Abstract
IL-33 is a novel member of the IL-1 family and ligand for the IL-1 receptor-related protein, ST2. Recent evidence suggests that the IL-33/ST2 axis plays a critical role in several autoimmune and inflammatory disorders; however, its role in inflammatory bowel disease (IBD) has not been clearly defined. We characterized IL-33 and ST2 expression and modulation after conventional anti-TNF therapy in Crohn’s disease and ulcerative colitis (UC) patients and investigated the role of IL-33 in SAMP1/YitFc (SAMP) mice, a mixed Th1/Th2 model of IBD. Our results showed a specific increase of mucosal IL-33 in active UC, localized primarily to intestinal epithelial cells (IEC) and colonic inflammatory infiltrates. Importantly, increased expression of full-length IL-33, representing the most bioactive form, was detected in UC epithelium, whereas elevated levels of cleaved IL-33 were present in IBD serum. ST2 isoforms were differentially modulated in UC epithelium, and sST2, a soluble decoy receptor with anti-inflammatory properties, was also elevated in IBD serum. Infliximab (anti-TNF) treatment of UC decreased circulating IL-33 and increased sST2, whereas stimulation of HT-29 IEC confirmed IL-33 and sST2 regulation by TNF. Similarly, IL-33 significantly increased and correlated with disease severity, and potently induced IL-5, IL-6, and IL-17 from mucosal immune cells in SAMP mice. Taken together, the IL-33/ST2 system plays an important role in IBD and experimental colitis, is modulated by anti-TNF therapy, and may represent a specific biomarker for active UC.
Keywords: inflammatory bowel disease, anti-TNF therapy, SAMP1/YitFc mouse model
Inflammatory bowel disease (IBD), such as Crohn’s disease (CD) and ulcerative colitis (UC), are chronic, relapsing inflammatory disorders of the digestive tract resulting from dysregulated immune responses toward environmental factors in genetically predisposed individuals. Although the precise etiology is unknown, it is widely accepted that an imbalance of pro- and anti-inflammatory mediators is a key factor in IBD pathogenesis (1). In support of this hypothesis, selective blockade of proinflammatory cytokines is one of the most effective strategies to down-regulate mucosal inflammation in IBD; the best example is the use of anti-TNF therapies to successfully treat both CD and UC (2). Additional support comes from studies reporting an imbalance between the proinflammatory cytokine, IL-1, and its natural antagonist, IL-1 receptor antagonist, in the development of UC (3, 4). In fact, other IL-1 family members have been implicated in IBD pathogenesis, including IL-18, described as an important Th1-polarizing cytokine in CD (5, 6).
In 2005, IL-33 was recognized as a novel IL-1 family member (7). IL-33 is expressed in a variety of nonhematopoietic cells (e.g., fibroblasts, adipocytes, smooth muscle, endothelial, and bronchial epithelial cells) and in restricted populations of inflammatory cells (e.g., macrophages and dendritic cells) (7, 8). Similar to IL-1 and IL-18, it was originally proposed that IL-33 was synthesized as a 30-kDa precursor molecule and cleaved by caspase-1 upon inflammasome activation into an 18-kDa mature/bioactive form (7). Recent studies have challenged this paradigm, suggesting that full-length 30 kDa IL-33 (f-IL-33) is the actual bioactive form and that other processed, less active forms (20–22 kDa) result from caspase-3 and -7 cleavage (9–11).
The formerly orphaned IL-1 receptor-related protein, ST2, is the IL-33 receptor and exists in two different splice variants leading to the synthesis of ST2L, a transmembrane receptor that confers IL-33’s biologic effects, and sST2, a soluble molecule that likely serves as a decoy receptor for IL-33 (12). The biologic relevance of both ST2 and IL-33 isoforms is unknown in human disease.
The IL-33/ST2 axis appears to play an important role in several chronic inflammatory disorders, including asthma, rheumatoid arthritis, and anaphylactic shock (13–15); however, in contrast to IL-1 and IL-18, which predominantly promote Th1-type responses, IL-33 mainly induces Th2 cytokines (i.e., IL-5 and IL-13) (7). Relevant to our study, IL-33 exerts specific effects in the gut, because mice treated with recombinant (r)IL-33 displayed epithelial hyperplasia and eosinophil/neutrophil infiltration in the colonic mucosa (7). Similar to IL-1α, IL-33 may possess dual roles, functioning as an intracellular nuclear factor and a proinflammatory cytokine (16); in fact, IL-33 has been described as a chromatin-associated transcriptional regulating factor (17).
In the present study, we showed increased IL-33 and ST2 expression in inflamed colonic mucosa and serum of IBD patients. During active UC, intracellular epithelial accumulation of f-IL-33 and decreased ST2L were observed and likely regulated by TNF. In support of these findings, TNF had the ability to up-regulate both IEC-derived f-IL-33 and sST2 in vitro, and treatment of IBD patients with anti-TNF modulated circulating IL-33 and sST2 levels, particularly in UC patients. Up-regulation of IL-33 was also confirmed in SAMP mice, a spontaneous model of chronic intestinal inflammation characterized by a mixed Th1/Th2 immune phenotype (18), wherein IL-33 was increased and correlated with disease severity, induced IL-5, IL-6, and IL-17 from mesenteric lymph node (MLN) cells, and displayed a similar pattern of mucosal cell production as IBD patients. Taken together, our data suggest that the IL-33/ST2 system is strongly activated in IBD, and a specific imbalance between IL-33 and ST2 may play a pathogenic role particularly in UC.
Results
IL-33 and ST2 are Increased in Inflamed Mucosa of UC Patients.
