Abstract
Previously we showed that the ~2% of fetal liver cells reactive with an anti-CD3ε monoclonal antibody support ex vivo expansion of both fetal liver and bone marrow hematopoietic stem cells (HSCs); these cells express two proteins important for HSC ex vivo expansion, IGF2, and angiopoietin-like 3. Here we show that these cells do not express any CD3 protein and are not T cells; rather, we purified these HSC-supportive stromal cells based on the surface phenotype of SCF+DLK+. Competitive repopulating experiments show that SCF+DLK+ cells support the maintenance of HSCs in ex vivo culture. These are the principal fetal liver cells that express not only angiopoietin-like 3 and IGF2, but also SCF and thrombopoietin, two other growth factors important for HSC expansion. They are also the principal fetal liver cells that express CXCL12, a factor required for HSC homing, and also α-fetoprotein (AFP), indicating that they are fetal hepatic stem or progenitor cells. Immunocytochemistry shows that >93% of the SCF+ cells express DLK and Angptl3, and a portion of SCF+ cells also expresses CXCL12. Thus SCF+DLK+ cells are a highly homogenous population that express a complete set of factors for HSC expansion and are likely the primary stromal cells that support HSC expansion in the fetal liver.
Keywords: bone marrow transplantation, growth factors, hematopoiesis, stem cell niche
In adult animals, hematopoietic stem cells (HSCs) are normally quiescent. Following mitosis, on average at least one of the daughter cells becomes an HSC, and thus the number of HSCs remains constant over the animal’s lifetime. In contrast, in the fetal liver HSCs undergo a dramatic expansion between day 12 and 16. HSCs normally reside in specialized microenvironments—HSC niches—created by surrounding stromal cells (1–3). In the bone marrow there are thought to be at least two spatially and likely functionally distinct bone marrow HSC niches (2). Osteoblasts are the main supportive cell type in the endosteal HSC niche; osteoblasts produce many factors that are important in maintenance of hematopoiesis and the endosteal HSC niche is thought to contain mostly quiescent HSCs (3). The involvement of osteoblasts in HSC regulation was suggested by findings that animals with constitutively active parathyroid hormone receptor (PTHr) or PTH-related protein receptor (PPR), and therefore increased numbers of osteoblasts, have increased numbers of HSCs (4). In the bone marrow and spleen, many HSCs are associated with the sinusoidal endothelium (5). The stromal cells in that niche have not been identified, but it is hypothesized that HSCs in this niche constantly self-renew to maintain HSC numbers and at the same time contribute to normal hematopoiesis through asymmetrical divisions.
In the fetal liver, HSCs undergo a dramatic expansion between days 12 and 16. Several years ago we used a monoclonal antibody supposedly specific for the T-cell protein CD3e to purify from embryonic day 15.5 (E15.5) fetal livers a population of cells that supports expansion of HSC numbers in a culture medium that contains added stem cell factor (SCF), Flt3, and IL-6 (6). DNA array experiments showed that, among other proteins, insulin-like growth factor 2 (IGF2) and angiopoietin-like proteins 2 and 3 (Angptl2 and Angptl3) are specifically expressed in these CD3+ cells but not in several cells that do not support HSC expansion (6, 7). Based on this finding, we developed a simple, serum-free culture system that contains low levels of SCF, TPO, fibroblast growth factor–1 (FGF-1), IGF2, and either Angptl2 or Angptl3, which resulted in a 30-fold expansion of murine HSCs ex vivo (7). A similar “cocktail” resulted in a >20-fold increase in human cord blood HSC numbers after culture (8).
Here we show that these cells do not express any CD3ε protein and are not T cells; rather, we show that they are of hepatic lineage. They are the principal cell type in the fetal liver that expresses not only angiopoietin-like 3 and IGF2 but also SCF and thrombopoietin, two other growth factors important for HSC expansion. They are also the principal cells that express CXCL12, a factor required for HSC homing, and α-fetoprotein (AFP), a marker protein for fetal hepatic stem and progenitor cells.
Results
Because SCF and TPO are essential for HSC self-renewal (9), we were interested in determining which, if any, fetal liver cells produced these growth factors. In preliminary studies we used real-time PCR to examine the expression of many growth factors in different fetal liver cell populations characterized by specific lineage markers. Figure S1 shows that, when compared with several other nonHSC-supportive cell populations, fetal liver cells reactive with the CD3ε monoclonal antibody are enriched not only for the expression of the mRNAs encoding Angptl3 and IGF2, as shown previously, but also for SCF and TPO. The fifth growth factor used in our “cocktail,” FGF-1, is not expressed by these FL CD3+ cells. However, FGF-1 is not essential for ex vivo HSC expansion, as FL CD3+ cells expanded HSCs in a culture medium without FGF-1, and a combination of SCF, TPO, and IGF2 without FGF-1 also expanded HSCs (6). Thus, fetal liver CD3+ cells express four growth factors—SCF, TPO, Angptl3, and IGF2—that together support extensive HSC expansion.
