Abstract
Early B cell factor (EBF)1 is essential for B lineage specification. Previously, we demonstrated the synergistic activation of Cd79a (mb-1) genes by EBF1 and its functional partner, RUNX1. Here, we identified consequences of Ebf1 haploinsufficiency together with haploinsufficiency of Runx1 genes in mice. Although numbers of “committed” pro-B cells were maintained in Ebf1+/−Runx1+/− (ERhet) mice, activation of B cell-specific gene transcription was depressed in these cells. Expression of genes encoding Aiolos, κ0 sterile transcripts, CD2 and CD25 were reduced and delayed in ERhet pro-B cells, whereas surface expression of BP-1 was increased on late pro-B cells in ERhet mice. Late pre-B and immature and mature B cells were decreased in the bone marrow of Ebf1+/− (Ehet) mice and were nearly absent in ERhet mice. Although we did not observe significant effects of haploinsuficiencies on IgH or Igκ rearrangements, a relative lack of Igλ rearrangements was detected in Ehet and ERhet pre-B cells. Together, these observations suggest that B cell lineage progression is impaired at multiple stages in the bone marrow of Ehet and ERhet mice. Furthermore, enforced expression of EBF1 and RUNX1 in terminally differentiated plasmacytoma cells activated multiple early B cell-specific genes synergistically. Collectively, these studies illuminate the effects of reduced Ebf1 dosage and the compounding effects of reduced Runx1 dosage. Our data confirm and extend the importance of EBF1 in regulating target genes and Ig gene rearrangements necessary for B cell lineage specification, developmental progression, and homeostasis.
Keywords: B cell development, B lymphopoiesis, immunoglobulin gene rearrangements, transcription factor dosage, transcriptional networks
Multiple lines of evidence suggest that early B cell factor (EBF)1 (also known as EBF/O/E-1/COE1) is centrally important in B lineage specification (1). In EBF1 knockout mice, B cell development is arrested at an early progenitor stage (2). EBF1-deficient mice fail to rearrange Ig heavy (IgH) and light (IgL) chain genes and do not express essential proteins necessary for B cell development, e.g., Ig-α (Cd79a/mb-1), Ig-β (Cd79b/b29), CD19 (Cd19), λ5 (Igll1), VpreB1 (Vpreb1), Rag1 (Rag1), and Pax5 (Pax5). Evidence of EBF1’s primary role in B cell lineage specification includes its ability to drive the B cell fate at the expense of other cell lineages (3). Furthermore, B cell lineage development is promoted by EBF1 in the absence of upstream regulators including IL-7, IL-7 receptor α, PU.1, Ikaros, and E2A (4–9). Recent evidence suggests that EBF1 controls B lymphopoiesis in at least two ways: (i) by “pioneering” the activation of genes that are essential for the B lineage-specific program (10, 11) and (ii) by reinforcing B lineage commitment with Pax5 (12, 13).
RUNX1 (AML1/PEBP2αB/CBFα2) is a key regulator of hematopoiesis in multiple cell types of the blood (14). The absence of RUNX1 results in embryonic lethality due to a complete lack of hematopoiesis (15, 16). RUNX1 is required for the expression of Sfpi1 (PU.1), which in turn coordinates the development of myeloid cells, granulocytes, and lymphocytes (17). In T cell development, RUNX1 functions as both an activator and a repressor (18). The contributions of RUNX1 to B cell development have not been investigated extensively. RUNX1 is expressed in early B cell progenitors and in immature and mature B cells, where it increases cell survival (19, 20). The conditional ablation of Runx1 genes in adult mice reduces numbers of common lymphoid progenitors and B cells (21). Expression of a fusion protein comprising the partner protein of RUNX1, CBFβ, and smooth muscle myosin heavy chain (SMMHC) decreased numbers of pro-B and pre-B cells and transcripts of the Cd79a, Vpreb1, and Igll1 genes in mice (22). However, questions concerning the roles of RUNX1 in B cell development have not been addressed completely.
Previous work in our laboratory demonstrated DNA binding interactions between EBF1 and RUNX1, suggesting functional cooperation in vivo. EMSA and in vivo footprinting assays detected the binding of EBF1 and RUNX1 (with CBFβ) to tandem sites in the Cd79a promoter (23, 24). Coexpression of EBF1 and RUNX1 in terminally differentiated plasmacytoma cells reactivated Cd79a gene transcription in the context of hypermethylated DNA and inactive chromatin. EBF and RUNX1 function synergistically in B cells; however, little is understood concerning their coregulation of gene expression in these cells.
To determine the extent of gene regulation by EBF1 and RUNX1 in developing B cells, we turned to mice with reduced gene dosage of each factor. This step was necessitated by the extremely early arrest of B lymphopoiesis in Ebf1−/− mice and lack of hematopoiesis in Runx1−/− fetuses. To avoid these issues, we assessed the contributions of EBF1 and RUNX1 in the context of single and compound haploinsufficient mice. Such an approach reveals synergistic effects of reducing both genes simultaneously. Specific effects observed in compound, but not single, haploinsufficient mice, have provided evidence of cooperation between transcription factors and signaling proteins in multiple shared pathways (25, 26).
Unlike mice with homozygous knockouts of Ebf1 or Runx1 genes, Ebf1+/−Runx1+/− (ERhet) mice are viable, and analysis of B cells over the full course of their development is possible. B cell numbers are reduced greatly in the spleens and bone marrow of ERhet mice. The maintenance of pro-B cells and relative absence of pre-B and immature and mature B cells in the bone marrow of compound haploinsufficient animals indicate that EBF1 and RUNX1 are required for developmental transitions following the expression of Ig μ chains. Reasons for this impeded development include the reduced and/or delayed expression of stage-specific genes in pro-B cells, including Ikzf3 (Aiolos), κ0 sterile transcripts, Cd25 (CD25), and Cd2 (CD2). We confirmed that these genes are downstream targets of EBF1 and RUNX1 by activating their endogenous counterparts in plasmacytoma cells. Together, these data confirm the importance of synergistic interactions between EBF1 and RUNX1 and define new roles of EBF1 in B cell lineage specification and progression.