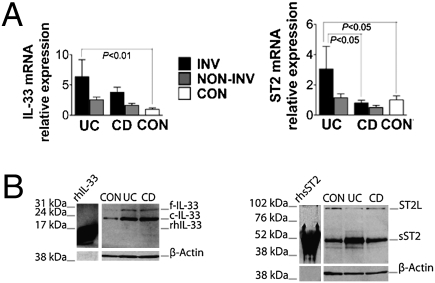
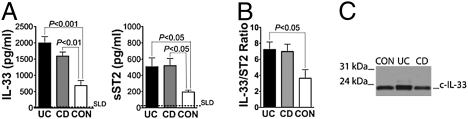
We initially measured IL-33 and ST2 in involved (INV) and noninvolved (NON-INV) endoscopic biopsies of colonic mucosa from CD and UC patients and noninflamed controls (CON). IL-33 mRNA transcripts were more abundant in INV UC compared with CON (6.4-fold increase, P < 0.01), with no differences among all noninflamed tissues (Fig. 1A Left). Western blots showed the presence of 30 and 20- to 22-kDa bands corresponding to f- and cleaved (c)-IL-33, respectively, which were both up-regulated in active UC and CD compared with CON (Fig. 1B Left). Increased total ST2 mRNA levels were observed in INV UC compared with CON (3.1-fold increase, P < 0.05) (Fig. 1A Right), with specific abundance of mRNA transcripts from the sST2 splice variant (2.3-fold increase, P < 0.05) and sST2 protein, whereas no significant changes were detected for ST2L (Fig. S1). Together, these data suggested that both IL-33 and ST2 expression are increased in IBD locally, within the colonic mucosa, and are indicative of active inflammation. In addition, increased IL-33 and sST2 may be more specific for disease activity in UC vs. CD.
Fig. 1.
IL-33 and ST2 are specifically up-regulated in inflamed UC mucosa. Colonic biopsies were harvested from INV and NON-INV areas of UC (n = 40), CD (n = 64), and noninflamed CON (n = 30) patients, and total RNA and protein were extracted and processed for RT-PCR and Western blots. (A) IL-33 (Left) and ST2 (Right) were normalized to GAPDH and relative mRNA levels calculated as fold increase over control. Statistical analysis was performed by using one-way ANOVA. (B) Representative Western blots of 6 separate experiments for IL-33 (Left) and ST2 (Right) on INV IBD and CON patients. rhIL-33 and rsST2 were used as positive controls, and β-actin was used as an internal housekeeping control. Full-length and cleaved IL-33, f- and c-IL-33.
Differential Localization/Expression of IEC-Derived IL-33 and ST2 in IBD.
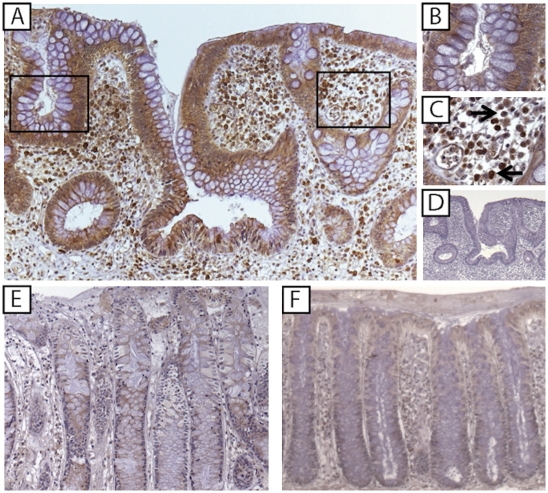
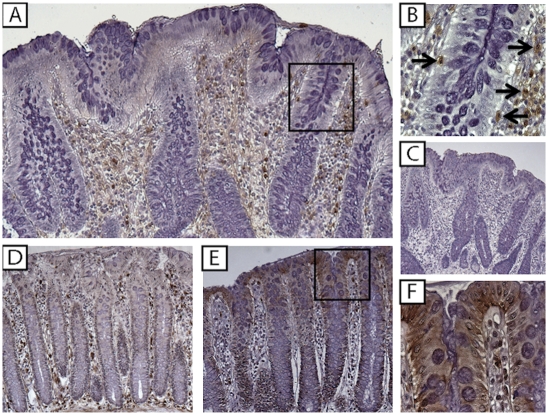
To localize IL-33 and ST2 in the gut mucosa, IHC was performed on surgically resected full-thickness colon specimens from IBD and noninflamed CON patients. IL-33 was detected in the cytoplasm and nucleus of IEC and lamina propria mononuclear cells (LPMC) in all specimens studied, although intensity and distribution varied among experimental groups (Fig. 2 and Fig. S2A). Localization within cytoplasmic and nuclear fractions of IEC was confirmed by IL-33 Western blots in HT-29 IEC (Fig. S2B). IL-33 was most prominently detected in inflamed UC specimens (Fig. 2 A–C), with intense staining mainly localized to the epithelium (Fig. 2B) and infiltrating LPMC with abundant cytoplasm and large, kidney-shaped, granular nuclei, and smaller, condensed cells with eccentrically placed cartwheel nuclei, morphologically consistent with tissue macrophages (histiocytes) and plasma cells, respectively (Fig. 2C and Fig. S2C, arrows). FACS analysis of LPMC isolated from UC-involved mucosa confirmed these results, identifying CD11b+ (macrophages) and CD19+ (B cell lineage) as IL-33 producing immune cells (Fig. S2D). In contrast to UC, less intense staining was observed in CD (Fig. 2E) and noninflamed CON (Fig. 2F). IL-33 also localized to gut-associated adipocytes during active disease and endothelial cells (Fig. S3), consistent with previous reports describing IL-33 in other tissues/organ systems (7, 17). Intense ST2 staining was observed in inflamed UC colons, although mainly limited to the LP compartment (Fig. 3 A and B), in large mononuclear (kidney-shaped) cells with abundant cytoplasm, and round cells with large condensed nuclei and little cytoplasm, morphologically consistent with tissue macrophages and lymphocytes, respectively (Fig. 3B and Fig. S4 A and B, arrows). FACS analysis confirmed these results, localizing ST2 primarily to CD11b+ and CD4+ cells (Fig. S4C). Importantly, ST2 was absent/decreased in UC-associated IEC. Although less intense, a similar pattern was observed in Crohn’s colitis (Fig. 3D). Interestingly, in noninflamed CON, although detected in scattered LPMC, the primary source for ST2 was the gut epithelium (Fig. 3E). These data indicated that IL-33 is produced by IEC and LPMC within the gut mucosa and is up-regulated during active UC compared with CD and CON. However, although ST2 was present and increased in LPMC from inflamed UC colons, epithelial loss of ST2 may represent specificity for IBD because IEC-derived ST2 was detected in other self-limiting colitides, such as infectious colitis and diverticulitis (Fig. S5).