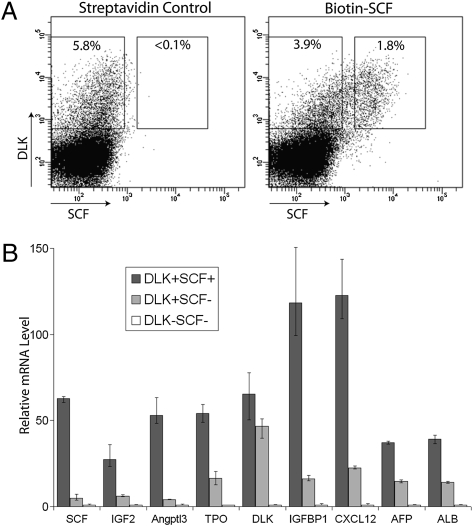
Because of the low apparent level of CD3 expressed on these fetal liver cells (Fig. S1) and because using RT-PCR we were unable to detect the expression of CD3ε, the protein targeted by the monoclonal anti-CD3 antibodies that we were using, in these CD3+ cells, we concluded the CD3+ surface phenotype of these cells is likely an artifact even though the antibody is selecting a discrete population of ~1–2% of fetal liver cells. Thus we sought to use other methods to purify these presumed stromal cells. SCF is fabricated as a transmembrane plasma membrane protein that normally binds to its receptor, c-Kit, on the surface of adjacent cells (2, 10–12). As all HSCs in fetal liver express c-Kit (13), and as the fetal liver–supportive “CD3+” cells are enriched for the expression of SCF mRNA (Fig. S1), we presumed that HSC stromal cells would express SCF on their surface. To test whether these cells can be purified by their surface SCF expression, we stained total fetal liver E15.5 cells with a biotin-conjugated SCF antibody followed by APC-conjugated streptavidin; control cells were stained with APC-streptavidin only. FACS analysis showed that the biotin-SCF antibody clearly stained a population of ~2% of fetal liver cells compared with the streptavidin control sample (Fig. 1 and Fig. S2A, Panel 2 and 3).
Fig. 1.
Potential stromal cells for HSC expansion in E15.5 fetal liver can be purified by SCF+DLK+ surface phenotype. (A) FACS analysis of total fetal liver cells stained with biotin-SCF and DLK antibodies. Approximately 1.8% fetal liver cells are SCF+, most of which are also DLK+. Approximately 3.9% of fetal liver cells are DLK+SCF–. (B) Relative expression of mRNAs encoding HSC expansion factors IGF2, Angptl3, TPO, IGFBP1, and CXCL12 and hepatocyte markers AFP and ALB. SCF+DLK+, SCF–DLK+, and SCF–DLK– cells were sorted by flow cytometry. The mRNA level of each gene was determined by qPCR and normalized against ribosomal RNA. The relative expression of each gene in each population was calculated, setting the level of each mRNA in SCF–DLK– cells as 1 (SCF+ cells were gated more stringently in this analysis than in Fig. S2A). Numbers are average of several individual repeats (n = 3); error bars indicate maximal and minimal values scored.
Next we sorted SCF+ cells and SCF– cells and analyzed their mRNA expression by real-time PCR. As anticipated, SCF+ cells were enriched >20-fold for mRNAs encoding SCF compared with SCF– cells (Fig. S2B), which affirms that the antibody correctly and specifically recognizes membrane-bound SCF on the surface of SCF+ fetal liver cells. Moreover, the mRNAs for other key HSC expansion factors, namely, IGF2, Angptl3, and TPO, were also enriched to a similar extent in these fetal liver SCF+ cells (Fig. S2B). This result indicates the potential stromal cells previously identified as “CD3+” can be more efficiently purified by their SCF+ surface phenotype.
DLK is another membrane-bound cytokine that we previously identified as being enriched in the fetal liver CD3+-supportive cells (6). DLK was also shown to promote the maintenance and self-renewal of HSCs (14, 15), and DLK mRNA is also enriched in fetal liver SCF+ cells (Fig. S2B). FACS analysis with an FITC-conjugated DLK antibody showed that >5% E15.5 fetal liver cells are DLK+, which is similar to that reported for E14 rat fetal liver cells (16). Approximately 2% (varies between 1% and 2% in different samples) of E15.5 fetal liver cells are both SCF+ and DLK+ (Fig. 1A); importantly, the vast majority of SCF+ cells are also DLK+.