Results
Production of B Cells Is Defective in ERhet Mice.
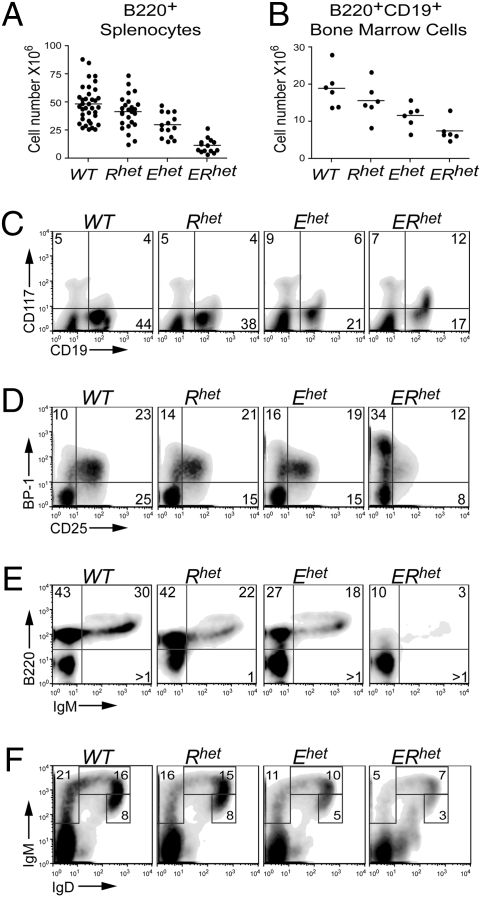
To evaluate the impact of EBF1 and RUNX1 haploinsufficiencies on B cell development in vivo, we generated WT (Wild Type), Rhet (Ebf1+/+Runx1+/−), Ehet (Ebf1+/−Runx1+/+), and ERhet mice. Flow cytometric analysis of B220+ cells isolated from spleens of adult mice (4–6 weeks) revealed only a small difference between total numbers of B cells in WT relative to Rhet spleens; however, splenic B cell numbers in Ehet mice were reduced by 62% (P = 0.0003) relative to those in WT mice (Fig. 1A). We observed an even greater reduction (by 75%; P = 1.4e-10) in B cell numbers in spleens of ERhet mice. The difference between Ehet and ERhet mice suggests that EBF1 and RUNX1 are both necessary to obtain normal numbers of peripheral B cells. In contrast, splenic T cell numbers were relatively unaffected by Ebf1 or Runx1 haploinsufficiencies (Fig. S1).
Fig. 1.
Development of bone marrow B cells in WT and haploinsufficient mice. All mice were 4–6 weeks old (n ≥ 5 per group). (A) Numbers of B220+ splenocytes as determined by flow cytometry. (B) Numbers of B220+CD19+ bone marrow cells as determined by flow cytometry. (C–F) Representative flow cytometry assessing (C) CD117 (c-kit) and CD19 expression on IgM– bone marrow cells, (D) BP-1 and CD25 expression on CD19+IgM– bone marrow cells, (E) B220 and IgM expression on CD43– bone marrow cells, and (F) IgM and IgD expression on CD19+ bone marrow cells.
We hypothesized that the reduction of B cell numbers in the spleen was a consequence of defective B lymphopoiesis in the bone marrow. To assess this, we used flow cytometry to evaluate the populations of bone marrow cells expressing the definitive marker of committed B cells, CD19. The numbers of CD19+ cells decreased to less than half between WT and ERhet mice (Fig.1B). Proportions of CD19+ cells were similar in the bone marrow of WT and Rhet mice (48% and 42%, respectively) (Fig.1C). In contrast, decreased proportions of CD19+ cells were observed in bone marrow from Ehet and ERhet mice (decreased from 48% to 27% and 29%, respectively) relative to that from WT mice (P = 0.015 and 0.001, respectively). An intermediate effect was observed in Ehet mice. Moreover, the composition of the CD19+ populations changed considerably between ERhet mice and the other genotypes. Coexpression of CD19 and CD117 (c-kit) indicates “committed” pro-B cells (27). ERhet mice exhibited 2- to 3-fold greater frequencies of CD19+CD117+ cells, although absolute numbers of these cells were relatively constant among the four genotypes (Fig.1C and Fig. S2A). The enhanced detection of CD117+ cells is likely due to decreased representation of later stages of B cell development in ERhet bone marrow.
The overall reductions in the numbers of CD19+ cells in the bone marrow of single and double haploinsufficient mice suggested an impediment in the generation of pre-B cells. To dissect the status of the pre-B cell compartment, we analyzed cell surface markers indicative of this bone marrow-derived population, BP-1 and CD25 (28–30). BP-1+CD25–CD19+IgM– cells represent late pro-B cells, whereas BP-1+CD25+CD19+IgM– cells represent the majority of pre-B cells. At a very late point in pre-B cell development, the cells become BP-1–CD25+CD19+IgM– cells. We detected increased expression of BP-1 and proportions of BP-1+CD25–CD19+IgM– cells in ERhet mice (Fig.1D); however, the numbers of these late pro-B cells were similar among all four genotypes (Fig. S2B). In contrast, both the proportions and the numbers of pre-B cells (BP-1+CD25+ and BP-1–CD25+) were reduced significantly (by 78 and 92%, respectively; P = 0.0008 and 0.0001) in ERhet mice. Intermediate effects were observed in Ehet bone marrow, which also exhibited approximately half the number of pre-B cells observed in WT mice (P = 0.03 and 0.001). Significantly reduced numbers of BP-1–CD25+CD19+ IgM– cells were detected as well in Rhet bone marrow (by 62%; P = 0.001). Furthermore, we assessed the fraction D pre-B cell compartment as defined by Hardy et al. (30). As indicated in Fig.1E, we detected a substantial reduction in B220+IgM–CD43– bone marrow cells (P = 0.04). Together, these data suggest an inability of pro-B cells to transition efficiently to later stages of B cell development due to compound effects of Rhet and Ehet haploinsufficiencies.
