Abstract
Large conductance voltage- and Ca2+-activated potassium channels (BK channels) are important feedback regulators in excitable cells and are potently regulated by protein kinases. The present study reveals a dual role of protein kinase C (PKC) on BK channel regulation. Phosphorylation of S695 by PKC, located between the two regulators of K+ conductance (RCK1/2) domains, inhibits BK channel open-state probability. This PKC-dependent inhibition depends on a preceding phosphorylation of S1151 in the C terminus of the channel α-subunit. Phosphorylation of only one α-subunit at S1151 and S695 within the tetrameric pore is sufficient to inhibit BK channel activity. We further detected that protein phosphatase 1 is associated with the channel, constantly counteracting phosphorylation of S695. PKC phosphorylation at S1151 also influences stimulation of BK channel activity by protein kinase G (PKG) and protein kinase A (PKA). Though the S1151A mutant channel is activated by PKA only, the phosphorylation of S1151 by PKC renders the channel responsive to activation by PKG but prevents activation by PKA. Phosphorylation of S695 by PKC or introducing a phosphomimetic aspartate at this position (S695D) renders BK channels insensitive to the stimulatory effect of PKG or PKA. Therefore, our findings suggest a very dynamic regulation of the channel by the local PKC activity. It is shown that this complex regulation is not only effective in recombinant channels but also in native BK channels from tracheal smooth muscle.
Keywords: phosphorylation, protein kinase A, protein kinase G, protein phosphatase 1, tracheal smooth muscle cells
Large conductance Ca2+-activated potassium channels (BK channels) are unique in their regulation by both intracellular Ca2+ and membrane voltage. They are expressed in many tissues, and are particularly abundant in nerve and smooth muscle, where they play a key role as negative and positive feedback regulators of cell excitability by conducting repolarizing and hyperpolarizing outward currents, as has been impressively demonstrated in mice with targeted deletion of the pore-forming α-subunit (1–5). Alternative splicing of premRNA and protein phoshorylation generates structural and functional diversity of BK channels. Several serine/threonine kinases, such as the cAMP-(PKA)- and cGMP-dependent protein kinase (PKG) and protein kinase C (PKC), potently regulate BK channel activity. Their phosphorylation sites at the pore-forming α-subunit are fully conserved in almost all mammalian alternative splice variants, and mutation of the PKA and PKG phosphorylation sites abolished the kinase effect on channel activity in heterologous expression systems (6–10). In smooth muscle, PKA and PKG predominantly activate BK channels by increasing the apparent voltage and Ca2+ sensitivity of the channel, whereas PKC exerts opposite effects (11). Experimental evidence indicates that hormones and drugs that activate PKA or PKG contribute to smooth muscle relaxation by activation of BK channels. In contrast, activators of PKC seem to reinforce contraction by inhibiting BK channels as negative feedback regulators (12). Despite the potential physiological and pathophysiological relevance, the molecular mechanism of BK channel inhibition by PKC has remained elusive. This is surprising because numerous hormones, neurotransmitters, and drugs bind to G protein-coupled receptors, which signal through pathways leading to activation of PKC.
We show in this study that PKC inhibits the open-state probability of BK channel α-subunits. This inhibition depends on a sequential phosphorylation of two distinct serines in the C terminus of the channel protein. In addition, both PKC phosphorylation sites have important effects on the regulation of BK channels by PKA and PKG in primary cells, e.g., freshly isolated smooth muscle cells of the mouse trachea.
Results
Inhibition of BK Channel Activity by PKC.