Thus, we purified SCF+DLK+, SCF–DLK+, and SCF–DLK– cells by flow cytometry and analyzed the levels of mRNAs that encode several relevant cytokines (Fig. 1B). As expected, SCF mRNA is highly enriched in SCF+DLK+ cells, and SCF+DLK+ and SCF–DLK+ cells have similar elevated levels of DLK mRNA relative to SCF–DLK– cells. Importantly, mRNAs encoding IGF2, Angptl3 and TPO are highly enriched in SCF+DLK+ cells relative to SCF–DLK+ and SCF–DLK– cells. Comparing SCF+DLK+ with SCF–DLK– cells, the enrichments of these mRNAs average >50-fold. Furthermore, these SCF+DLK+ cells are highly enriched for the expression of CXCL12, a chemoattractant for HSCs (17). CXCL12 is secreted by stromal cells in bone marrow and regulates trafficking of HSCs (18). SCF+DLK1+ cells are also highly enriched for expression of IGFBP1. IGFBP1 shares significant similarity (34.3% identity) with another IGF binding protein family member, IGFBP2, which we previously showed is able to support ex vivo HSC expansion (19). Since IGFBP2 is very poorly expressed in fetal liver, it is possible that IGFBP1 plays a role in stimulating the expansion of fetal liver HSCs. Thus SCF+DLK1+ cells are the principal cells in fetal liver that synthesize seven cytokines that support HSC maintenance, expansion, and trafficking.
Figure 2B shows that sorted SCF+DLK1+ cells are able to support HSC maintenance using an ex vivo coculture method similar to that in our earlier study of fetal liver CD3+ cells (6). A total of 25 sorted CD150+CD48–CD41– HSCs from E15.5 fetal liver were cocultured with or without 2,000 sorted E15.5 fetal liver SCF+DLK1+ cells for 4 days in a serum-containing medium with added SCF, IL6, and FLT3. The content of each well was transplanted into lethally irradiated mice competitively with freshly isolated bone marrow cells from CD45.1 mice. Figure 2B shows that HSCs cultured alone almost completely lost HSC activity (average 0.8% repopulation, n = 8), whereas HSCs cocultured with SCF+DLK1+ cells (Fig. 2C) maintained HSC repopulating activity (average 16% repopulation, n = 9) at a level similar to that of uncultured HSCs (average 20% repopulation, n = 9) (Fig. 2A). Donor-derived CD45.2+ cells in the peripheral blood showed multilineage reconstitution, as determined by FACS analysis using antibodies specific for B220 (B cells), CD3 (T cells), CD11b (monocytes), and Gr-1 (granulocytes) (Fig. S3), thus indicating that they derived from repopulating HSCs.
Fig. 2.
Coculture of purified fetal liver HSCs with fetal liver E15.5 SCF+DLK+ cells. A total of 25 sorted E15.5 fetal liver HSCs (CD45.2) were cultured in a serum-containing medium supplemented with SCF, IL6, and FLT3 for 4 days either without (B) or with (C) 2,000 sorted E15.5 SCF+DLK+ cells. A total of 25 sorted HSCs were also mixed with 2,000 sorted fetal liver E15.5 SCF+DLK+ cells and were transplanted directly into CD45.1 mice as a control (A). Each filled triangle in the figure represents the percentage repopulation of one recipient mouse, which is calculated as percentage of CD45.2+ cells divided by total number of CD45.1+ plus CD45.2+ cells in the peripheral blood. Average percentage of reconstitution for each experiment is also indicated (n = 7–9).
We also cultured and transplanted 2,000 SCF+DLK1+ cells and observed no repopulating activity, excluding the possibility of contaminating HSCs from the sorted SCF+DLK1+ cells. This result clearly demonstrated the ability of SCF+DLK1+ cells to maintain HSCs in culture, a result similar that obtained with the CD3+ cells. This further establishes that the CD3+ and SCF+DLK1+ cells have, as expected, similar supportive activities.
DLK in fetal liver is expressed by hepatic stem and progenitor cells that are progenitors of both hepatocytes and biliary epithelial cells; a DLK antibody has been used to highly enrich these cells (16, 20). The majority of rat E14.5 fetal liver DLK+ cells also stain positive for AFP, a specific maker for hepatic stem and progenitor cells (16). Our data show that SCF+DLK1+ cells are highly enriched for mRNAs encoding both AFP and albumin (ALB), another specific maker for both hepatic stem/progenitors and mature hepatocytes; SCF–DLK+ cells express significant but lower levels of ALB and AFP mRNAs (Fig. 1B). Thus, the SCF+DLK+ putative stromal cells likely are a subset of fetal hepatic stem or progenitor cells that express AFP and ALB at a high level.