We next examined immature and mature B cells in the bone marrow of mice with each of the four genotypes. We used flow cytometry to delineate IgM+IgD–, IgMhiIgD+, and IgMloIgD+ B cells (Fig.1F). Reduced numbers of each of these populations were observed in mice with the mutant genotypes (Fig. S2C). The three IgM+ B cell populations were reduced slightly in Rhet bone marrow and dramatically in Ehet (60, 54, and 57%, respectively; P = 0.001, 0.01, 0.001) and ERhet bone marrow (91, 93, and 88%, respectively; all P < 0.0001). We conclude that the production of mIg+ B cells is defective in ERhet mice due to compound effects of Ebf1 and Runx1 haploinsufficiencies.
Haploinsufficiencies Alter Gene Expression in Ehet, Rhet, or ERhet Mice.
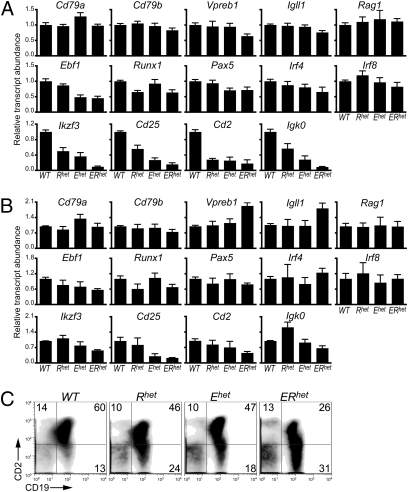
Because EBF1 and RUNX1 are transcription factors, alterations in pre-B and mIg+ cells in single and double haploinsufficient mice suggest perturbations of the expression of B cell stage-specific genes. We purified CD117+CD19+ pro-B or CD117–CD19+mIgM– late pro-B/pre-B cells and quantitated transcripts using quantitative real-time PCR (qRT-PCR). The assays focused on known targets of EBF1 and genes that reflect developmental transitions during B lymphopoiesis. Despite the presence of similar numbers of CD117+CD19+ cells in mice with each of the four genotypes, differences in gene expression were evident (Fig. 2A). The expression of known targets of EBF1 including Cd79a, Cd79b, the surrogate light (ψL) chain genes Vpreb1 and Igll1 (λ5), and Recombination activating gene 1 (Rag1) was largely unaffected in CD117+CD19+ cells isolated from WT, Rhet, or Ehet mice. However, significant reductions of Vpreb1 (36%; P = 0.0048) and Igll1 (25%; P = 0.0061) transcripts were detected in ERhet mice relative to WT mice. As expected, expression of Ebf1 and Runx1 was reduced (52–55% and 35–36%, respectively; P < 0.0034) in the context of the mutant alleles. No additional decrease in transcripts of either gene was evident in CD117+CD19+ cells of ERhet mice. Reduced expression of Pax5 transcripts (30%) was noted in CD117+CD19+ cells of Ehet and ERhet mice. A similar decrease in Irf4 transcripts (35%; P = 0.061) was noted in the cells from ERhet mice (changes in Irf8 were not significant). Transcripts of Ikzf3 (Aiolos), which is important for the development of pre-B cells (31, 32), were reduced by half in Rhet and Ehet mice and were nearly absent in CD117+CD19+ cells of ERhet mice (P < 0.0001). Other genes that encode markers of pre-B cells were expressed at low levels in CD117+CD19+ cells of each mutant genotype. Expression of Cd25 was reduced in Rhet (44%), Ehet (73%), and ERhet (84%; P < 0.0001) CD117+CD19+ cells relative to WT CD117+CD19+ cells. Expression of Cd2 genes was reducted in Rhet (72%), Ehet (75%), and ERhet (82%; P = 0.0021) CD117+CD19+ cells. Igκ0 sterile transcripts, which precede κ IgL gene rearrangements, were reduced greatly in Rhet (43%), Ehet (72%), and ERhet (91%; P < 0.0001) CD117+CD19+ cells. These findings suggest that pre-B cell stage-specific genes, which are expressed significantly in committed pro-B cells, are reduced in ERhet mice.
Fig. 2.
Expression of stage-specific markers in CD117+CD19+mIgM– and CD117–CD19+mIgM– bone marrow cells of WT and haploinsufficient mice. (A) Quantitative PCR (qRT-PCR) of transcripts in CD117+CD19+mIgM– “committed” pro-B cells (n = 3–5 mice per group). All transcript levels were normalized to levels of β-actin transcripts. (B) qRT-PCR of transcripts in CD117–CD19+mIgM– late pro-B/total pre-B cells (n = 3–5 mice per group). All transcript levels were normalized to levels of Hprt transcripts. (C) Analysis of CD2 and CD19 expression on B220+IgM– cells by flow cytometry.
We examined the same set of gene transcripts using cDNAs derived from CD117–CD19+mIgM– late pro-B/pre-B cells from mice with each of the four genotypes (Fig.2B). Several significant differences were noted in these cells in comparison with gene expression in CD117+CD19+ cells. First, levels of Vpreb1 and Igll1 transcripts were elevated (1.9- and 1.8-fold, respectively; P = 0.0043 and 0.025) in CD117–CD19+mIgM– cells of ERhet mice relative to WT mice. This likely reflects the skewing of these populations to early pre-B cells, which express pre-B cell receptors (pre-BCR). Interestingly, although Ebf1, Runx1, and other transcription factors show small differences in expression, these changes are muted in CD117–CD19+mIgM– cells relative to CD117+CD19+ cells. For example, differences in the expression of Ikzf3 transcripts are reduced, but only by 43%, between pre-B cells of WT and ERhet mice. Levels of Igκ0 sterile transcripts were affected less and even elevated (in Rhet mice). However, Cd25 transcripts were greatly reduced in Ehet and ERhet (by 70 and 77%; P = 0.028 and 0.013) mice.