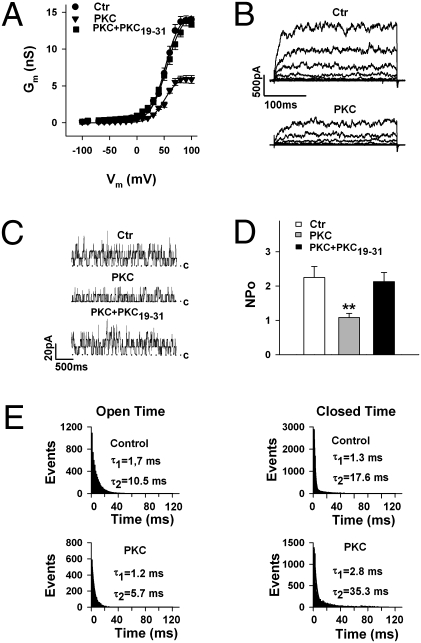
The PKC-dependent BK channel regulation was investigated with the pore-forming BK channel α-subunit (KCNMA1) cloned from bovine trachea (BKA, corresponding to the BKzero variant) (9). When inside-out membrane patches were superfused at the cytosolic side with 30 nM of the catalytically active fragment of PKC (PKCc), a decrease of macroscopic currents was observed at all voltages tested (−100 to +100 mV; Fig. 1 A and B). The current decline started 30 s after the application of PKCc and reached a stable level within 4–5 min. At +80 mV, PKC reduced membrane conductance (Gm) from 13.7 ± 0.7 to 5.9 ± 0.5 nS, i.e., by 57.2 ± 3.4% (n = 7). Reduced Gmax could not be reversed by increasing voltage or Ca2+. The inhibitory PKC effect was abolished when inside-out patches were concurrently superfused for at least 5 min with PKCc and 5 μM of the PKC pseudosubstrate inhibitor peptide PKC19–31 (Fig. 1A). Representative macroscopic BK currents before (Ctr) and after application of 30 nM PKCc are shown in Fig. 1B. To avoid effects on channel activity of the storage buffers in which the purified protein kinases were dissolved, freshly excised inside-out patches were superfused with control solutions containing the same amount of storage buffer as the solution with the respective kinase (SI Materials and Methods). Experiments carried out on inside-out patches with low channel density in the presence of symmetrically high potassium (140 mM) revealed that PKC decreased channel open probability, NPo, by shortening channel open time and prolonging the closed state of the channel (Fig. 1 C–E). Channel open and closed times were well fitted by two exponentials, corresponding to a short (τ1 ∼1 ms) and a longer duration (τ2 ∼10 ms for open time and ∼20 ms for closed time). PKC affected only τ2; it shortened the channel open time from 12.0 ± 5.3 to 7.0 ± 2.9 ms (n = 9) and prolonged the closed time from 23.9 ± 3.8 to 47.5 ± 4.7 ms (n = 7). The single-channel conductance, the voltage dependence, and the calcium sensitivity of BK channels were not affected by PKC (Fig. S1).
Fig. 1.
PKC inhibits BK channel activity by decreasing channel open state probability. (A) Conductance-voltage relationships obtained from inside-out membrane patches of HEK293 cells expressing BK channels. Curves before (Ctr, n = 15) and in the presence of 30 nM PKCc (PKC, n = 7) are shown. The PKC pseudosubstrate inhibitor peptide PKC19–31 (5 μM; n = 8) abolished the PKC effect. (B) Representative macroscopic BK channel currents before (Ctr) and in the presence of 30 nM PKCc (−40 to +80 mV in 20-mV increments). (C) Decrease of NPo by 30 nM PKCc and abolition of this effect by the PKC inhibitor peptide; c indicates the closed state of the channel. Shown are single-channel currents from an inside-out patch with low channel density in the presence of symmetrically high potassium of 140 mM at +40 mV. (D) Summary of NPo before (Ctr; n = 11) and in the presence of PKCc (n = 6), or PKCc plus PKC19–31 (n = 5). Data are means ± SEM. (E) PKC shortens the open state and prolongs the closed state of BK channels. Shown are open- and closed-time histograms fitted by two exponentials (τ1 and τ2). Recording conditions as in C, except that the data used for the histograms were taken from inside-out patches with only one active channel. **P < 0.01 vs. control (Ctr). The intracellular (bath) Ca2+ concentration was 1 μM in A and B, 0.3 μM in C–E.
PKC-Induced Inhibition Depends on Phosphorylation of Ser695 and Ser1151.