Fig. 2.
IL-33 is localized to colonic IEC and LPMC, and markedly increased in active UC. Full-thickness colon tissues from UC, CD, and noninflammatory CON patients (n = 4 per experimental group) were evaluated by IHC for IL-33 expression and immunolocalization. (A) UC inflamed mucosa. (B and C) Higher power magnification (mag) of A showing IL-33-specific IEC (B) and LPMC (C, arrows) staining. (D) Isotype control Ab staining of serial section from A. (E) CD inflamed mucosa. (F) Noninflamed CON. (Original magnification: A and D–F: ×10; B and C, ×80.)
Fig. 3.
ST2 is specifically decreased in IBD epithelium. Full-thickness colon tissues from UC, CD, and noninflammatory CON patients (n = 4 per experimental group) were evaluated by IHC for ST2 expression and immunolocalization. (A) UC inflamed mucosa. (B) Higher power magnification of A showing ST2-specific LPMC staining (arrows). (C) Isotype control Ab staining of serial section from A. (D) CD inflamed mucosa. (E) Noninflamed CON. (F) Higher power magnification of E showing IEC-specific ST2 staining. (Original magnification: A and C–E: ×10; B and F, ×80.)
Increased IL-33/Decreased ST2L Expression in IEC from UC Patients.
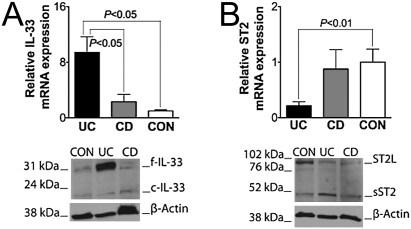
To further examine epithelial-specific IL-33 and ST2 in IBD, IEC were isolated from surgically resected UC, CD, and CON colon specimens, and IL-33 and ST2 was measured. Evaluation of mRNA expression confirmed IHC results: Increased IL-33 mRNA transcripts were measured in UC IEC compared with either CD or CON (both P < 0.05) (Fig. 4A Upper). Western blots revealed an abundance of f-IL-33 in UC IEC compared with CD and CON, with little posttranslational modification to the cleaved form, which was equally low in all groups (Fig. 4A Lower). Total ST2 mRNAs were decreased in UC IEC vs. CON (P < 0.05), while significant variability was present in CD (Fig. 4B Upper). Specifically, ST2L was consistently more abundant in CON IEC compared with UC (P < 0.05), and sST2 was slightly elevated in IBD IEC (Fig. S6 and Fig. 4B Lower). Taken together, these data indicated that f-IL-33 is the predominant form expressed by IEC during active UC, and epithelial loss of ST2 during inflammatory changes characteristic of IBD is due to a decrease in ST2L. In addition, the small, but increased presence of sST2 protein in UC IEC was consistent with our previous results demonstrating overall elevated sST2 levels in the colonic mucosa of active UC.
Fig. 4.
Full-length IL-33 is abundantly expressed, whereas ST2L is decreased in IEC from UC patients. IEC were harvested from full-thickness inflamed colons of UC (n = 18) and CD (n = 10) and noninflamed CON (n = 7) patients. Total RNA and protein were extracted and processed for RT-PCR and Western blots. (A) IL-33 (Upper) and ST2 (Lower) mRNA levels. Statistical analysis was performed by using one-way ANOVA. (B) Representative Western blots of 4 separate experiments for IL-33 (Upper) and ST2 (Lower) on IEC from inflamed IBD colons and noninflamed CON. β-actin was used as an internal housekeeping control.
Serum IL-33 and ST2 are Increased in IBD.
After establishing that both IL-33 and ST2 were increased locally within inflamed IBD colons, particularly in UC, we investigated whether either protein could serve as a circulating biomarker for UC or CD. Serum IL-33 and sST2 protein levels were measured in IBD patients with active disease and in healthy CON. IL-33 levels were elevated in UC and CD patients compared with CON (P < 0.001 and P < 0.01, respectively); although an increased trend was observed in UC compared with CD, this difference was not statistically significant (Fig. 5A Left). Similarly, increased circulating sST2 levels were found in both UC and CD patients compared with CON (both P < 0.05) (Fig. 5A Right). Given the described role of sST2 as a decoy receptor, we also calculated the IL-33/sST2 ratio for each subject to estimate the potential bioavailability of circulating IL-33. Both UC and CD patients demonstrated higher IL-33/sST2 ratios compared with CON, but only UC was statistically significant (P < 0.05) (Fig. 5B). Only 20- to 22-kDa IL-33 was detectable in human serum (Fig. 5C), demonstrating that c-IL-33 likely represents the sole form of circulating IL-33, and although both c-IL-33 and sST2 serum concs were greater in IBD patients, the systemic IL-33/sST2 ratio was significantly increased only in UC.