Our data suggest that, with a few notable exceptions, cells that make the transition from pro-B to pre-B cells have activated genes of the pre-B stage-specific program successfully. To confirm that the generation of late pre-B cells is impeded in the mutant mice, we examined the expression of CD2 on B220+IgM– pre-B cells of mice with each of the four genotypes using flow cytometry (Fig.2C). CD2 is displayed on small resting pre-B cells in response to pre-BCR signaling (33, 34). In WT mice, CD19+CD2+ B cells constitute a discrete population including both late pre-B and mIgM+ cells. In Ehet and ERhet mice, CD2 expression is decreased on B220+IgM– cells expressing slightly higher levels of CD19. In ERhet mice this population includes a high proportion of CD19+ cells, which exhibit a continuous range of CD2 expression. These data suggest that the transition between pre-B and immature B cells is inefficient in Ehet and ERhet bone marrow, possibly from inadequate pre-BCR signaling.
Decreased Igλ Gene Rearrangements in Ehet and ERhet Mice.
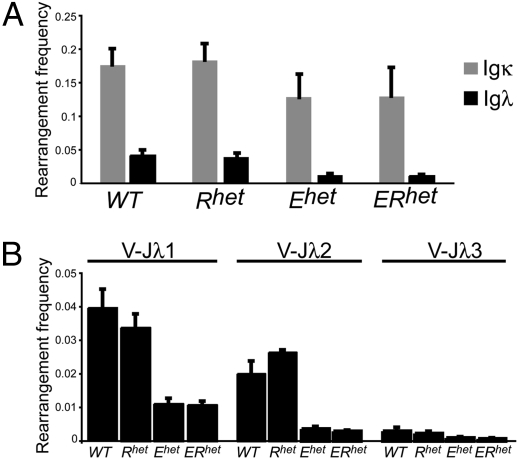
A hallmark of pre-B cell development is the activation of IgL gene rearrangements in response to pre-BCR signaling. Productive IgL gene rearrangements result in the replacement of ψ L chain proteins (VpreB1 and λ5) with κ or λ L chains, resulting in immature B cells expressing a mature BCR. To assess effects of Ebf1 and Runx1 haploinsufficiencies on the processes of IgL gene rearrangements, we isolated genomic DNA from purified CD117+CD19+ pro-B cells and used quantitative PCR assays to assess Ig heavy (IgH) chain gene rearrangements. We did not detect significant differences between frequencies and patterns of IgH rearrangements including proximal VH7183 or distal VHJ558 variable regions (Fig. S3). Next, we isolated genomic DNA from purified CD117–CD19+mIgM– pre-B cells for analysis of IgL chain gene rearrangements. Igκ rearrangements were decreased by 25% in Ehet and ERhet mice, but not in Rhet mice (Fig.3A). Igλ rearrangements were decreased more significantly, with reductions of 75% each in Ehet and ERhet mice (P = 0.0062 and 0.0018). The overall decrease in Igλ rearrangements included reduced utilization of each of the three available Jλ loci in Ehet mice (28.0, 18.0, and 31.6% of WT; P = 0.001, 0.011, and 0.059) and ERhet mice (27.0, 14.6, and 22.5% of WT; P = 0.0002, 0.0002, and 0.018) (Fig. 3B). We conclude that IgL chain, but not IgH chain, rearrangements are impaired in mice with a single functional Ebf1 allele.
Fig. 3.
Ig λ light chain gene rearrangements are reduced by Ebf1 haploinsufficiency. (A) Frequencies of Igκ and Igλ gene rearrangements in pre-B cells of WT and haploinsufficient mice. (B) Reduced frequencies of Igλ gene rearrangements including Jλ1, Jλ2, and Jλ3 in pre-B cells of Ehet and ERhet mice (n = 3 mice per group).
Early B Cell-Specific Genes Are Activated by EBF1 and RUNX1 in Terminally Differentiated Plasmacytoma Cells.
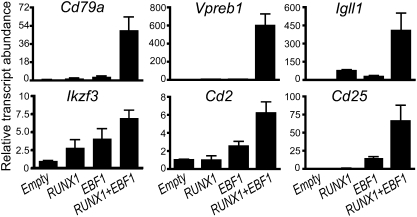
We demonstrated previously that retroviral expression of EBF1 and RUNX1 in μM.2 plasmacytoma cells activates endogenous Cd79a and Vpreb1 gene transcription (24, 35). To determine whether EBF1 and RUNX1 similarly regulate other genes that are decreased in ERhet mice, we infected μM.2 cells with retroviruses for expression of EBF1 or RUNX1, separately or simultaneously, and used qRT-PCR to measure the levels of specific transcripts at 48 h following infection (Fig. 4). Similar to previous results, Cd79a genes were activated only minimally by EBF1 or RUNX1 alone. However, in combination, they increased Cd79a transcripts by 49.7-fold (P = 0.0042). EBF1 and RUNX1 also strongly activated expression of Vpreb1 and Igll1 genes (596-fold and 405-fold, respectively; P = 0.011 and 0.052). As we have demonstrated previously for the Cd79a gene (24), this robust activity likely reflects the high affinity of EBF1 and RUNX1 for the promoters of these genes. Thus, it was not surprising that haploinsufficiency of EBF1 and RUNX1 resulted in minor changes in transcript levels of these genes (Fig.2 A and B). When expressed separately, RUNX1 or EBF1 activated Ikzf3 genes 2.8- or 4-fold, respectively, although synergistically increasing transcription of Ikzf3 genes 6.9-fold (P = 0.0004). Robust induction of two other markers of pre-B cells, Cd2 and Cd25, was dependent on cooperative effects of EBF1 and RUNX1 as well (6- and 66-fold, respectively; P = 0.0038 and 0.044). However, Cd2 and Cd25 transcripts were stimulated 2.5- and 15-fold, respectively, by EBF1 alone. Therefore, the combination of EBF1 and RUNX1 activates multiple silenced genes of the early B cell-specific program in terminally differentiated plasmacytoma cells. We conclude that this ability reflects the functional cooperation of EBF1 and RUNX1 in early B cell progenitors in the bone marrow.