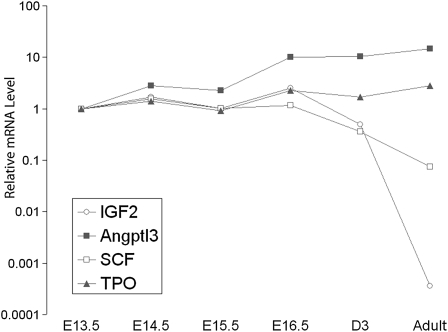
Figure 3 shows that the total mRNA levels of the HSC expansion factors SCF, TPO, and Angptl3 increased gradually from E13.5–16.5 in fetal liver. Expression of TPO and Angptl3 mRNA in liver remained high after birth and was even higher in adult liver (Fig. 2). In adults, TPO indeed is produced mainly by hepatocytes and supposedly reaches bone marrow hematopoietic cells via the circulation (21). The data in Fig. 2 suggest that, in adults, hepatocytes are also the source of Angptl3. Interestingly, expression of the two HSC expansion factors, SCF and DLK, which normally signal in a paracrine fashion while tethered to the plasma membrane, decline in liver soon after birth, as does expression of the imprinted IGF2 gene. Presumably, in adults, when bone marrow HSCs need to be expanded, some as-yet-unidentified cell type produces SCF and DLK to support expansion of adjacent HSCs, whereas secreted HSC expansion factors such as TPO and Angptl3 are produced by liver or other types of cells.
Fig. 3.
Expression of HSC expansion factors during liver development. Total RNAs were extracted from fetal liver E13.5–16.5 cells, neonatal liver (D3), and adult liver. The mRNA level of each gene was normalized to 18S RNA and the expression level of each gene at E13.5 was set as 1 (n = 3).
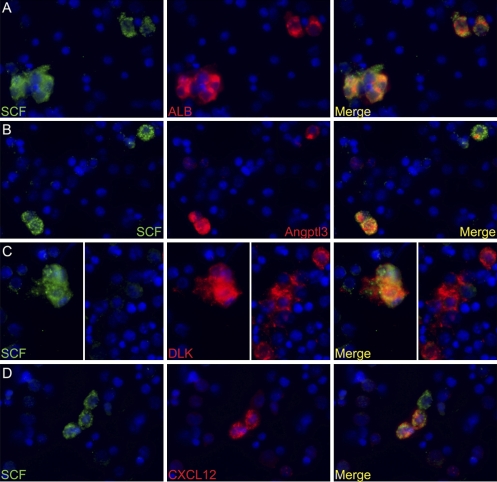
To determine whether fetal liver SCF+DLK+ cells are homogenous and whether they are indeed hepatic cells, we analyzed unfractionated fetal liver cells by dual immunocytochemistry for SCF together with DLK, ALB, Angptl3, or CXCL12. Approximately 2% of total fetal liver cells stain positive for SCF (FITC channel; green in Fig. 4). We then examined each of ~100 SCF+ cells to determine whether they are also positive for DLK, ALB, Angptl3, or CXCL12 (Rhodamine Red X channel). Figure 4 shows representative images of the dual staining. Table 1 shows the result of analysis of ~100 SCF+ cells for their ability to express the second marker protein. The data in Fig. 4 A–C and Table 1 show that >93% of the SCF+ cells in E15.5 fetal liver are also positive for ALB, Angptl3, and DLK expression. This indicates the SCF+DLK+ cells are homogenous for ALB and Angptl3 expression. In contrast, only ~34% of the SCF+ cells stain for CXCL12 and thus likely express this cytokine. We found that ~80% (27/34) of the CXCL12+ cells are also positive for SCF expression, indicating that the CXCL12+ cells are mostly a subpopulation of SCF+ fetal liver cells. These results establish that these supportive cells for HSC expansion are indeed a mostly homogenous population of hepatic lineage. Consistent with this conclusion, costaining of SCF and CD45 antibodies shows that SCF+ and CD45+ cells are mutually exclusive of each other, proving that SCF+ cells are not of a hematopoietic lineage (Fig. S4 and Table 1).
Fig. 4.
SCF+ cells in E15.5 fetal liver are also positive for ALB, Angptl3, and DLK expression but are heterogeneous for CXCL12 expression. (A) Double immunocytochemistry for SCF (green) and ALB (red) expression in total fetal liver cells. DAPI was used to stain nuclei (blue). SCF+ cells are also positive for ALB expression. (B) Double staining for SCF and Angptl3. Three SCF+ cells are shown, all of which are positive for Angptl3 expression. (C) Double staining for SCF and DLK. (Left) Cluster of SCF+ cells that are also DLK+. DLK is also expressed by other cells, as indicated (Right) by a group of DLK+ cells that are SCF–. (D) Only a fraction of SCF+ cells are also CXCL12+. Shown are three SCF+ cells, two of which are CXCL12+ and one is CXCL12– (white arrow).
Table 1.
Quantification of coexpression of SCF with other hematopoietic growth factors in mouse E15.5 liver
| No. of SCF+ cells positive for other marker | No. of SCF+ cells negative for other marker | Percentage of double-positive cells | |
| ALB | 160 | 10 | 94% |
| Angptl3 | 87 | 7 | 93% |
| DLK | 92 | 6 | 94% |
| CD45 | 1 | 121 | 1% |
| CXCL12 | 30 | 57 | 34% |
Total dissociated fetal liver cells were double stained for SCF and other markers, as shown in Fig. 4. SCF+ cells were identified visually and examined for the expression of the other immunostained protein, including ALB, Angptl3, DLK, and CXCL12. Column 2 shows numbers of SCF+ cells that are also positive for the other marker; column 3 shows the numbers of SCF+ cells that are negative for the other marker. More than 94% of SCF+ cells are also ALB+, Angptl3+, and DLK+ (column 4), whereas only ~34% of SCF+ cells are also CXC12+.