Fig. 5.
Circulating IL-33 and sST2 are significantly increased in IBD, and only cleaved IL-33 is detectable in human sera. Serum samples were obtained from active UC (n = 59) and CD (n = 72) patients and healthy CON (n = 19) patients. IL-33 and sST2 protein levels were measured by ELISA and Western blots. IL-33 (A Left), sST2 (A Right), and (B) IL-33/sST2 ratio. Statistical analysis was performed by using Kruskal-Wallis test. Dotted line, standard limit of detection (SLD). (C) Representative blot of four separate experiments for IL-33 on active IBD patients and healthy CON.
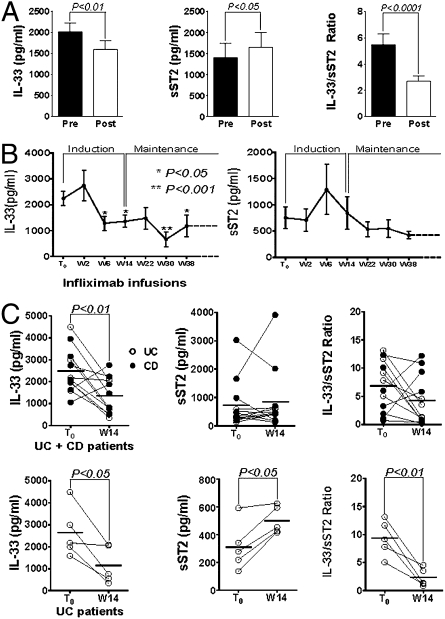
Anti-TNF Therapy Modulates Circulating IL-33 and ST2 Levels in IBD.
We next determined whether circulating IL-33, sST2, and/or the IL-33/sST2 ratio could be modulated during a conventional treatment protocol for IBD, i.e., use of anti-TNF mAbs (Infliximab). Initially, to evaluate the acute effects of TNF blockade, serum samples were collected from IBD patients 1 h before (pre-infusion) and 2 h after (post-infusion) Infliximab treatment. Acute neutralization of TNF decreased systemic IL-33 (P < 0.01), while raising sST2 levels (P < 0.05), reducing the overall IL-33/sST2 ratio (P < 0.0001) (Fig. 6A). We then explored the modulation of IL-33/sST2 for the duration of the anti-TNF protocol, from pre-infusion (T0), during the induction regimen, and through the maintenance phase (up to wk 38). Serum IL-33 was reduced, comparing baseline levels (T0) with those taken before the last infusion of the induction regimen at wk 6 (P < 0.05). Circulating IL-33 remained at reduced levels throughout maintenance therapy, at infusions performed at 14, 30, and 38 wks (Fig. 6B Left). In contrast, serum sST2 did not show significant changes from T0 throughout anti-TNF therapy (Fig. 6B Right); however, a decrease in the IL-33/sST2 ratio from T0 was achieved at wk 30 (P < 0.05) (Fig. S7A). Analysis of individual data from T0 until the end of the induction regimen (wk 14) showed differential modulation of the IL-33/sST2 axis when comparing UC vs. CD. In fact, after the induction phase at wk 14, IL-33 levels were decreased in the overall IBD group (P < 0.01) (Fig. 6C Upper Left); upon separating by disease subgroups, the significance was maintained for UC (P < 0.05), but not for CD patients (Fig. 6C Lower Left and Fig. S7B Left). Similarly, no differences were observed in serum sST2 levels and the IL-33/ST2 ratio for the overall group (Fig. 6C Upper Center and Upper Right); however, UC, but not CD, showed an increase in circulating sST2 (P < 0.05) and a decrease in the IL-33/sST2 ratio (P < 0.01) (Fig. 6C Lower Center and Lower Right and Fig. S7B Center and Right). Thus, these data provided support that neutralization of TNF modulated the IL-33/ST2 axis in vivo during a human disease state, such as IBD. In addition, anti-TNF therapy had both acute and long-term effects on systemic IL-33 and ST2 levels, with a greater impact on UC vs. CD patients.
Fig. 6.
Anti-TNF modulates IL-33 and sST2 serum levels in IBD patients. Serum samples were assayed from IBD patients (UC, n = 9; CD, n = 11) undergoing Infliximab therapy (5–10 mg/kg, i.v.). IL-33 and sST2 protein levels were measured by ELISA. (A) IL-33 (Left), sST2 (Center), and IL-33/sST2 ratio (Right) observed 1 h prior (Pre) and 2 h after (Post) anti-TNF infusion. (B) IL-33 (Left) and sST2 (Right) measured 1 h before each Infliximab infusion throughout treatment protocol (T0, n = 15; W2, n = 15; W6, n = 11; W14, n = 12; W22, n = 10; W30, n = 9; W38, n = 5); W, week. (C) Individual patient data at T0 and at end of induction regimen (W14) from CD and UC patients (n = 12). IL-33 (Left), sST2 (Center), and IL-33/sST2 ratio (Right) are shown for all IBD patients (Upper) and only for UC (n = 5) (Lower). Statistical analysis was performed by using paired Student’s t test for paired data.
TNF Stimulation Directly Modulates IL-33 and ST2 in IEC.