Fig. 4.
Enforced expression of EBF1 and RUNX1 activates expression of early B cell-specific genes in plasmacytoma cells. μM.2 plasmacytoma cells were transduced with retroviruses for expression of EBF1 and/or RUNX1 as shown. Transduced cells were purified 48 h postinfection and analyzed using qRT-PCR. Data were normalized to control Hprt transcripts and represent three to five independent experiments.
Discussion
Here, we demonstrated the importance of regulatory gene dosage and functional cooperation between transcription factors in B lymphopoiesis. EBF1 drives expression of the B cell-specific transcriptional program in synergy with factors including PU.1, E2A, RUNX1, and Ikaros. The primary consequences of this process include the initiation of V(D)J recombination and assembly of the pre-BCR and BCR. Successful expression of these receptors allows cells to traverse checkpoints in developmental progression. Here, we demonstrated the importance of regulatory gene dosage and functional cooperation between transcription factors in B lymphopoiesis. B cell progenitors with lower than normal levels of EBF1 and RUNX1 are impeded during their transit through early B cell development, which results in greatly reduced pre-B and mIg+ B cells in the bone marrow and mature B cells in the periphery.
In the original study of mice with targeted Ebf1 alleles, Ebf1 haploinsufficiency resulted in a 50% decrease in Hardy fractions B and C and a 10–30% reduction in B cells in the spleen (2). It was found subsequently that the effects of Ebf1 haploinsufficiency are compounded by haploinsufficiency of Tcfe2a (encoding the E proteins E12 and E47) in Ebf1+/−Tcfe2a+/− (EThet) mice (25). Our studies revealed similarities and significant differences between ERhet and EThet mice. In both models, B cell development is impeded by the combination of haploinsufficient loci, with decreased pre-B and mIg+ cells in the bone marrow and spleen. However, unlike in EThet mice, numbers of pro-B cells were not grossly affected in ERhet mice. In EThet mice, late pre-B cell development was decreased, whereas mIgM+ cells were nearly absent. Analysis of transcript levels in purified pro-B cells of EThet mice demonstrated significant decreases in target genes including Cd79a, Cd79b, Igll1, Vpreb1, and Rag2. Pax5 expression was decreased to nearly background levels. In contrast, there was little or no change in the expression of Cd79a and Cd79b and there were moderate changes in expression of Igll1, Vpreb1, and Pax5 in pro-B cells of ERhet mice. Similar to that in EThet mice, we observed potent effects of Ebf1 haploinsufficiency on pre-B cells in ERhet mice. Although BP-1+CD25– cells were not reduced in number in ERhet mice, they expressed greatly increased levels of BP-1. Numbers of BP-1+CD25+ and BP-1–CD25+ pre-B cells were decreased profoundly in Ehet and ERhet mice. In part, reduction of these cells may be due to decreased expression of Cd25, which we identified as a transcriptional target of EBF1 and RUNX1. We also observed reduced numbers of B220+CD43–IgM– pre-B cells (Hardy fraction D). All mIg+ stages of bone marrow B cell development were reduced as well. This deficiency likely contributes to the dearth of peripheral B cells in young ERhet mice.
We also identified changes in the expression of transcription factors and target genes that impact the development of pre-B cells in ERhet mice. Irf4 transcripts were decreased modestly. Expression of Ikzf3 (Aiolos) was delayed, as indicated by its reduced expression in ERhet CD117+CD19+ cells. Whereas Ikzf3 expression initiates at the pro-B stage (32), its levels increase dramatically in pre-B cells, where it is important for silencing Igll1 gene transcription. The delay in activating Ikzf3 transcription could contribute to the increased expression of Igll1 transcripts in pre-B cells of these mice. Transcripts of genes that are expressed at high levels in WT pre-B cells, including Cd2, were reduced in CD117+CD19+ cells, but increased in cells that successfully progressed to pre-B cells. Interestingly, graded expression of CD2 was observed on the surface of ERhet pre-B cells. Together, the data suggest that signals originating from the pre-BCR in ERhet mice are insufficient to drive the up-regulation of genes including Ikzf3 and Cd2 in pre-B cells, resulting in delayed or unstable activation of the pre-B cell program. In explanation of these effects, substantial up-regulation of Ebf1 transcripts was detected in B220+CD43+CD24+BP-1+ cells (Hardy fraction C′) (30, 36). We propose that the low concentrations of EBF1 in Ehet and ERhet mice slow the transition from early to late pre-B cells and hence to immature B cells. These data are consistent with stochastic mechanisms that function at the pre-B cell developmental boundary. Subsequently, cells that do succeed in activating the pre-B cell program progress to immature and mature B cells. Furthermore, our data highlight difficulties with rigidly associating a scheme of stage-specific markers with developmental subpopulations of cells and the fluid process of lymphopoiesis.