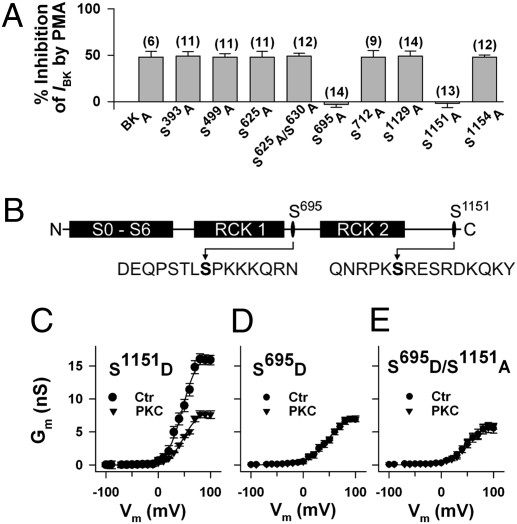
By screening the BK channel α subunit for PKC consensus sequences (13), we found in addition to the prominent tandem motif with Ser1151 and Ser1154, seven further serines—Ser390, Ser499, Ser625, Ser630, Ser695, Ser712, and Ser1129—as putative PKC phosphorylation sites. Whole-cell IBK was measured in mutants where the respective serines were replaced by alanine. With the exception of the mutants S695A and S1151A, at which the PKC activator phorbol 12-myristate 13 acetate (PMA) had lost its inhibitory effect, the activation of PKC reduced IBK at all potentials by ∼50% (Fig. 2A). In contrast, the inactive phorbol ester analog 4α-PMA (100 nM) had no significant effect on BK channel currents. The positions of the critical residues Ser695 and Ser1151 and the respective flanking regions within the BKA isoform are shown in Fig. 2B.
Fig. 2.
Inhibition of BK channel activity by PKC depends on serines 695 and 1151. (A) Mutation of the PKC phosphorylation sites Ser695 and Ser1151 to alanine abolished the inhibitory effect of the PKC activator phorbol 12-myristate 13 acetate (100 nM PMA) on BK channel whole-cell currents in transfected HEK293 cells. Percent inhibition of BK currents (IBK) at +80 mV is shown with number of cells in parentheses. The pipette solution contained 0.3 μM Ca2+. IBK before adding PMA was not significantly different between mutants (range: 121.2 ± 8.0 for S625A and 127.7 ± 12.3 pA pF−1 for S499A) and BKA (125.3 ± 13.1 pA pF−1). (B) Schematic representation of the BK channel with the positions of Ser695 and Ser1151 and their flanking regions, with the first methionine being in the context MANGG according to GenBank accession no. AAK54352.1. (C–E) Conductance-voltage relationships of mutants obtained from inside-out membrane patches before (Ctr) and after application of 30 nM PKCc (PKC). Means ± SEM of six (S1151D and S695D) and eight (S695D/S1151A) cells, respectively. Note, Gmax of channels inhibited by PKC resembles Gmax of phosphomimetic mutants. The intracellular (bath) Ca2+ concentration was 1 μM.
To examine whether the negative charge added by phosphorylation of Ser695 and Ser1151 is responsible for BK channel inhibition by PKC, we constructed mutants in which either serine was replaced by the negatively charged amino acid aspartate (S695D and S1151D). Surprisingly, the S1151D mutant did not significantly differ from the nonmutated BK channel with respect to membrane conductance, the half-maximal activating voltage (V1/2) and inhibition of conductance by 30 nM PKCc (cf. Fig. 2C with Fig. 1A). Introducing a negative charge at position 695 (S695D), however, resulted in channels with a strongly reduced membrane conductance resembling that of nonmutated BK channels in the presence of PKC (cf. Fig. 2D with Fig. 1A). At +80 mV, the mean membrane conductance of the S695D mutant was 6.4 ± 0.5 nS (n = 6), which is 47.4 ± 2.1% of the conductance measured in the nonmutated BK channel at the same potential. The mutant was insensitive toward PKC; a V1/2 of 47.9 ± 3.6 mV (n = 6) vs. 51.6 ± 4.6 mV in seven controls indicates that its voltage-dependent activation was unchanged. Nearly identical results as those shown in Fig. 2D were obtained with the double mutant S695D/S1151D. To determine the contribution of Ser1151 to the inhibitory effect of PKC, we created the double mutant S695D/S1151A. This mutant showed exactly the same electrophysiological characteristics as the mutants S695D and S695D/S1151D, its membrane conductance was strongly reduced with respect to the nonmutated BK channel (by 59.8 ± 3.9% at +80 mV; n = 8), and PKC was ineffective (Fig. 2E). Taken together, these findings indicate that phosphorylation of Ser1151 is constitutive (or unconditional) in nonmutated BK channels and a prerequisite for PKC-induced BK channel inhibition by phosphorylation of Ser695 (conditional phosphorylation). The decrease of membrane conductance depends solely on a negative charge at the amino acid position 695.