To study the direct effects of TNF on IEC-derived IL-33 and ST2, we performed in vitro experiments by using HT-29 cells stimulated with rhTNF. Increased amounts of IL-33 mRNA and f-IL-33 protein were observed after TNF stimulation for 48 h (Fig. S8 A Left and B Left); interestingly, IL-33 remained intracellular without detectable amounts in cell culture supernatants (Fig. S8 A–C Left). A similar pattern of IL-33 expression was observed in freshly isolated IEC from UC patients, wherein robust levels of f-IL-33 were detected intracellularly with little modulation of the cleaved form (Fig. 4A Lower). Intracellular IEC sST2 accumulation was also increased at 24 and 48 h of TNF stimulation (Fig. S8 A Right and B Right), which was comparable with the pattern observed in UC-derived IEC (Fig. 4B). In addition, no overt changes were detected for ST2L after TNF stimulation (Fig. S8 A Right and B Right). These data suggested that TNF stimulation of IEC induces the intracellular accumulation, but not secretion, of f-IL-33, and at the same time enhances sST2 intracellular production.
IL-33 is Up-Regulated and Correlates with Disease Severity in Experimental Th1/Th2-Mediated Enteritis.
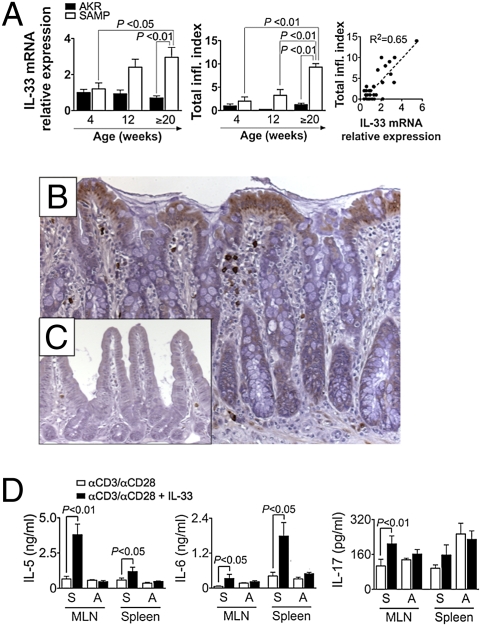
To determine whether IL-33 contributes to the development chronic intestinal inflammation, SAMP mice were studied at a preinflammatory state (4 wk), at the onset of inflammation (12 wk), and during established disease (>20 wk) (19). Increasing IL-33 mRNA and protein (both f- and c-IL-33) levels were detected in full-thickness ilea of SAMP mice vs. AKR (parental control strain) with age (20 wks, P < 0.01) (Fig. 7A Left and Fig. S9A), with elevated mRNA expression positively correlating with the severity of gut inflammation (Fig. 7A Center and Right). Consistent with the human data, IL-33 localized to LPMC (morphologically resembling macrophages and plasma cells) and IEC in inflamed SAMP ilea and was virtually absent in AKR (Fig. 7 B and C and Fig. S9C). Only f-IL-33 was detected in IEC, whereas systemic c-IL-33 was increased in SAMP vs. AKR (P < 0.05) and was the sole form found in serum (Fig. S9 D and E). In addition, IL-33 potently induced IL-5 and IL-6, cytokines known to be important in the pathogenesis of SAMP enteritis (20, 21), from anti-CD3/CD28-activated MLN and spleen cells in SAMP (P < 0.05), but not AKR, mice (Fig. 7D Left and Center). Interestingly, activated MLN cells, but not splenocytes, from SAMP produced high levels of IL-17 after IL-33 stimulation (P < 0.01) (Fig. 7D Right), suggesting that IL-33 may initiate enhanced proinflammatory and Th2-type immune responses critical to the pathogenesis of chronic intestinal inflammation.
Fig. 7.
Increased IL-33 correlates with disease severity in SAMP ileitis and induces the production of proinflammatory cytokines from activated mucosal immune cells. Age-matched SAMP and control AKR ilea (n ≥ 4 per age) were collected and processed for total RNA extraction and histologic/IHC evaluation. MLN and spleen cells from 20-wk-old SAMP and AKR (n = 5 per group) were cultured with or without IL-33 (10 ng/mL) in the presence of anti-CD3 (αCD3)/αCD28 for 48 h. (A) Linear regression analysis (Right) of IL-33 mRNA levels normalized to β-actin (Left) and total inflammatory index (Total infl. Index, Center). (B and C) IL-33 IHC in SAMP (B) and AKR (C) ileum. (Original magnification: ×20.) (D) IL-5, IL-6, and IL-17 protein levels from cultured MLN and spleen cell supernatants. S, SAMP; A, AKR. Statistical analyses were performed by using one-way ANOVA, linear regression, and paired Student t test for paired data.
Discussion
Increasing evidence has supported a critical role of the Toll/IL-1 receptor family in the pathogenesis of IBD (22, 23). In the present study we report a specific dysregulation of the novel IL-1 family member, IL-33, and its receptor, ST2, during chronic intestinal inflammation. We also address the potential biologic relevance of IL-33 and ST2 isoforms (11, 24) in a specific disease setting. The specificity for increased IL-33 in UC and in SAMP enteritis is consistent with the relative predominance of Th2 polarization in UC as opposed to CD, which is characterized primarily by a Th1 phenotype (25), and in SAMP mice, a model of Th1/Th2-driven gut inflammation (18). It is therefore conceivable that IL-33 overexpression may represent one of the earlier events in IBD pathogenesis that may polarize colitis toward a Th2/UC vs. a Th1/CD phenotype.
During active UC and SAMP enteritis, IL-33 is markedly up-regulated, mainly in IEC and infiltrating LPMC, and although similar gut mucosal cells express ST2, the pattern of expression is remarkably different in noninflamed CON compared with IBD patients. In macroscopically noninflamed CON colons, ST2 is primarily found in the epithelium, whereas in chronically inflamed colitis in either UC or CD, the major source of ST2 is in the LP compartment with expression virtually absent in IEC. Interestingly, the decrease or absence of ST2 in both UC and CD IEC may be specific for IBD because nonspecific colitides do not appear to display this epithelial-specific deficit.