Although significant differences in IgH or Igκ gene rearrangements were not detected, Igλ rearrangements were reduced significantly in Ebf1 haploinsufficient pre-B cells. This did not appear to be due to decreased Rag (i.e., Rag1) gene expression in pre-B cells; however, we cannot rule out decreased V(D)J recombinase activity in a select population of B cells. The reduction in Igλ gene rearrangements may be a consequence of (i) decreased expression of EBF1 itself, (ii) decreased expression of other transcription factors that are regulated by EBF1, and/or (iii) defective pre-BCR signaling in Ehet and ERhet pre-B cells. In regard to (i), ectopic expression of EBF1 in nonlymphoid cells preferentially induced Igλ rearrangements (37, 38). Together, these data suggest a role for EBF1 in the control of Igλ rearrangements.
Our data confirmed the importance of synergistic interactions between EBF1 and RUNX1 during early B cell development. We observed effects of Ebf1 and Runx1 haploinsufficiencies on previously unsuspected gene targets including Cd2, Cd25, and Ikzf3, which are expressed at low levels in CD117+CD19+ cells before their up-regulation in pre-B cells. Roles of EBF1 and RUNX1 in regulating these genes were confirmed by activation of their expression in μM.2 plasmacytoma cells. We demonstrated that EBF1 and RUNX1 activated Cd79a, Igll1, and Cd25 transcription synergistically in μM.2 cells. Surprisingly, Cd79a expression was unaffected in ERhet mice. The lack of effects may be due to the high degree of DNA binding cooperativity between EBF1 and RUNX1 on Cd79a promoters, which compensates for their reduced dosage. We conclude that expression of EBF1 and RUNX1 is sufficient for the reprogramming of early B cell-specific genes in terminally differentiated plasmacytoma cells. However, details of how EBF1 and RUNX1 activate these genes are currently unknown and will be the subject of future investigations.
The effects of Ebf1 and Runx1 haploinsufficiencies have implications for human disease. Haploinsufficiencies of Runx1 are associated with reduced hematopoietic stem cells in mice and familial thrombocytopenia and acute myelogenous leukemia in humans (39–41). Less is known concerning naturally occurring murine Ebf1 haploinsufficiency; however, somatic mutations in EBF1 genes are associated with human acute lymphoblastic leukemia (42). It will be interesting to determine whether these mutations result in phenotypes similar to those of Ehet and Rhet mice.
Materials and Methods
Mice.
Ebf1+/− mice were obtained on a mixed 129Sv/C57BL/6J background from Y. Zhuang (Duke University). Runx1+/− mice were obtained on a mixed 129Sv/C57BL/6J background from Dan Littman (New York University). Mice were backcrossed onto a C57BL/6 background for >10 generations. Genotyping of Ebf1 and Runx1 haploinsufficent mice was performed by PCR using the following primers: (Ebf1) 5′-GGAGCCTCACCATTGCTGTAGAG-3′, 5′ATGGCGATGCCTGCTTGCCGAATA-3′, and 5′-AAAACGAGCGGAACCCTACTTG-3′ and (Runx1) 5′-TTAGCAGTAGATAGGTATGAGTCCC-3′, 5′-AAGGGGGCTCACTTACTTCG-3′, and 5′-TCGCAGCGCATCGCCTTCTA-3′. Tail DNA was amplified for 28 cycles of 1 min at 94 °C, 1 min at 55 °C, and 2 min at 72 °C. Ebf1+/− mice were bred with Runx1+/− mice to generate compound haploinsufficent mice. Mice were bred and housed in the Biological Resources Center at National Jewish Health. All experiments received prior approval from the National Jewish Health Institutional Animal Care and Use Committee.
Antibodies and Flow Cytometry.
Cell staining was conducted using the following antibodies: CD45R (B220) (various fluorochromes) (Caltag Laboratories, eBiosciences, and BD Pharmingen); IgM-PE/Cy5 (Caltag Laboratories); CD117-PE-Cy5, CD25-Alexa Fluor 488, CD19-FITC/PE, CD2-FITC/PE, and CD117-FITC (eBioscience); CD19-APC, CD43-PE/APC, CD117-PE, IgM-FITC, and IgD-FITC (BD-Pharmingen); and IgM-Cy5 (Jackson Laboratories). Analytical flow cytometry was conducted using a CyAn cytometer. Data were analyzed using Summit (Dako Colorado) and FloJo software (Tree Star). Cell sorting was conducted using a MoFloXDP (Beckman Coulter) and analyzed using Summit software. For FACS analysis, bone marrow of adult mice (4–6 weeks) was harvested in Iscove's modified Dulbecco's medium (IMDM) (GIBCO/Invitrogen) supplemented with 1× Glutamax and 50 μg/mL gentamycin (Invitrogen) and 10% FBS (Biosource). Red blood cells were lysed in 1 mL of 144 mM NH4Cl, 13.3 mM Tris-HCl (pH 7.2) buffer for 10 min and quenched with 10 mL complete IMDM. Cells were harvested, washed with FACS buffer (FB) [1× PBS (pH 7.6), 0.5% BSA, 0.02% NaN3], and stained in FB containing antibodies and Fc block (anti-CD16) (BD-Pharmingen) at 20 × 106 cells/mL for 20 min at room temperature. Cells were washed three times with FB and analyzed. For cell sorting, bone marrow of adult mice (4–6 weeks) was harvested and treated as above; however, FB was replaced with MoFlo buffer [1× PBS (pH 7.6), 2% FBS]. Sorted cells were collected in a 50:50 mixture of complete IMDM and FBS.
Cell Transduction, RNA Isolation, and qRT-PCR.