Stoichiometry of BK Channel Phosphorylation by PKC.
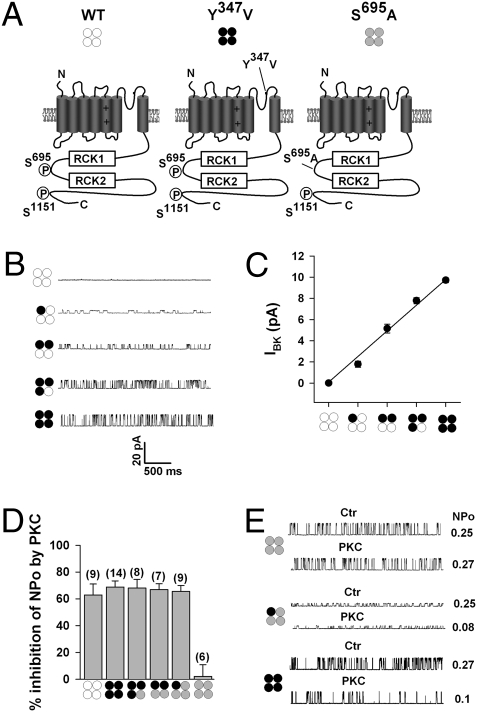
BK channel α-subunits are assembled as tetramers. To investigate the stoichiometry of BK channel tetramer phosphorylation by PKC, we employed a strategy that allowed us to determine electrophysiologically the subunit composition of each BK channel from the single-channel current amplitude by introducing a mutation to the tetraethylammonium (TEA)-sensitive site (10, 14, 15). Introducing the point mutation Y347V in the subunit pore (Fig. 3A) strongly reduces the sensitivity of BK channels expressed in outside-out patches to extracellular TEA (2 mM), whereas nonmutated channels (WT) were completely blocked (Fig. 3 B and C). The stoichiometry of mixtures of TEA-sensitive and TEA-insensitive α-subunits could thus be predicted from the single-channel current amplitude in the presence of 2 mM TEA (Fig. 3 B and C). We then examined in inside-out patches with 2 mM TEA in the patch-pipette channels formed from mixtures of BK α-subunits containing the TEA mutation (Y347V, which retains the two PKC phosphorylation sites) coexpressed with TEA-sensitive BK channels in which either Ser695 (Fig. 3A) or Ser1151 had been mutated to alanine. The TEA sensitivity of S695A and S1151A homotetramers was not significantly different from nonmutated BK channels. The application of 30 nM PKCc to the cytosolic side of patches expressing either the homotetrameric Y347V mutant or the heterotetrameric channels, resulted in a robust inhibition of NPo by ∼60% (Fig. 3D; note that the data shown here for the mutant S695A are nearly identical with those obtained from the S1151 mutant and which are therefore omitted). For comparison, the PKC-induced inhibition of NPo in homotetrameric nonmutated BK channels (WT), and the lacking PKC effect in homotetrameric S695A mutants, is also shown, albeit in the absence of TEA. Taken together, the findings show that only one BK channel α-subunit within the tetramer needs to be phosphorylated for PKC-induced channel inhibition (Fig. 3 D and E).
Fig. 3.