In these experiments, we used a biotin-conjugated SCF antibody followed by FITC-streptavidin to detect the anti-SCF antibody. Before incubation with the biotin-conjugated SCF antibody, the sections were treated with an avidin-biotin endogenous biotin removal kit to block background staining from endogenous biotin. Interestingly, if we did not block endogenous biotin staining and treated the sections with FITC-streptavidin (with or without prior anti SCF antibody), ~4% of the fetal liver cells stained positively. Almost every one of these positive cells was also stained by the ALB antibody (Fig. S5 A and C, FITC channel). After treatment with the biotin removal kit, only sections incubated with the anti-SCF antibody had cells clearly staining with FITC-streptavidin (Fig. S5 B and D, FITC channel), attesting to the specificity of the SCF staining. As hepatocytes are known for having high levels of endogenous biotin, the staining of the ALB+ cells by FITC-streptavidin in the absence of removal of endogenous biotin attests to the identity of these cells as hepatic stem or progenitor cells.
In Fig. 5, we used a different way to purify fetal hepatic stem and progenitor cells and confirmed that the HSC-supportive stromal cells are indeed of hepatic lineage. We analyzed fetal liver cells harvested from a Tg(AFP-GFP) mouse line, in which the GFP gene is under the control of the promoter for the AFP gene (22). Approximately 5% of total liver cells express this transgene, a number roughly equal to the fraction of fetal liver cells that stain with an antibody to albumin. Of these GFP+ cells, approximately one-third, or 1.6% of total fetal liver cells, express SCF on their surface (Fig. 5B). The vast majority of the SCF+ cells expressed GFP, confirming our result (Table 1) that virtually all SCF+ cells also express AFP and thus are hepatic cells.
Fig. 5.
AFP+ fetal hepatobasts are enriched in stromal cells that express seven growth factors that support HSC maintenance, expansion, or homing. (A) FACS analysis of E15.5 fetal liver cells from Tg(AFP-GFP) mice stained by an SCF antibody as in Fig. 1; AFP+ cells are detected by GFP expression. (B) Relative expression of mRNAs encoding HSC expansion factors IGF2, Angptl3, TPO, IGFBP1, and CXCL12 and hepatocyte markers AFP and ALB in GFP+ and GFP– cells sorted by flow cytometry. The mRNA level of each gene was determined by qPCR and normalized against ribosomal RNA. The relative expression of each gene in each population was calculated setting the level of each mRNA in GFP– cells as in Fig. 1 (n = 3).
We next purified the GFP+ and GFP– populations by FACS. Similar to the data in Fig. 1 on SCF+DLK+ cells, GFP+ cells are enriched >15-fold for mRNAs encoding all seven HSC expansion factors (SCF, Angptl3, TPO, IGF2, DLK1, IGFBP1, and CXCL12), as well as for albumin and, as expected, AFP (Fig. 5B). Thus, the fetal liver HSC stromal cells are indeed hepatic stem or progenitor cells, and can equivalently be purified by either the SCF+DLK+ or the SCF+ Tg(AFP-GFP)+ phenotype.
Discussion
Embryonic day 15.5 fetal liver cells that are positive for the surface markers SCF and DLK comprise a subset of 1–2% of fetal hepatic stem or progenitor cells that are the principal fetal liver cells that express, in addition to SCF and DLK, four other growth factors that support HSC expansion, homing, or maintenance: TPO, Angptl3, IGF2, and CXCL12. Among these, SCF is the only essential HSC growth factor (9). They also are the principal fetal liver cells that express IGFBP1, a secreted protein closely related to the known HSC expansion factor IGFBP2. We believe that these SCF+DLK+ cells are the same cells that we purified several years ago from embryonic day 15.5 (E15.5) fetal livers using a monoclonal antibody supposedly specific for the T-cell protein CD3ε. These cells secrete growth factors that are sufficient to expand HSCs ex vivo: a serum- free culture medium containing just SCF, TPO, Angptl3 and IGF-2 can support >20-fold expansion ex vivo of both fetal liver and adult bone marrow HSCs (7), as assayed by long-term competitive bone marrow transplantation experiments.
Moreover, by competitive repopulating assay, we showed that SCF+DLK+ cells supported ex vivo maintenance of HSCs when cultured in a medium containing SCF, IL-6, and FLT3 (Fig. 2), similar to what we found with the CD3+ cells. Clearly these stromal cells produce factors that support cocultured HSCs, as all HSC activity was lost in the absence of these cells. However, with the current coculture method that we use, we cannot be certain that the SCF+DLK+ cells do support net HSC expansion rather than maintenance of their numbers, although this seems very likely. One likely problem is that many of these SCF+DLK+ cells died during culture, perhaps not surprising because cells of hepatic origin are notoriously difficult to culture. Moreover, the cells that remained alive in culture might have decreased expression of key HSC expansion factors. For example, DLK expression in hepatic stem/progenitor cells is significantly down-regulated in culture (16, 23). To demonstrate possible ex vivo expansion of HSCs by fetal liver SCF+DLK+ cells, we need to develop culture conditions that improve the survival of these cells yet still allow the maintenance and expansion of HSCs.
