed on log AUC of the features. To properly distinguish between biological replicates (samples from different subjects) from technical replicates (aliquots of samples from the same subject), we first average the three technical replicates for each feature within each subject. Two-sample t tests were then performed on the three low HDL-c subjects (N = 3) vs. the three high HDL-c subjects, for each feature. Those features with p values lower than 0.02 for two or more isotopes and charge state 2+ or greater were selected (Table S1).
Protein Identification and Characterization.
ETD-MS/MS spectra were analyzed by ProsightPC v1.0 in order to identify the protein and to characterize posttranslational modifications (41). Briefly, ETD-MS/MS spectra were deisotoped to generate a list of monoisotopic, neutral fragment masses and then searched against a well-annotated human protein database (Uniprot) consisting of 130,248 protein sequences with known and potential posttranslational modifications to generate a total of 2,985,079 distinct protein forms (887 Mb). ProsightPC searches were performed using 2,000 Da and 20 ppm tolerances for precursor and fragment ion masses, respectively. Expectation values < 1 × 10-5 were manually verified and considered true positive identifications.
Supplementary Material
Acknowledgments.
The authors thank Betsy Frantz-Wattley for the preparation of HDL3 samples, Brian Hubbard and Thomas Roddy for their helpful discussions of the experiment, Yi Du and Fanyu Meng for the helpful comments on the manuscript, and Daniel Bloomfield and the CVD clinical team for helpful discussion on future next steps. We extend our special appreciation to Michele McColgan in Merck creative services for the expert assistance with the figures.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910776107/DCSupplemental.
References
- 1.Cravatt BF, Simon GM, Yates JR., III The biological impact of mass-spectrometry-based proteomics. Nature. 2007;450:991–1000. doi: 10.1038/nature06525. [DOI] [PubMed] [Google Scholar]
- 2.Domon B, Aebersold R. Mass spectrometry and protein analysis. Science. 2006;312:212–217. doi: 10.1126/science.1124619. [DOI] [PubMed] [Google Scholar]
- 3.Mann M, Hendrickson RC, Pandey A. Analysis of proteins and proteomes by mass spectrometry. Annu Rev Biochem. 2001;70:437–473. doi: 10.1146/annurev.biochem.70.1.437. [DOI] [PubMed] [Google Scholar]
- 4.de Godoy LM, et al. Comprehensive mass-spectrometry-based proteome quantification of haploid versus diploid yeast. Nature. 2008;455:1251–1254. doi: 10.1038/nature07341. [DOI] [PubMed] [Google Scholar]
- 5.Florens L, et al. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419:520–526. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- 6.Vaisar T, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everley PA, Krijgsveld J, Zetter BR, Gygi SP. Quantitative cancer proteomics: Stable isotope labeling with amino acids in cell culture (SILAC) as a tool for prostate cancer research. Mol Cell Proteomics. 2004;3:729–735. doi: 10.1074/mcp.M400021-MCP200. [DOI] [PubMed] [Google Scholar]
- 8.Zhao X, et al. Differential mass spectrometry of rat plasma reveals proteins that are responsive to 17beta-estradiol and a selective estrogen receptor modulator PPT. J Proteome Res. 2008;7:4373–4383. doi: 10.1021/pr800309z. [DOI] [PubMed] [Google Scholar]
- 9.McLafferty FW, Fridriksson EK, Horn DM, Lewis MA, Zubarev RA. BIOCHEMISTRY: Biomolecule Mass Spectrometry. Science. 1999;284:1289–1290. doi: 10.1126/science.284.5418.1289. [DOI] [PubMed] [Google Scholar]
- 10.Kelleher NL, et al. Top down versus bottom up protein characterization by tandem high-resolution mass spectrometry. J Am Chem Soc. 1999;121:806–812. [Google Scholar]
- 11.Siuti N, Kelleher NL. Decoding protein modifications using top-down mass spectrometry. Nat Methods. 2007;4:817–821. doi: 10.1038/nmeth1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikesh LM, et al. The utility of ETD mass spectrometry in proteomic analysis. BBA—Proteins Proteom. 2006;1764:1811–1822. doi: 10.1016/j.bbapap.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wysocki VH, Resing KA, Zhang Q, Cheng G. Mass spectrometry of peptides and proteins. Methods. 2005;35:211–222. doi: 10.1016/j.ymeth.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Zubarev RA, Kelleher NL, McLafferty FW. Electron capture dissociation of multiply charged protein cations. A nonergodic process. J Am Chem Soc. 1998;120:3265–3266. [Google Scholar]
