Abstract
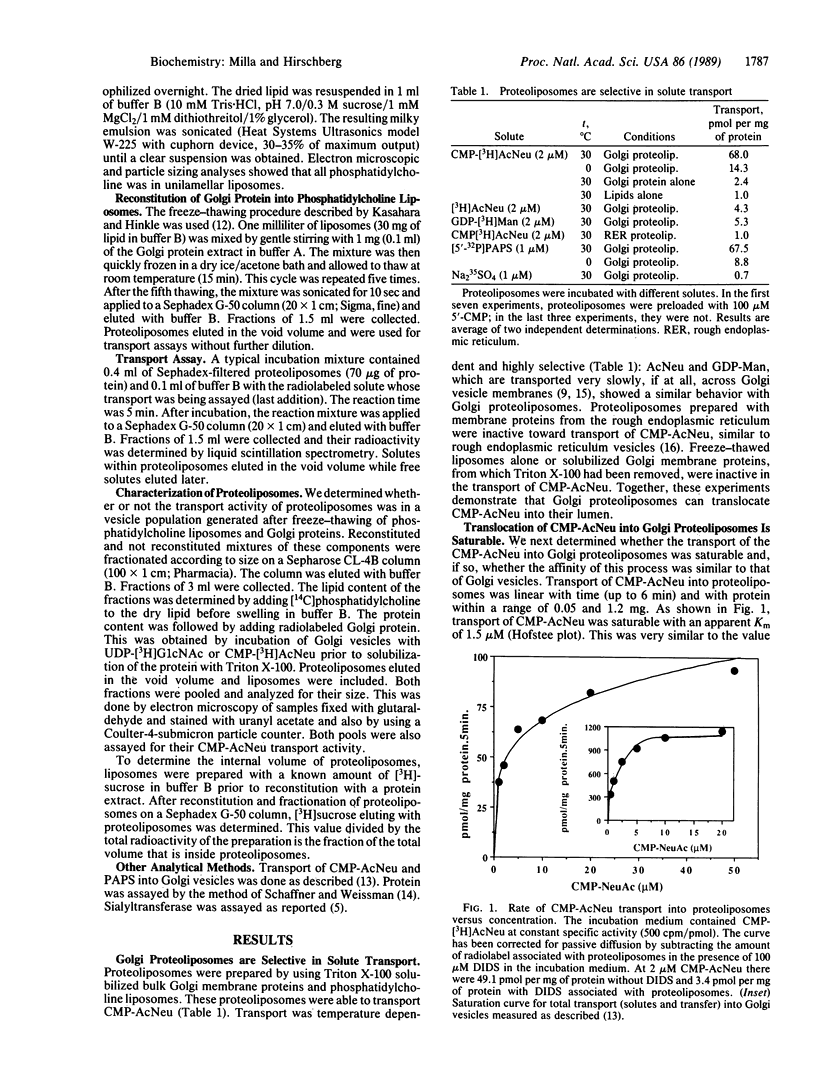
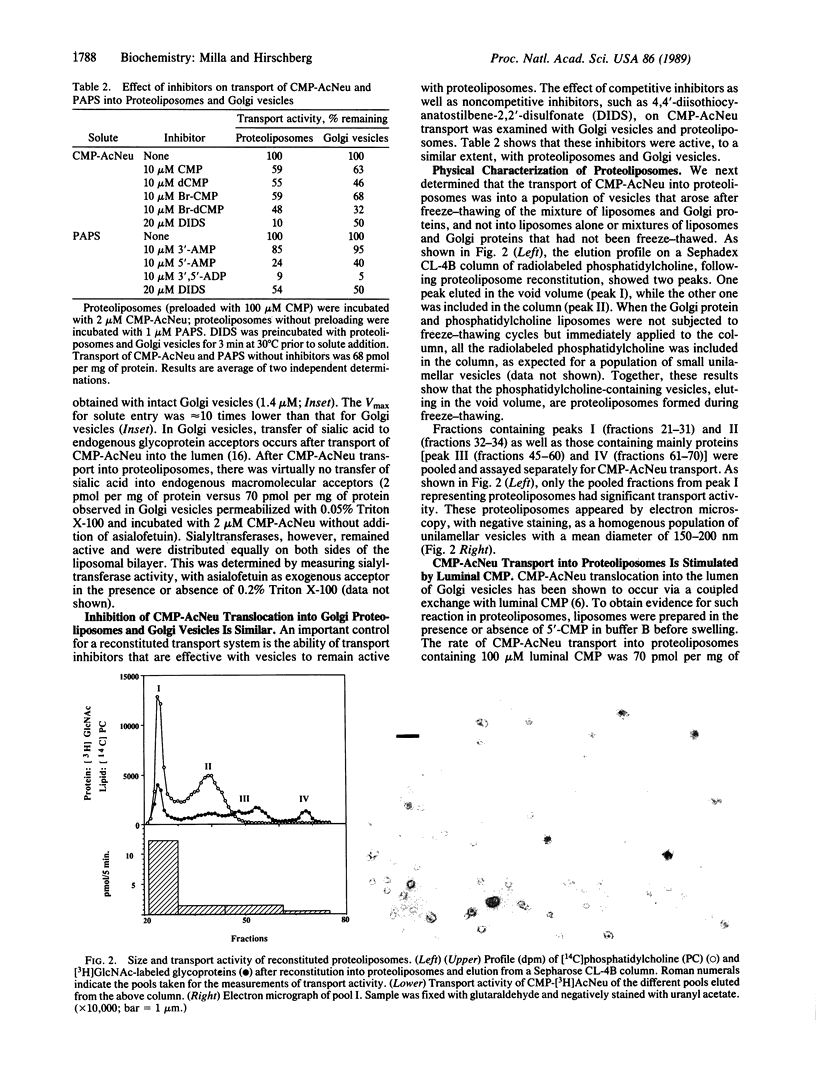
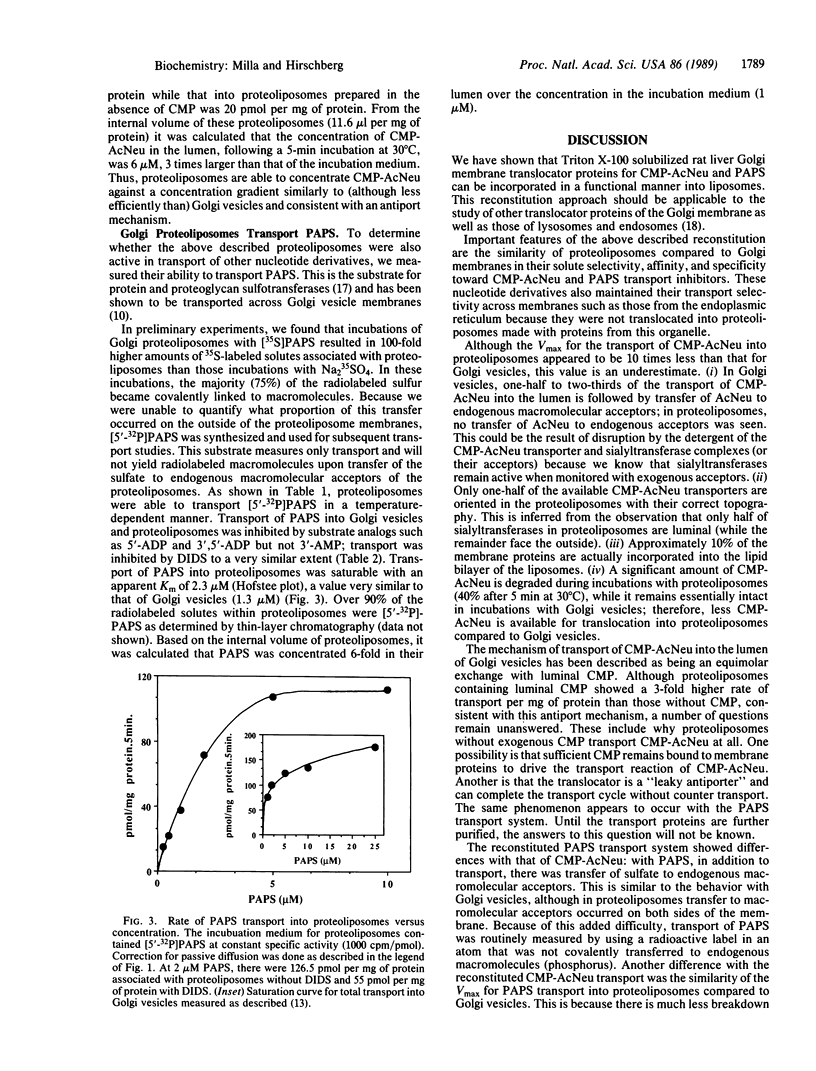
We have previously shown that Golgi apparatus vesicles transport nucleotide sugars and nucleotide sulfate into their lumen. These transport activities are organelle and substrate specific and are characterized by apparent Km for nucleotide derivatives in the low micromolar range. As part of our goal of purifying and characterizing the above transport proteins, we have reconstituted a protein extract from rat liver Golgi membranes into phosphatidylcholine liposomes. The resulting proteoliposomes transport CMP-N-acetylneuraminic acid (CMP-AcNeu) and adenosine 3'-phosphate 5'-phosphosulfate with very similar affinity and inhibition characteristics as intact Golgi vesicles. Sialic acid and sodium sulfate, which are transported only very slowly into the lumen of Golgi vesicles, are transported at low rates by the reconstituted proteoliposomes. Neither rough endoplasmic reticulum-derived vesicles nor proteoliposomes made from proteins of the rough endoplasmic reticulum transport CMP-AcNeu. The above results demonstrate that this reconstituted system can be used for further purification and characterization of nucleotide sugar and nucleotide sulfate translocator proteins. This approach should also be useful to study membrane transport proteins of lysosomes and endosomes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capasso J. M., Hirschberg C. B. Mechanisms of glycosylation and sulfation in the Golgi apparatus: evidence for nucleotide sugar/nucleoside monophosphate and nucleotide sulfate/nucleoside monophosphate antiports in the Golgi apparatus membrane. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7051–7055. doi: 10.1073/pnas.81.22.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey D. J., Hirschberg C. B. Topography of sialoglycoproteins and sialyltransferases in mouse and rat liver Golgi. J Biol Chem. 1981 Jan 25;256(2):989–993. [PubMed] [Google Scholar]
- Carey D. J., Sommers L. W., Hirschberg C. B. CMP-N-acetylneuraminic acid: isolation from and penetration into mouse liver microsomes. Cell. 1980 Mar;19(3):597–605. doi: 10.1016/s0092-8674(80)80036-5. [DOI] [PubMed] [Google Scholar]
- Coates S. W., Gurney T., Jr, Sommers L. W., Yeh M., Hirschberg C. B. Subcellular localization of sugar nucleotide synthetases. J Biol Chem. 1980 Oct 10;255(19):9225–9229. [PubMed] [Google Scholar]
- D'Souza M. P., Ambudkar S. V., August J. T., Maloney P. C. Reconstitution of the lysosomal proton pump. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6980–6984. doi: 10.1073/pnas.84.20.6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher S. L., Hirschberg C. B. Mechanism of galactosylation in the Golgi apparatus. A Chinese hamster ovary cell mutant deficient in translocation of UDP-galactose across Golgi vesicle membranes. J Biol Chem. 1986 Jan 5;261(1):96–100. [PubMed] [Google Scholar]
- Deutscher S. L., Nuwayhid N., Stanley P., Briles E. I., Hirschberg C. B. Translocation across Golgi vesicle membranes: a CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell. 1984 Dec;39(2 Pt 1):295–299. doi: 10.1016/0092-8674(84)90007-2. [DOI] [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J. N-Linked glycoprotein assembly. Evidence that oligosaccharide attachment occurs within the lumen of the endoplasmic reticulum. J Biol Chem. 1980 Apr 25;255(8):3600–3604. [PubMed] [Google Scholar]