Because SCF+DLK+ cells support HSCs in culture and because they express a complete set of HSC growth factors such as SCF, TPO, IGF2, and Angptl3, which are sufficient to highly stimulate the expansion of HSCs ex vivo, we conclude that SCF+DLK+ cells are most likely the major fetal liver cells that support the extensive expansion of HSCs that occurs during late embryonic development. Although we have not yet determined whether these SCF+DLK+ stromal cells physically interact with HSCs, the fact that they express membrane-bound forms of DLK and SCF on their surface suggests that they are able to interact with HSCs via binding to the SCF receptor c-kit and the still-unknown receptor for DLK on HSCs. We do note that both the FACS analysis with SCF and DLK antibodies and our immuno-cytochemistry study indicate that a small percentage of SCF+ cells are DLK negative (Fig. 1A) and/or ALB negative (7%; Table 1). We do not know the identity of these presumably nonhepatic cells, and we cannot exclude the possibility that some of these cells might also be part of HSC niche in fetal liver. As an example, three different type of cells, namely, osteoblasts (4, 24), endothelial cells (5), and CXCL12hi reticular cells (18), have been identified as part of HSC niches in adult bone marrow, and our data do not exclude the possibility that, in fetal liver, SCF+DLK+ cells cooperate with other types of cells to stimulate HSC expansion.
Our study provides strong evidence that the SCF+DLK+ stromal cells for HSCs are actually hepatic progenitor cells, not CD3+ hematopoietic cells as previously reported. The most well-studied hepatic progenitors in the fetal liver are hepatoblasts, bipotential progenitors capable of differentiating into either hepatocytes or biliary cholangiocytes (25). We do not know whether these SCF+DLK+ cells still possess the potential to differentiate into both hepatocytes and cholangiocytes, or whether they are progenitors at a later developmental stage and are restricted to hepatic lineage only. Chagraoui et al. showed that a stromal cell line derived from E14 fetal liver that is capable of maintaining long-term HSC activity actually consisted of cells that bore markers for both epithelial and mesenchymal cells (26). Induced hepatocytic maturation of these cells by oncostatin M resulted in loss of mesenchymal marker expression, decreased AFP expression, and diminished ability to support hematopoiesis (26). The authors presented evidence that similar cells existed in the fetal liver. Although caution is needed when dealing with transformed cell lines and more definitive results are needed to determine whether such cells exist in vivo, this work nevertheless supports our finding that fetal liver cells bearing hepatic markers are stromal cells for HSCs.
In contrast to the developing embryo, in the adult, HSCs are mostly quiescent. Whether cells similar to fetal liver SCF+DLK+ cells are present in the bone marrow is not known. However, using RT-PCR analysis of total bone marrow mRNA, we could not detect transcripts from the TPO, Angptl3, IGF2, DLK1, or IGFBP1 genes. The mRNA for SCF is detectable but is at a level less than one-tenth that of fetal liver. Also, CXCL12, a chemokine used by stromal cells to attract HSCs, is expressed in both fetal liver (Fig. 1B) and bone marrow, although we do not know whether SCF+ bone marrow cells also express CXCL12. Therefore, although fetal liver and adult bone marrow HSC niches share some similarities such as CXCL12 expression, presumably for proper homing of HSCs, growth factors for HSC expansion are mostly absent from the bone marrow niche. In adults TPO is also essential for HSC maintenance (27). Our data showed that both TPO and Angptl3 are produced by adult hepatocytes at levels comparable to those in fetal liver and thus these endocrine hormones could support HSC maintenance in the bone marrow niches, although the concentration of these factors in bone marrow is likely much lower than in fetal liver, in which these factors are secreted from cells nearby. Other HSC expansion factors, such as IGF2, SCF, and DLK, are either not expressed in adult liver or are expressed but at a reduced level compared with fetal liver (Fig. 2). Clearly, adult hepatocytes, unlike their fetal counterparts, are unlikely to form supportive HSC niches.