μM.2 plasmacytoma cells were grown and transduced as described previously (35). Sequences of primers used for qRT-PCR are in Table S1. Sorted cells were washed once with 1 mL of 1× PBS, 2 mM EDTA, 0.5% BSA. RNA was isolated using a PicoPure RNA Isolation Kit (Arcturus) according to the manufacturer's instructions. Preparation of cDNA used 2 μL of RNA eluate in the recommended reaction mixture for SuperScript II RT (Invitrogen). The mixture was incubated at 37 °C for 1 h and quenched with 1.4 mM EDTA/0.6 μg tRNA at 90 °C for 10 min. qRT-PCR used 12.5 μL SYBR Green Master Mix (Applied Biosystems), 50 nM primers, and 2 μL of cDNA per 25-μL sample, amplified and detected using an Applied Biosystems 7300 system. All samples were evaluated in triplicate. Data were analyzed for significance using Student's two-tailed t test.
Quantitative Analysis of Ig Gene Rearrangements.
B cell populations were purified as above. Cells were snap frozen and lysed in 10 mM Tris-HCl, pH 8.3. Cell lysates were treated with 100 μg of proteinase K at 56 °C for 2 h. Proteinase K was inactivated at 95 °C for 10 min. Optical density of DNA samples was measured at 260 nm. Semiquantitative analysis of IgH gene rearrangements was performed using AmpliTaq Gold polymerase (Applied Biosystems) with primers listed in Table S1. DNA amplification was carried out in a final volume of 50 μL containing 15 mM Tris-HCl (pH 8.05), 50 mM KCl, 1.5 mM MgCl2, 200 mM dNTPs, 0.2 μM of each primer, 1.25 units of AmpliTaq polymerase, and 50 ng of template DNA. qPCR conditions were 10 min at 95 °C and 28 cycles of 30 sec at 94 °C, 30 sec at 60 °C, and 2 min at 72 °C. Ten-microliter aliquots of PCR were taken at 28, 32, and 38 cycles. PCR products were run on a 1.2% agarose gel and stained with EtBr. Quantitative analysis of κ, λ1, λ2, and λ3 rearrangements was performed by real-time PCR using a QuantiTect SYBR Green PCR Kit (Qiagen), using 0.2 μM of each primer and 10–50 ng of DNA template. Samples were amplified on an ABI7900 HT real-time PCR system (Applied Biosystems) and data were analyzed using SDS 2.1 software. Igκ, λ1, λ2, and λ3 (IgL) rearrangement frequencies were calculated as 2ΔCt with ΔCt = CtActin – CtIgL.
Supplementary Material
Acknowledgments
We thank Cornelis Murre (University of California, San Diego) and Holly Maier and James DeGregori (University of Colorado, Denver) for helpful discussions, Mikael Sigvardsson (Linköping University) and John Cambier (National Jewish Health) for critically reading the manuscript, and H. Singh for sharing unpublished data. We are indebted to Yuan Zhuang, Rudolf Grosschedl, Dan Littman, and Nancy A. Speck for providing the Ebf1+/− and Runx1+/− mice. J.H. was supported by National Institutes of Health Grants R01 AI54661 and P01 AI22295 and by a generous award from the Rocky Mountain Chapter of the Arthritis Foundation. S.F. and J.R. were supported by National Institutes of Health Postdoctoral Training Grant 5 T32 AI07405. A.J.F. and M.C. were supported by National Institutes of Health Grant R01 AI29672.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1003525107/DCSupplemental.
References
- 1.Nutt SL, Kee BL. The transcriptional regulation of B cell lineage commitment. Immunity. 2007;26:715–725. doi: 10.1016/j.immuni.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Lin H, Grosschedl R. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature. 1995;376:263–267. doi: 10.1038/376263a0. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Z, Cotta CV, Stephan RP, deGuzman CG, Klug CA. Enforced expression of EBF in hematopoietic stem cells restricts lymphopoiesis to the B cell lineage. EMBO J. 2003;22:4759–4769. doi: 10.1093/emboj/cdg464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seet CS, Brumbaugh RL, Kee BL. Early B cell factor promotes B lymphopoiesis with reduced interleukin 7 responsiveness in the absence of E2A. J Exp Med. 2004;199:1689–1700. doi: 10.1084/jem.20032202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medina KL, et al. Assembling a gene regulatory network for specification of the B cell fate. Dev Cell. 2004;7:607–617. doi: 10.1016/j.devcel.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Dias S, Silva H, Jr, Cumano A, Vieira P. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J Exp Med. 2005;201:971–979. doi: 10.1084/jem.20042393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kikuchi K, Lai AY, Hsu C-L, Kondo M. IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF. J Exp Med. 2005;201:1197–1203. doi: 10.1084/jem.20050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kikuchi K, Kasai H, Watanabe A, Lai AY, Kondo M. IL-7 specifies B cell fate at the common lymphoid progenitor to pre-proB transition stage by maintaining early B cell factor expression. J Immunol. 2008;181:383–392. doi: 10.4049/jimmunol.181.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynaud D, et al. Regulation of B cell fate commitment and immunoglobulin heavy-chain gene rearrangements by Ikaros. Nat Immunol. 2008;9:927–936. doi: 10.1038/ni.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagman J, Lukin K. Early B-cell factor ‘pioneers’ the way for B-cell development. Trends Immunol. 2005;26:455–461. doi: 10.1016/j.it.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Gao H, et al. Opposing effects of SWI/SNF and Mi-2/NuRD chromatin remodeling complexes on epigenetic reprogramming by EBF and Pax5. Proc Natl Acad Sci USA. 2009;106:11258–11263. doi: 10.1073/pnas.0809485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pongubala JM, et al. Transcription factor EBF restricts alternative lineage options and promotes B cell fate commitment independently of Pax5. Nat Immunol. 2008;9:203–215. doi: 10.1038/ni1555. [DOI] [PubMed] [Google Scholar]