Indeed, the epithelium is a critical component of the gut mucosal immune system because it represents a primary barrier between host and environmental antigens. IEC have the ability to recognize pathogens through innate immune receptors, respond by releasing antimicrobial peptides (26), and modulate initial events of the immune response by, for example, secretion of pro/anti-inflammatory mediators and cytokines (27). The IEC-specific increase of IL-33 in inflamed UC colons and SAMP ilea, particularly the 30-kDa form with increased proinflammatory activity (11), may be a consequence of interactions with commensal and/or luminal pathogens, thereby triggering inflammatory responses and perpetuating a chronic, Th2-mediated colitis. To date, helminth infection, representing a canonical Th2-type immune response, is the only identified stimulus known to induce gut-derived IL-33, which is thought to be pivotal in mounting appropriate immunity to clear the infection (28). In addition, several genetic studies in IBD patients and mouse models of colitis emphasize that innate epithelial defects can lead to IEC stress, barrier dysfunction, and subsequent development of chronic intestinal inflammation (29–31). In this context, IL-33 may also function as a novel epithelial “alarmin” (9, 11), because mounting evidence suggests that similar to HMGB1 and IL-1α, IL-33 can be released as a danger signal by damaged, stressed, or necrotic cells to alert the immune system of a local threat. Remarkably, IL-33 mRNA levels correlate with disease severity in SAMP mice, and in vitro IL-33 stimulation of activated MLN cells, primarily trafficking from the inflamed gut, markedly increases the production of IL-5 and IL-6, cytokines critical to SAMP enteritis because anti-IL-5 and -IL-6 strategies have been reported to ameliorate gut inflammation in this IBD model (20, 21). In addition, although its role in SAMP ileitis has yet been defined, it is well known that IL-17 and Th17 immune responses play a central role in chronic intestinal inflammation (32), and its induction by IL-33 is consistent with what has been described in other organ systems (13, 33). Thus, locally produced IL-33 may represent a primary mediator important in promoting a robust proinflammatory mucosal immune response, capable of triggering chronic gut inflammation.
Perhaps in response to either increased IL-33 or a simple intrinsic deficit in ST2, we observed a significant decrease of ST2L, IL-33’s transmembrane signaling receptor, in UC epithelium. Conversely, sST2 is increased in both colonic biopsies and in the systemic circulation of UC patients, primarily produced by mucosal LPMC, to a lesser extent IEC, and potentially by circulating PBMC. In fact, increased sST2 (and IL-33) serum levels may be indicative of an active inflammatory state and serve as potential biomarkers for IBD, particularly UC, wherein systemic IL-33/sST2 ratios were significantly elevated.
An important concept of the IL-33/ST2 system and shared by other IL-1 family members (e.g., IL-1 and IL-18) is the balance among cytokine, receptor, and competing receptor antagonist/decoy receptor/negative regulator that impacts the cytokine’s overall bioactivity (34). Imbalances in both IL-1 and IL-18 systems have been implicated in the pathogenesis of UC and CD, respectively (22, 35). The sST2 protein has been reported to possess anti-inflammatory properties, such as inhibition of IL-1R type I and TLR4 (36); more importantly, sST2 is thought to serve as a decoy receptor to “trap” IL-33, thus competing with membrane bound ST2L (12). Indeed, although we found the IL-33/ST2 balance significantly increased and overall bioavailable IL-33 may be greater in UC, considerable amounts of sST2 were still detected in both the local gut mucosa and serum of UC patients vs. CON, which is conceptually counterintuitive to promoting proinflammatory Th2 immune responses. To date, however, the direct effects of IL-33 on regulating either its signaling or its decoy receptor have not been firmly established. It has also been reported that binding of cytokine to its soluble receptor(s) can result in increasing the circulating half-life and overall bioactivity of the cytokine; this phenomenon has, for example, been described for IL-6/sIL-6R pairing (37). Although this type of interaction between IL-33 and sST2 has not been reported, the possibility may exist. Interestingly, we detected in both human and mouse sera only c-IL-33 that has markedly reduced bioactivity vs. f-IL-33 (9, 11), suggesting the existence of specific extracellular proteases that cleave 30-kDa IL-33 into its 20- to 22-kDa form because significant amounts of f-IL-33 were detected in inflamed UC and SAMP mucosa, particularly intracellularly in IEC. It can thus be speculated that the presence of this mechanism may be to reduce the overall deleterious effects that can be triggered with high levels of systemic IL-33, such as that reported in IL-33-induced anaphylactic shock (14).
TNF has the ability to regulate IL-33 production in vitro (8, 33). As such, we were presented with an ideal situation to evaluate whether IL-33 and/or ST2 can be modulated by TNF in vivo, because anti-TNF therapy represents “state-of-the-art” treatment for IBD (2). Overall, our results demonstrated that neutralization of TNF had the ability to alter both IL-33 and sST2 in human disease, and had both acute and long-term effects on IBD patients, systemically decreasing IL-33 and increasing sST2 levels, resulting in an overall net decrease in the IL-33/sST2 ratio. A greater impact of IL-33/sST2 modulation was observed in UC vs. CD after treatment with Infliximab, suggesting a different mechanism of response to anti-TNF therapy. In fact, in all UC patients evaluated, anti-TNF administration significantly decreased circulating IL-33, whereas the response in CD was less predictable. Also, serum sST2 levels in UC, but not CD, uniformly increased after anti-TNF therapy, although the range in baseline levels of systemic sST2 before treatment was much more variable in CD than UC. It is therefore conceivable that TNF/anti-TNF-dependent regulation of the IL-33/ST2 axis differs in UC vs. CD, with a more predictable profile of decreasing the IL-33/ST2 ratio in UC.