PKC inhibition of BK channels requires phosphorylation of a single α-subunit within the tetramer. (A) Schematic of the nonmutated BK channel α-subunit (WT, open circles), the TEA-insensitive Y347V α subunit (black circles), and the PKC site mutant S695A (gray circles). The critical PKC phosphorylation sites S695, located between regulator of K+ conductance (RCK) domains, and S1151 are indicated. Representative single-channel traces (B) and summary plot of single-channel amplitude (C) of BK channels in the presence of 2 mM TEA, assembled as heterotetramers of BK and Y347V α-subunits. Pictograms illustrate predicted channel stoichiometry. Recordings were at +40 mV in symmetrically high potassium (140 mM) from outside-out patches of transfected cells, means ± SEM, 6–14 patches per group. (D) Summary of effects of 30 nM PKCc on NPo by using BK-Y347V plus S695A heterotetramers with (3:1), (2:2), and (1:3) stoichiometry. For comparison, the PKC effect on homotetrameric WT−, Y347V−, and S695A− channels are also shown. Data from inside-out patches with symmetrically high potassium (140 mM) at +40 mV. With the exception of WT− and S695A homotetrameric channels, the recording pipette contained 2 mM TEA. Means ± SEM; n in parentheses. (E) Representative single records before (Ctr) and NPo after application of 30 nM PKCc (PKC) to channels composed as indicated by the pictograms. Recordings as in D. Intracellular free Ca2+ concentration was 0.3 μM.
PKC Modulates the Effects of PKA and PKG on BK Channels.
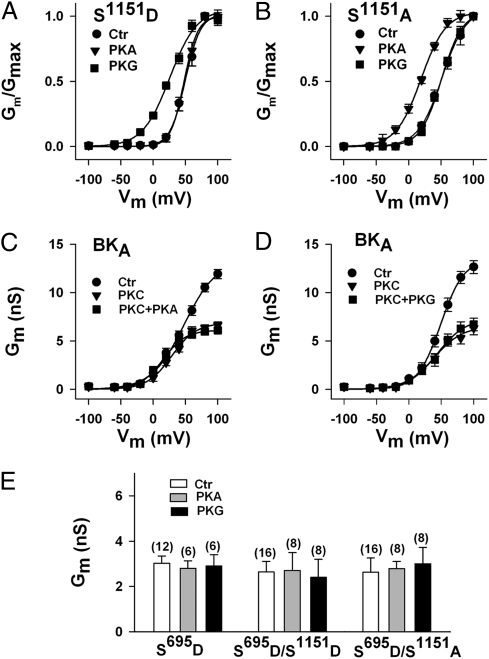
We have shown earlier that the mutation of the tandem PKC motif in the C terminus to alanine (S1151A/S1154A) switches BK channel regulation from PKG to PKA (9). When we applied 300 nM PKG to the cytosolic face of excised inside-out membrane patches expressing the S1151D mutant, we observed a 26 mV shift to the left of the normalized conductance-voltage relation (V1/2 before, 51.4 ± 4.2 mV, n = 12; V1/2 after PKG 25.1 ± 4.4 mV; n = 6). Opposite to PKG, the catalytic subunit of PKA (300 nM) had no significant influence on the S1151D mutant (V1/2 before 51.4 ± 4.2 mV, n = 12; V1/2 after PKA 48.5 ± 3.8 mV; n = 6; Fig. 4A). These findings resemble those obtained earlier with the nonmutated BK channel (9). Inside-out membrane patches expressing the S1151A mutant, however, proved insensitive to stimulation by PKG but were responsive to PKA. V1/2 obtained from normalized conductance-voltage relations was 52.1 ± 5.1 mV before (control, n = 14) and 50.7 ± 5.8 mV after the application of PKG (n = 7; not significant). In contrast, PKA shifted V1/2 by 29 mV from 52.1 ± 5.1 (n = 14) to 22.7 ± 4.2 mV (n = 7; Fig. 4B). Nearly identical results as those shown in Fig. 4 A and B were obtained when Ser1151 was mutated on the S695A background (Fig. S2). Because phosphorylation/dephosphorylation of Ser1151 seems to determine whether BK channels are regulated by PKG or PKA, we wondered whether BK channels under the influence of 30 nM PKCc are still regulated by the two cyclic nucleotide-dependent kinases. When in the presence of PKCc, PKG or PKA was applied to excised inside-out patches expressing the nonmutated BK channel, neither kinase enhanced membrane conductance at any potential (Fig. 4 C and D). The same result was obtained with the mutant S695D (n = 6) and the double mutants S695D/S1151D (n = 7) and S695D/S1151A (Fig. 4E; n = 8). Mutation of Ser695 in alanine abolished the inhibitory PKC effect on BK channels but had no influence on the stimulatory effect of additionally applied PKG (Fig. S3). Thus, BK channels in which Ser695 is phosphorylated by PKC or contain a negatively charged amino acid at this position are no longer regulated by cyclic nucleotide-dependent protein kinases.