SCF+DLK+ cells comprise ~1–2% of E15 fetal liver cells, greatly outnumbering HSCs, which at this stage comprise <0.01% of fetal liver cells. Therefore not every one of these cells can be in contact with an HSC. We found that ~30% of the SCF+DLK+ cells secrete the HSC chemo-attractant CXCL12 and thus could specifically attract HSCs. These CXCL12+ SCF+DLK+ cells might have a greater chance of establishing close cell–cell contacts with HSCs and thus stimulating their expansion; however it is likely that the rest of the SCF+DLK+ cells, although not in direct contact with HSCs, also contribute to their expansion by secreting HSC expansion factors. In adult bone marrow, the numbers of HSC stromal cells is limited, as experimental approaches to alter the number of stromal cells also alter the number of HSCs accordingly (4). In contrast, in fetal liver, the stromal cells are in excess. This would allow the newly divided HSCs to quickly find other stromal cells with which to interact. Furthermore, the larger number of stromal cells in fetal liver likely also contributes to a higher concentration of HSC expansion factors, allowing HSCs to expand efficiently in fetal liver.
SCF is required for the survival not only of HSCs but also of immature hematopoietic progenitors such as burst-forming units erythroid (BFU-Es) (28). This suggests that the SCF+ fetal hepatoblasts support not only HSC expansion but also survival or expansion of many hematopoietic progenitors as well as other fetal hepatoblasts. It is possible that the same SCF+DLK+ cells support not only HSCs but also other cell types. Alternatively, a subpopulation of the SCF+ DLK+ cells might be specialized in supporting HSC expansion. It would be of interest to see whether a subpopulation of SCF+DLK+ cells secrete other growth factors that support development of immature hematopoietic progenitors and thus are capable of supporting them alone. Interestingly, adult liver is also the primary site of EPO production, suggesting that a portion of fetal hepatoblasts might also be able to produce EPO to support early stages of erythropoiesis (21).
Methods
Mice.
Mice of the C57 BL/6 background were used for harvesting bone marrow and fetal liver cells. Tg(AFP-GFP) mice were generous gifts from Margaret Baron (Mount Sinai School of Medicine, New York).
Purification and Expression Analysis of Fetal Liver Stromal Cells.
E15.5 fetal liver cells were incubated with a biotin conjugated SCF antibody (Pepro Tech). The cells were washed and then incubated with APC-conjugated streptavidin (Ebioscience). For double staining with DLK, a FITC-conjugated DLK1 antibody (MBL International) was also added. The stained samples were analyzed using a LSR II FACS analyzer (Becton Dickinson).
For FACS analysis of the stromal population, E15.5 fetal liver cells were stained the same way as above and sorted for desired populations by a custom Aria FACS sorter (Becton Dickinson). Total RNAs from each sorted population were extracted and used to synthesize first strand cDNA using an Affinity Script QPCR kit from Stratagene. Analysis of synthesized cDNA samples was performed on a 7900HT fast real-time PCR analyzer. The PCR primers for the genes of interest were purchased from Qiagen.
FACS Analysis of Fetal Liver HSCs.
Total fetal liver E15.5 cells were first treated with ammonium chloride to lyse erythrocytes. We then depleted Ter119+ cells using magnetic beads (anti-Ter119 beads, BD). The remaining cells were stained with APC-conjugated CD150 (Biolegend), FITC-conjgated CD48 (Biolegend) and FITC-conjugated CD41 antibodies (Ebioscience). CD150+ CD48–CD41– HSCs were sorted as described previously (29).
Culture of HSCs and Repopulating Analysis of HSC Activity.
HSCs were cultured as previously described with minor changes (6). HSCs were cultured in 100 μL IMDM medium containing 10% serum, 1% BSA, 50 μM β-mercaptoethanol in a well of 96 U-well plate for 4 days. The culture medium was supplemented with 20 ng/mL SCF (Peprotech), 10 ng/mL IL6 (Peprotech), and 30 ng/mL FLT3 (Peprotech).
For competitive repopulating analysis, cultured HSCs (CD45.2) were mixed with 4 × 105 freshly isolated total bone marrow cells and injected into mice irradiated with a lethal dose of 1000 rad. Peripheral blood sample were collected 4 months after transplantation and analyzed with antibodies against CD45.1, CD45.2, B220 (B cells), CD3 (T cells), Gr-1 (granulocytes), and CD11b (granulocytes and monocytes).
Immunocytochemistry of Total Fetal Liver Cells.
Total E15.5 fetal liver cells were spread onto lysine-coated glass slide using a cytospin. Cells were then fixed with 4% paraformaldehyde and permeabilized by 100% methanol. Endogenous biotin was blocked by an avidin-biotin blocking kit from Abcam before staining with biotin-SCF antibody. For staining of SCF, the same biotin-conjugated SCF antibody used for FACS analysis was used and was followed by FITC-conjugated streptavidin. The antibodies for ALB (Abcam), Angptl3 (R&D systems), CXCL12 (Santa Cruz) and CD45 (R&D systems) staining are all polyclonal goat antibodies. For their staining, a Rhodamine Red X (RRX)–conjugated donkey antigoat secondary antibody was used (Jackson Immunoresearch). An RRX-conjugated donkey antirat secondary antibody (Jackson Immunoresearch) was used for DLK (MBL International) staining.