Our in vitro experiments showed that TNF induced intracellular accumulation of f-IL-33, but not its extracellular secretion, from IEC. These results were consistent with previous studies showing that TNF alone or in synergy with IL-1β increased IL-33 production from fibroblasts (33) and adipocytes/preadipocytes (8), wherein only intracellular, but not secreted, IL-33 was detected. Therefore, a two-step model may be hypothesized for TNF-induced IL-33 production in that TNF may be an effective stimulant for intracellular IL-33 production, but a different mechanism may be responsible for active IL-33 secretion, such as other cytokines, specific pattern recognition receptor activation or as proposed by others, direct cell injury (9, 11). Alternatively, IL-33 secretion may be restricted to select cell types and intracellular IL-33 may function mainly as a nuclear factor and alarmin (17), which may be the case in IEC.
In summary, our studies provide evidence that IL-33 and ST2 may be critical mediators in the pathogenesis of UC, specifically displaying an increased IL-33/ST2 imbalance. In the setting of UC, we also provide evidence that IL-33 isoforms may differentially contribute to disease pathogenesis, with IEC-derived f-IL-33 potently initiating and sustaining local Th2 mucosal immune responses, whereas specific extracellular proteases cleave IL-33 into its less active 20- to 22-kDa forms. c-IL-33 is detected at high levels in the serum of IBD patients and may serve as a circulating biomarker, particularly for UC. We also provide rationale for the potential use of anti-IL-33 strategies to treat UC. In fact, this approach has been tested in mouse models of airway inflammation and arthritis with encouraging results (12, 13). Neutralization of IL-33 in experimental models of colitis and patients with UC will thus provide definitive proof to support the pathogenic role of the IL-33/ST2 system in IBD.
Materials and Methods
Human Endoscopic Biopsies, Surgical Specimens, and Serum Samples.
Tissues from male and female patients, 18–65 years of age and affected by CD and UC, as well as CON were used for the present study. For full description, see SI Materials and Methods.
Tissue Harvest and Histologic Assessment of SAMP Ileitis.
SAMP and AKR mice were maintained in SPF conditions at the animal facilities of Case Western Reserve University. Terminal ilea were removed from mice and histologically evaluated as described (18). For full description, see SI Materials and Methods.
Isolation and Culture of MLN and Spleen Cells.
MLN and spleen cells were isolated from experimental mice and cultured in the presence or absence of immobilized anti-CD3 (5 μg/mL) and soluble anti-CD28 (1 μg/mL) with or without rmIL-33 (10 ng/mL) for 48 h as described (18). For full description, see SI Materials and Methods.
Isolation of IEC and LPMC.
Surgically resected colon specimens and mouse ilea were harvested and processed for IEC and human LPMC as described (5, 38). For full description, see SI Materials and Methods.
Flow Cytometry.
LPMC from UC patients were stained for FACS analysis by using specific antibodies against IL-33, ST2, CD4, CD8, CD11b and CD19. For full description, see SI Materials and Methods.
Cell Culture.
The HT-29 IEC line was grown to 80% confluency and cultured with or without rhTNF (10 ng/mL) for 0, 2, 6, 12, 24, and 48h. For full description, see SI Materials and Methods.
IHC.
Immunohistochemical staining was performed as described (5), using specific detecting antibodies for IL-33 and ST2. For full description, see SI Materials and Methods.
IL-33 and ST2 mRNA Expression.
Total RNA was extracted from human colonic biopsies, mouse ilea, freshly isolated IEC and HT-29 cells and RT-PCR was performed by using specific primers, as described (38–41). For full description, see SI Materials and Methods.
Western Blot Analysis.
Western blots were performed on total protein extracts or cell fractions of colonic biopsies, mouse ilea, freshly isolated and HT-29 IEC, or on diluted (1:100) human and mouse sera as described (5), using specific antibodies for IL-33 and ST2. For full description, see SI Materials and Methods.
ST2 and Cytokine Protein Levels.
Human IL-33 and ST2, and mouse cytokine concentrations were quantified by commercially available sandwich and multiplex ELISA kits. For full description, see SI Materials and Methods.
Statistical Analysis.
Statistical analyses were performed by using one-way ANOVA, Kruskal-Wallis test, paired Student’s t test for paired data, and linear regression, as appropriate.
Supplementary Material
Acknowledgments
The authors acknowledge the UVA Biorepository and Tissue Research Facility for select tissue specimens, resources provided by the Silvio O. Conte Digestive Diseases Center at the University of Virginia (National Institutes of Health Grant DK067629), and Brian Reuter, Paul Guarino, Fabio Cominelli, and Xiao-Ming Wang for technical/scientific input. This work was supported by National Institutes of Health Grants DK056762 and DK057880/PPG5 (T.T.P.) and an Italian Ministry of University and Research PRIN Grant 2007K4HZEJ_004 (M.V.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912678107/DCSupplemental.