Fig. 4.
Phosphorylation of serines 695 and 1151 determines the sensitivity of BK channels to PKA and PKG. (A and B) Normalized conductance-voltage relationships before (Ctr) and after the application of 300 nM PKA and PKG, respectively. The phosphomimetic mutation S1151D renders BK channels sensitive to PKG but not to PKA, whereas the mutant S1151A is sensitive to PKA only. Inside-out patches are 12 (Ctr) and 6 for PKA and PKG, respectively (S1151D), and 14 (Ctr) and 7 for PKA and PKG, respectively (S1151A). (C and D) PKC inhibits PKA- and PKG-induced BK channel activation. Conductance-voltage relationships of nonmutated BK channels before (Ctr), after the application of 30 nM PKCc, and after the additional application of either 300 nM PKA (C, n = 6) or PKG (D, n = 8). (E) The phosphomimetic mutation S695D prevents the activating effects of PKA and PKG on BK channels irrespective of whether residue 1151 contains a negative charge. Conductances from inside-out patches at +40 mV with n in parentheses. Intracellular-free Ca2+ concentration was 1 μM; means ± SEM.
PKC-Dependent Inhibition of BK Channels in Tracheal Smooth Muscle Cells.
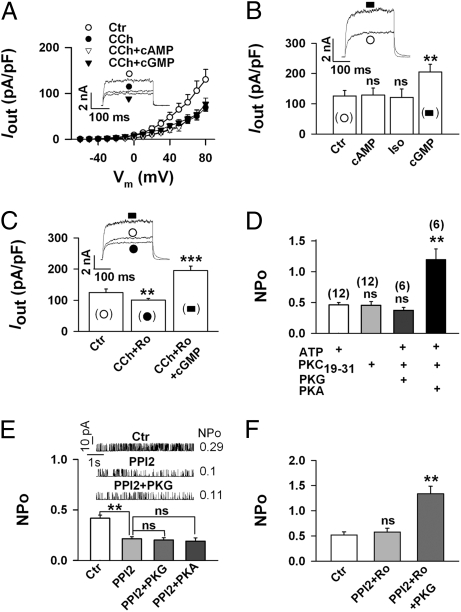
To verify that the regulation of BK channels described above occurs not only in a heterologous expression system but also under physiological conditions, we investigated the PKC-dependent regulation of BK channel activity in native cells. Recently, we showed that stimulation of muscarinic M2 receptors by carbamylcholine (CCh) inhibits BK channels in isolated smooth muscle cells of the mouse trachea (TSMCs) by a dual mechanism involving PKC and Giβγ (12). When we elicited whole-cell outward currents (Iout) in freshly isolated TSMCs, addition of the specific BK channel-blocking peptide iberiotoxin (300 nM) revealed that more than 90% of Iout was conducted by BK channels (Fig. S4). The application of 10 μM CCh decreased within 5 min Iout at all potentials by ∼50%. At 80 mV, current densities decreased from 129.9 ± 22.3 to 69.0 ± 8.3 pA pF−1, i.e., by 46.3 ± 4.7% (Fig. 5A; n = 16). Additional application of the membrane permeant-specific activators of PKG (300 μM 8-pCPT-cGMP) or PKA (300 μM Sp-5,6-DCI-cBIMPS) for 10 min, produced no further significant change of Iout (Fig. 5A). In contrast, when the protein kinase activators were applied in the absence of CCh, only the PKG activator enhanced Iout by 63.1 ± 5.2% at 80 mV (n = 6), whereas the PKA activator or the β-adrenoreceptor agonist isoproterenol (10 μM) were ineffective (Fig. 5B). To demonstrate that the abolition of the PKG effect in the presence of CCh was due to activation of PKC, we superfused TSMCs for 5 min with the PKC inhibitor Ro 31-8220 (1 μM), which did not significantly affect Iout. When 10 μM CCh was added thereafter, inhibition of Iout was not completely prevented because Giβγ subunits partially inhibit BK channels (12). Further application of 300 μM 8-pCPT-cGMP, however, induced within 3–4 min an increase of BK channel activity by 66.0 ± 8.9% (n = 6), which is comparable to the increase obtained in control cells in the absence of CCh (cf. Fig. 5C with Fig. 5B). Taken together, the findings clearly show that similar to recombinant BK channels, PKG but not PKA regulates BK channels in TSMCs, and that CCh via activation of PKC abolishes the kinase effect.