- Hirschberg C. B., Snider M. D. Topography of glycosylation in the rough endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 1987;56:63–87. doi: 10.1146/annurev.bi.56.070187.000431. [DOI] [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution of D-glucose transport catalyzed by a protein fraction from human erythrocytes in sonicated liposomes. Proc Natl Acad Sci U S A. 1976 Feb;73(2):396–400. doi: 10.1073/pnas.73.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez M., Hirschberg C. B. Topography of glycosylation reactions in the rough endoplasmic reticulum membrane. J Biol Chem. 1986 May 25;261(15):6822–6830. [PubMed] [Google Scholar]
- Perez M., Hirschberg C. B. Transport of sugar nucleotides into the lumen of vesicles derived from rat liver rough endoplasmic reticulum and Golgi apparatus. Methods Enzymol. 1987;138:709–715. doi: 10.1016/0076-6879(87)38061-9. [DOI] [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Isolation and identification of active sulfate. J Biol Chem. 1957 Dec;229(2):837–851. [PubMed] [Google Scholar]
- Rodriguez Boulan E., Kreibich G., Sabatini D. D. Spatial orientation of glycoproteins in membranes of rat liver rough microsomes. I. Localization of lectin-binding sites in microsomal membranes. J Cell Biol. 1978 Sep;78(3):874–893. doi: 10.1083/jcb.78.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Schwarz J. K., Capasso J. M., Hirschberg C. B. Translocation of adenosine 3'-phosphate 5'-phosphosulfate into rat liver Golgi vesicles. J Biol Chem. 1984 Mar 25;259(6):3554–3559. [PubMed] [Google Scholar]
- Sommers L. W., Hirschberg C. B. Transport of sugar nucleotides into rat liver Golgi. A new Golgi marker activity. J Biol Chem. 1982 Sep 25;257(18):10811–10817. [PubMed] [Google Scholar]