References
- 1.Cominelli F. Cytokine-based therapies for Crohn’s disease—new paradigms. N Engl J Med. 2004;351:2045–2048. doi: 10.1056/NEJMp048253. [DOI] [PubMed] [Google Scholar]
- 2.Rutgeerts P, Vermeire S, Van Assche G. Biological therapies for inflammatory bowel diseases. Gastroenterology. 2009;136:1182–1197. doi: 10.1053/j.gastro.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Casini-Raggi V, et al. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J Immunol. 1995;154:2434–2440. [PubMed] [Google Scholar]
- 4.Andus T, et al. Imbalance of the interleukin 1 system in colonic mucosa—association with intestinal inflammation and interleukin 1 receptor antagonist [corrected] genotype 2. Gut. 1997;41:651–657. doi: 10.1136/gut.41.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pizarro TT, et al. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn’s disease: Expression and localization in intestinal mucosal cells. J Immunol. 1999;162:6829–6835. [PubMed] [Google Scholar]
- 6.Monteleone G, et al. Bioactive IL-18 expression is up-regulated in Crohn’s disease. J Immunol. 1999;163:143–147. [PubMed] [Google Scholar]
- 7.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Wood IS, Wang B, Trayhurn P. IL-33, a recently identified interleukin-1 gene family member, is expressed in human adipocytes. Biochem Biophys Res Commun. 2009;384:105–109. doi: 10.1016/j.bbrc.2009.04.081. [DOI] [PubMed] [Google Scholar]
- 9.Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci USA. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem. 2009;284:19420–19426. doi: 10.1074/jbc.M901744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lüthi AU, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Oshikawa K, Yanagisawa K, Tominaga S, Sugiyama Y. Expression and function of the ST2 gene in a murine model of allergic airway inflammation. Clin Exp Allergy. 2002;32:1520–1526. doi: 10.1046/j.1365-2745.2002.01494.x. [DOI] [PubMed] [Google Scholar]
- 13.Palmer G, et al. Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis Rheum. 2009;60:738–749. doi: 10.1002/art.24305. [DOI] [PubMed] [Google Scholar]
- 14.Pushparaj PN, et al. The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci USA. 2009;106:9773–9778. doi: 10.1073/pnas.0901206106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: The new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 16.Werman A, et al. The precursor form of IL-1alpha is an intracrine proinflammatory activator of transcription. Proc Natl Acad Sci USA. 2004;101:2434–2439. doi: 10.1073/pnas.0308705101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carriere V, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci USA. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bamias G, et al. Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology. 2005;128:654–666. doi: 10.1053/j.gastro.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 19.Rivera-Nieves J, et al. Emergence of perianal fistulizing disease in the SAMP1/YitFc mouse, a spontaneous model of chronic ileitis. Gastroenterology. 2003;124:972–982. doi: 10.1053/gast.2003.50148. [DOI] [PubMed] [Google Scholar]
- 20.Takedatsu H, et al. Interleukin-5 participates in the pathogenesis of ileitis in SAMP1/Yit mice. Eur J Immunol. 2004;34:1561–1569. doi: 10.1002/eji.200324680. [DOI] [PubMed] [Google Scholar]
- 21.Mitsuyama K, et al. STAT3 activation via interleukin 6 trans-signalling contributes to ileitis in SAMP1/Yit mice. Gut. 2006;55:1263–1269. doi: 10.1136/gut.2005.079343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reuter BK, Pizarro TT. Commentary: The role of the IL-18 system and other members of the IL-1R/TLR superfamily in innate mucosal immunity and the pathogenesis of inflammatory bowel disease: friend or foe? Eur J Immunol. 2004;34:2347–2355. doi: 10.1002/eji.200425351. [DOI] [PubMed] [Google Scholar]
- 23.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 24.Yanagisawa K, Takagi T, Tsukamoto T, Tetsuka T, Tominaga S. Presence of a novel primary response gene ST2L, encoding a product highly similar to the interleukin 1 receptor type 1. FEBS Lett. 1993;318:83–87. doi: 10.1016/0014-5793(93)81333-u. [DOI] [PubMed] [Google Scholar]
- 25.Fuss IJ, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]
- 26.Wehkamp J, Koslowski M, Wang G, Stange EF. Barrier dysfunction due to distinct defensin deficiencies in small intestinal and colonic Crohn’s disease. Mucosal Immunol. 2008;1(Suppl 1):S67–S74. doi: 10.1038/mi.2008.48. [DOI] [PubMed] [Google Scholar]
- 27.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–2449. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 29.Pastorelli L, Vecchi M, Cominelli F, Pizarro TT. Genetic regulation of epithelial barrier function: Recent insights into the pathogenesis of inflammatory bowel disease. IBD Monitor. 2008;8:124–133. [Google Scholar]
- 30.Kaser A, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heazlewood CK, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahern PP, Izcue A, Maloy KJ, Powrie F. The interleukin-23 axis in intestinal inflammation. Immunol Rev. 2008;226:147–159. doi: 10.1111/j.1600-065X.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 33.Xu D, et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci USA. 2008;105:10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 35.Corbaz A, et al. IL-18-binding protein expression by endothelial cells and macrophages is up-regulated during active Crohn’s disease. J Immunol. 2002;168:3608–3616. doi: 10.4049/jimmunol.168.7.3608. [DOI] [PubMed] [Google Scholar]
- 36.Brint EK, et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–379. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- 37.Peters M, et al. The function of the soluble interleukin 6 (IL-6) receptor in vivo: Sensitization of human soluble IL-6 receptor transgenic mice towards IL-6 and prolongation of the plasma half-life of IL-6. J Exp Med. 1996;183:1399–1406. doi: 10.1084/jem.183.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olson TS, et al. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med. 2006;203:541–552. doi: 10.1084/jem.20050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwab A, et al. Functional role of Na+-HCO3- cotransport in migration of transformed renal epithelial cells. J Physiol. 2005;568:445–458. doi: 10.1113/jphysiol.2005.092957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verri WA, Jr, et al. IL-33 mediates antigen-induced cutaneous and articular hypernociception in mice. Proc Natl Acad Sci USA. 2008;105:2723–2728. doi: 10.1073/pnas.0712116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lécart S, et al. Activated, but not resting human Th2 cells, in contrast to Th1 and T regulatory cells, produce soluble ST2 and express low levels of ST2L at the cell surface. Eur J Immunol. 2002;32:2979–2987. doi: 10.1002/1521-4141(2002010)32:10<2979::AID-IMMU2979>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.