Fig. 5.
Carbachol-induced inhibition of BK channels in tracheal smooth muscle cells (TSMCs) shows the same characteristics as PKC-induced inhibition of BK channels in HEK293 cells. (A–C) Whole-cell outward currents (Iout) were elicited from a holding potential of −10 mV by depolarizing freshly isolated TSMCs every 5 s for 300 ms from −60 to +80 mV in 10-mV increments. Means ± SEM are shown. The original current recordings are at +80 mV, and reflect interventions as indicated by the symbols in the corresponding figure. The pipette solution contained 0.3 μM Ca2+. (A) Carbachol (CCh, 10 μM) decreases Iout and prevents channel regulation by activators of PKA (cAMP = 300 μM Sp-5,6-DCI-cBIMPS) and PKG (cGMP = 300 μM 8-pCPT-cGMP). Current-voltage relationships of 16 cells from 5 mice (Ctr and CCh), of 10 cells from 4 mice (CCh + cGMP), and of 6 cells from 3 mice (CCh + cAMP) are shown. (B) In the absence of CCh, only the activator of PKG (cGMP) enhances Iout. Ctr (19 cells from 9 mice), cAMP (6 cells from 3 mice), Iso (10 μM isoproterenol; 7 cells from 3 mice), and cGMP (6 cells from 3 mice). (C) The PKC inhibitor Ro31-8220 (1 μM Ro) partially inhibits the CCh effect and restores the stimulatory action of the PKG activator (cGMP). Iout of 6 cells from 3 mice is presented at +80 mV. (D) Dephosphorylation of BK channels by exposing inside-out patches from TSMCs to ATP-free solution containing 5 μM of the PKC inhibitor peptide PKC19–31, switches NPo activation from PKG to PKA. Means ± SEM of NPo at +40 mV with n (from 4 mice) in parentheses. (E and F) The phosphatase 1 inhibitor peptide (20 nM PPI2) decreases NPo of inside-out patches from TSMCs via a PKC-dependent pathway and prevents activation by PKG and PKA. Means ± SEM of NPo at +40 mV. The original traces in E show single-channel recordings from a representative patch. (E) 13 patches from 6 mice (Ctr and PPI2), 7 patches from 3 mice (PPI2 + PKG), 6 patches from 3 mice (PPI2 + PKA), and (F) 6 patches from 4 mice. (D–F) The intracellular (bath) Ca2+ concentration was 0.3 μM. **P < 0.01; ***P < 0.001; ns, not significant.
To provide supporting evidence, that dephosphorylation of a PKC-specific site, presumably Ser1151, switches the regulation of BK channels also in TSMCs from PKG to PKA, inside-out patches were superfused for 5 min with the inhibitory peptide PKC19–31. To allow complete dephosphorylation of PKC sites, inside-out patches were first superfused for 5 min with ATP-free solution to which 5 μM PKC19–31 had been added. This solution, which did not significantly alter NPo, was then changed for another 5 min to a solution containing ATP, PKC19–31, and either PKG or PKA. Similar to the results obtained with the mutant BK channel S1151A (Fig. 4B), PKC19–31 prevented the stimulatory action of PKG in TSMCs and switched the responsiveness of the channel toward PKA. At a holding potential of 40 mV, NPo was not significantly changed by PKG (control NPo 0.44 ± 0.05; after PKG 0.37 ± 0.05, n = 6), whereas a 2.4-fold increase was induced by PKA (control NPo 0.47 ± 0.03; after PKA 1.19 ± 0.17, n = 6; Fig. 5D).