- 13.Thal MA, et al. Ebf1-mediated down-regulation of Id2 and Id3 is essential for specification of the B cell lineage. Proc Natl Acad Sci USA. 2009;106:552–557. doi: 10.1073/pnas.0802550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Speck NA. Core binding factor and its role in normal hematopoietic development. Curr Opin Hematol. 2001;8:192–196. doi: 10.1097/00062752-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, et al. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci USA. 1996;93:3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada H, et al. AML1(-/-) embryos do not express certain hematopoiesis-related gene transcripts including those of the PU.1 gene. Oncogene. 1998;17:2287–2293. doi: 10.1038/sj.onc.1202151. [DOI] [PubMed] [Google Scholar]
- 18.Taniuchi I, et al. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell. 2002;111:621–633. doi: 10.1016/s0092-8674(02)01111-x. [DOI] [PubMed] [Google Scholar]
- 19.North TE, Stacy T, Matheny CJ, Speck NA, de Bruijn MF. Runx1 is expressed in adult mouse hematopoietic stem cells and differentiating myeloid and lymphoid cells, but not in maturing erythroid cells. Stem Cells. 2004;22:158–168. doi: 10.1634/stemcells.22-2-158. [DOI] [PubMed] [Google Scholar]
- 20.Blyth K, et al. Runx1 promotes B-cell survival and lymphoma development. Blood Cells Mol Dis. 2009;43:12–19. doi: 10.1016/j.bcmd.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Growney JD, et al. Loss of Runx1 perturbs adult hematopoiesis and is associated with a myeloproliferative phenotype. Blood. 2005;106:494–504. doi: 10.1182/blood-2004-08-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo YH, Gerstein RM, Castilla LH. Cbfbeta-SMMHC impairs differentiation of common lymphoid progenitors and reveals an essential role for RUNX in early B-cell development. Blood. 2008;111:1543–1551. doi: 10.1182/blood-2007-07-104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sigvardsson M, et al. Early B-cell factor, E2A, and Pax-5 cooperate to activate the early B cell-specific mb-1 promoter. Mol Cell Biol. 2002;22:8539–8551. doi: 10.1128/MCB.22.24.8539-8551.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maier H, et al. Early B cell factor cooperates with Runx1 and mediates epigenetic changes associated with mb-1 transcription. Nat Immunol. 2004;5:1069–1077. doi: 10.1038/ni1119. [DOI] [PubMed] [Google Scholar]
- 25.O'Riordan M, Grosschedl R. Coordinate regulation of B cell differentiation by the transcription factors EBF and E2A. Immunity. 1999;11:21–31. doi: 10.1016/s1074-7613(00)80078-3. [DOI] [PubMed] [Google Scholar]
- 26.Ma L, et al. Genetic analysis of Pten and Tsc2 functional interactions in the mouse reveals asymmetrical haploinsufficiency in tumor suppression. Genes Dev. 2005;19:1779–1786. doi: 10.1101/gad.1314405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delogu A, et al. Gene repression by Pax5 in B cells is essential for blood cell homeostasis and is reversed in plasma cells. Immunity. 2006;24:269–281. doi: 10.1016/j.immuni.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Cooper MD, Mulvaney D, Coutinho A, Cazenave PA. A novel cell surface molecule on early B-lineage cells. Nature. 1986;321:616–618. doi: 10.1038/321616a0. [DOI] [PubMed] [Google Scholar]
- 29.Rolink A, Grawunder U, Winkler TH, Karasuyama H, Melchers F. IL-2 receptor alpha chain (CD25, TAC) expression defines a crucial stage in pre-B cell development. Int Immunol. 1994;6:1257–1264. doi: 10.1093/intimm/6.8.1257. [DOI] [PubMed] [Google Scholar]
- 30.Hardy RR, Kincade PW, Dorshkind K. The protean nature of cells in the B lymphocyte lineage. Immunity. 2007;26:703–714. doi: 10.1016/j.immuni.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Morgan B, et al. Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation. EMBO J. 1997;16:2004–2013. doi: 10.1093/emboj/16.8.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson EC, et al. Ikaros DNA-binding proteins as integral components of B cell developmental-stage-specific regulatory circuits. Immunity. 2007;26:335–344. doi: 10.1016/j.immuni.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Sen J, Rosenberg N, Burakoff SJ. Expression and ontogeny of CD2 on murine B cells. J Immunol. 1990;144:2925–2930. [PubMed] [Google Scholar]
- 34.Young F, et al. Influence of immunoglobulin heavy- and light-chain expression on B-cell differentiation. Genes Dev. 1994;8:1043–1057. doi: 10.1101/gad.8.9.1043. [DOI] [PubMed] [Google Scholar]
- 35.Fields S, et al. The ‘zinc knuckle’ motif of Early B cell Factor is required for transcriptional activation of B cell-specific genes. Mol Immunol. 2008;45:3786–3796. doi: 10.1016/j.molimm.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roessler S, et al. Distinct promoters mediate the regulation of Ebf1 gene expression by interleukin-7 and Pax5. Mol Cell Biol. 2007;27:579–594. doi: 10.1128/MCB.01192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romanow WJ, et al. E2A and EBF act in synergy with the V(D)J recombinase to generate a diverse immunoglobulin repertoire in nonlymphoid cells. Mol Cell. 2000;5:343–353. doi: 10.1016/s1097-2765(00)80429-3. [DOI] [PubMed] [Google Scholar]
- 38.Goebel P, et al. Localized gene-specific induction of accessibility to V(D)J recombination induced by E2A and early B cell factor in nonlymphoid cells. J Exp Med. 2001;194:645–656. doi: 10.1084/jem.194.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.North TE, et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- 40.Song W-J, et al. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat Genet. 1999;23:166–175. doi: 10.1038/13793. [DOI] [PubMed] [Google Scholar]
- 41.Matheny CJ, et al. Disease mutations in RUNX1 and RUNX2 create nonfunctional, dominant-negative, or hypomorphic alleles. EMBO J. 2007;26:1163–1175. doi: 10.1038/sj.emboj.7601568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mullighan CG, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.