- 15.Syka JE, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc Natl Acad Sci USA. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAlister GC, et al. A proteomics grade electron transfer dissociation-enabled hybrid linear ion trap-orbitrap mass spectrometer. J Proteome Res. 2008;7:3127–3136. doi: 10.1021/pr800264t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng F, et al. Quantitative analysis of complex peptide mixtures using FTMS and differential mass spectrometry. J Am Soc Mass Spectrom. 2007;18:226–233. doi: 10.1016/j.jasms.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Wiener MC, Sachs JR, Deyanova EG, Yates NA. Differential mass spectrometry: A label-free LC-MS method for finding significant differences in complex peptide and protein mixtures. Anal Chem. 2004;76:6085–6096. doi: 10.1021/ac0493875. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, et al. Quantification of proteins and metabolites by mass spectrometry without isotopic labeling or spiked standards. Anal Chem. 2003;75:4818–4826. doi: 10.1021/ac026468x. [DOI] [PubMed] [Google Scholar]
- 20.Olsson AG. Is high HDL cholesterol always good? Ann Med. 2009;41:11–18. doi: 10.1080/07853890802609534. [DOI] [PubMed] [Google Scholar]
- 21.Miller NE, Thelle DS, Forde OH, Mjos OD. The Tromso heart-study. High-density lipoprotein and coronary heart-disease: A prospective case-control study. Lancet. 1977;1:965–968. doi: 10.1016/s0140-6736(77)92274-7. [DOI] [PubMed] [Google Scholar]
- 22.Miller GJ, Miller NE. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1975;1:16–19. doi: 10.1016/s0140-6736(75)92376-4. [DOI] [PubMed] [Google Scholar]
- 23.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 24.Barr DP, RUSS EM, EDER HA. Protein-lipid relationships in human plasma. II. In atherosclerosis and related conditions. Am J Med. 1951;11:480–493. doi: 10.1016/0002-9343(51)90183-0. [DOI] [PubMed] [Google Scholar]
- 25.Pollin TI, et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–1705. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scanu A. Studies on the conformation of human serum high-density lipoproteins HDL2 and HDL3. Proc Natl Acad Sci USA. 1965;54:1699–1705. doi: 10.1073/pnas.54.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scanu AM, Edelstein C. HDL: Bridging past and present with a look at the future. FASEB J. 2008;22:4044–4054. doi: 10.1096/fj.08-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heinecke JW. The HDL proteome: A marker—and perhaps mediator—of coronary artery disease. J Lipid Res. 2009;50:S167–S171. doi: 10.1194/jlr.R800097-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onat A, et al. Apolipoprotein C-III, a strong discriminant of coronary risk in men and a determinant of the metabolic syndrome in both genders. Atherosclerosis. 2003;168:81–89. doi: 10.1016/s0021-9150(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 30.Kypreos KE. ABCA1 promotes the de Novo biogenesis of apolipoprotein CIII-containing HDL particles in vivo and modulates the severity of apolipoprotein CIII-induced hypertriglyceridemiaΓÇá. Biochemistry. 2008;47:10491–10502. doi: 10.1021/bi801249c. [DOI] [PubMed] [Google Scholar]
- 31.Fan YX, et al. Discovery and identification of potential biomarkers of papillary thyroid carcinoma. Mol Cancer. 2009;8:79. doi: 10.1186/1476-4598-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paweletz CP, et al. Surface enhanced laser desorption ionization spectrometry reveals biomarkers for drug treatment but not dose. Proteomics. 2006;6:2101–2107. doi: 10.1002/pmic.200500569. [DOI] [PubMed] [Google Scholar]
- 33.Wang N, et al. ATP-binding cassette transporters G1 and G4 mediate cholesterol and desmosterol efflux to HDL and regulate sterol accumulation in the brain. FASEB J. 2008;22:1073–1082. doi: 10.1096/fj.07-9944com. [DOI] [PubMed] [Google Scholar]
- 34.Yvan-Charvet L, et al. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chi A, Bai DL, Geer LY, Shabanowitz J, Hunt DF. Analysis of intact proteins on a chromatographic time scale by electron transfer dissociation tandem mass spectrometry. Int J Mass Spectrom. 2007;259:197–203. doi: 10.1016/j.ijms.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeda H, Hashimoto RK, Ogura T, Hiraga S, Uzawa H. Molecular cloning of a human apoC-III variant: Thr 74----Ala 74 mutation prevents O-glycosylation. J Lipid Res. 1987;28:1405–1409. [PubMed] [Google Scholar]
- 37.Harvey SB, et al. O-glycoside biomarker of apolipoprotein C3: Responsiveness to obesity, bariatric surgery, and therapy with metformin, to chronic or severe liver disease and to mortality in severe sepsis and graft vs host disease. J Proteome Res. 2009;8:603–612. doi: 10.1021/pr800751x. [DOI] [PubMed] [Google Scholar]
- 38.Chapman MJ, Goldstein S, Lagrange D, Laplaud PM. A density gradient ultracentrifugal procedure for the isolation of the major lipoprotein classes from human serum. J Lipid Res. 1981;22:339–358. [PubMed] [Google Scholar]
- 39.Smith PK, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 40.Paweletz CP, et al. Application of an end-to-end biomarker discovery platform to identify target engagement markers in cerebro spinal fluid by high resolution differential mass spectrometry. J Proteome Res. 2010;9:1392–1401. doi: 10.1021/pr900925d. [DOI] [PubMed] [Google Scholar]
- 41.Zamdborg L, et al. ProSight PTM 2.0: Improved protein identification and characterization for top down mass spectrometry. Nucleic Acids Res. 2007;35:W701–W706. doi: 10.1093/nar/gkm371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.