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health (NIH) Grant DK067356 to H. L. and by an NIH National Research Service Award fellowship (to S. C.). We thank Dr. Margaret Baron (Mount Sinai School of Medicine) for a gift of the Tg(AFP-GFP) mice; Yachao Liu for helping with some of the experiments; and Drs. Beiyan Zhou, Bill Piu Wong, and Peng Ji for critical reading of the manuscript.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1003586107/DCSupplemental.
References
- 1.Martinez-Agosto JA, Mikkola HK, Hartenstein V, Banerjee U. The hematopoietic stem cell and its niche: A comparative view. Genes Dev. 2007;21:3044–3060. doi: 10.1101/gad.1602607. [DOI] [PubMed] [Google Scholar]
- 2.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 3.Taichman RS. Blood and bone: Two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–2639. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- 4.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 5.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 6.Zhang CC, Lodish HF. Insulin-like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood. 2004;103:2513–2521. doi: 10.1182/blood-2003-08-2955. [DOI] [PubMed] [Google Scholar]
- 7.Zhang CC, et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12:240–245. doi: 10.1038/nm1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang CC, Kaba M, Iizuka S, Huynh H, Lodish HF. Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/SCID transplantation. Blood. 2008;111:3415–3423. doi: 10.1182/blood-2007-11-122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang CC, Lodish HF. Cytokines regulating hematopoietic stem cell function. Curr Opin Hematol. 2008;15:307–311. doi: 10.1097/MOH.0b013e3283007db5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avraham H, et al. Interaction of human bone marrow fibroblasts with megakaryocytes: Role of the c-kit ligand. Blood. 1992;80:1679–1684. [PubMed] [Google Scholar]
- 11.Heissig B, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson DM, et al. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell. 1990;63:235–243. doi: 10.1016/0092-8674(90)90304-w. [DOI] [PubMed] [Google Scholar]
- 13.Orlic D, Fischer R, Nishikawa S, Nienhuis AW, Bodine DM. Purification and characterization of heterogeneous pluripotent hematopoietic stem cell populations expressing high levels of c-kit receptor. Blood. 1993;82:762–770. [PubMed] [Google Scholar]
- 14.Moore KA, Pytowski B, Witte L, Hicklin D, Lemischka IR. Hematopoietic activity of a stromal cell transmembrane protein containing epidermal growth factor-like repeat motifs. Proc Natl Acad Sci USA. 1997;94:4011–4016. doi: 10.1073/pnas.94.8.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Forman SJ, Bhatia R. Expression of DLK1 in hematopoietic cells results in inhibition of differentiation and proliferation. Oncogene. 2005;24:4472–4476. doi: 10.1038/sj.onc.1208637. [DOI] [PubMed] [Google Scholar]
- 16.Oertel M, et al. Purification of fetal liver stem/progenitor cells containing all the repopulation potential for normal adult rat liver. Gastroenterology. 2008;134:823–832. doi: 10.1053/j.gastro.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195:1145–1154. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Huynh H, et al. Insulin-like growth factor-binding protein 2 secreted by a tumorigenic cell line supports ex vivo expansion of mouse hematopoietic stem cells. Stem Cells. 2008;26:1628–1635. doi: 10.1634/stemcells.2008-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanimizu N, Nishikawa M, Saito H, Tsujimura T, Miyajima A. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J Cell Sci. 2003;116:1775–1786. doi: 10.1242/jcs.00388. [DOI] [PubMed] [Google Scholar]
- 21.Jelkmann W. The role of the liver in the production of thrombopoietin compared with erythropoietin. Eur J Gastroenterol Hepatol. 2001;13:791–801. doi: 10.1097/00042737-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Kwon GS, et al. Tg(Afp-GFP) expression marks primitive and definitive endoderm lineages during mouse development. Dev Dyn. 2006;235:2549–2558. doi: 10.1002/dvdy.20843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanimizu N, Saito H, Mostov K, Miyajima A. Long-term culture of hepatic progenitors derived from mouse Dlk+ hepatoblasts. J Cell Sci. 2004;117:6425–6434. doi: 10.1242/jcs.01572. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 25.Alison MR, et al. Hepatic stem cells: From inside and outside the liver? Cell Prolif. 2004;37:1–21. doi: 10.1111/j.1365-2184.2004.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chagraoui J, Lepage-Noll A, Anjo A, Uzan G, Charbord P. Fetal liver stroma consists of cells in epithelial-to-mesenchymal transition. Blood. 2003;101:2973–2982. doi: 10.1182/blood-2002-05-1341. [DOI] [PubMed] [Google Scholar]
- 27.Qian H, et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1:671–684. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Metcalf D. Hematopoietic cytokines. Blood. 2008;111:485–491. doi: 10.1182/blood-2007-03-079681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim I, He S, Yilmaz OH, Kiel MJ, Morrison SJ. Enhanced purification of fetal liver hematopoietic stem cells using SLAM family receptors. Blood. 2006;108:737–744. doi: 10.1182/blood-2005-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.