Finally, we wondered whether the effect of CCh on BK channel activity can be mimicked by the inhibition of channel-associated protein phosphatases (16–18). When we superfused inside-out patches from TSMCs with 20 nM of the protein phosphatase 1 inhibitor peptide (PPI2) for 5 min, NPo decreased by ∼50% at all potentials. At 40 mV, NPo was 0.42 ± 0.03 before and 0.21 ± 0.02 after the addition of PPI2 (Fig. 5E; n = 13). Additional application of 300 nM PKG or PKA for 5 min produced no further significant effect on NPo (Fig. 5E). When the experiments were repeated in the presence of the PKC inhibitor Ro 31–8220, PPI2 did not inhibit NPo, and additional application of 300 nM PKG enhanced NPo 2.6-fold at 40 mV from 0.52 ± 0.06 to 1.34 ± 0.15 (Fig. 5F; n = 6). Preferential inhibition of protein phosphatase 2A by 3 nM ocadaic acid for 10 min failed to induce BK channel inhibition and did not abolish PKG-dependent activation of BK channels (Fig. S5).
Discussion
The data reported in this study establish that inhibition of BK channels by PKC depends on the unconditional and conditional phosphorylation of the C-terminal serine residues 1151 and 695, respectively. The inhibition of BK channel conductance by PKC is due to a decrease of channel open probability without changing the unitary current amplitude. From the open- and closed-time histograms it is obvious that the inhibitory effect of PKC is mediated by altering both the opening and closing process to stabilize the nonconducting conformations of the channel. The voltage dependence and the Ca2+ sensitivity of the BK channel were not affected by PKC. In addition, the phosphorylation of the BK channel by PKC abolishes the stimulatory effects of PKA and PKG.
We investigated the mechanism underlying the PKC-induced BK channel inhibition using a BK channel isoform that contains no inserts at the alternative splice sites, and has been identified in a variety of species, including man (19, 20). Among nine putative PKC phophorylation sites, two serines, Ser695 and Ser1151, were identified by site-directed mutagenesis to be critical for PKC-dependent BK channel inhibition. Our experimental evidence indicates that Ser1151 is constitutively phosphorylated in HEK293 cells. First, the replacement of Ser1151 by aspartate (S1151D) to mimic PKC-dependent phosphorylation resulted in mutant BK channels that resembled nonmutated BK channels with respect to Gmax, V1/2, inhibition by PKC, and activation by PKG. Second, the BK channel mutant S1151A was insensitive to PKC and to stimulation by PKG. It was instead activated by PKA, which is in line with previous data (9) showing that phosphorylation/dephosphorylation of Ser1151 determines the channel's ability to respond to either PKG or PKA. The phosphorylation of the second critical serine, Ser695 by PKC, is conditional. It depends on the preceding phosphorylation of Ser1151. Most likely, this regulation relies on a conformational change within the C terminus that allows access of the PKC to Ser695 only when Ser1151 is phosphorylated, or when a negatively charged amino acid, like in the mutant S1151D, mimics the phosphorylation at this site. Ser695, which is highly conserved in BK channels of many vertebrates, is located in the linker between the two “regulator of K+ conductance” (RCK) domains. Both RCK1 and RCK2 domains contain high-affinity Ca2+-binding sites that form parts of a complex functional domain (gating ring) that converts the free energy of Ca2+ binding into mechanical work to open the channel (21–23). When Ser695 was replaced by aspartate to mimic phosphorylation at this position (S695D), BK currents were inhibited like under the influence of PKC. The same reduced channel conductance was also observed in the double mutant S695D/S1151A, in which the constitutive phosphorylation of Ser1151 was abandoned. The Ser695 mutants clearly demonstrate that a PKC-like inhibition of the BK channel depends exclusively on a negatively charged amino acid at position 695. BK channel α-subunits expressing a 59-amino acid stress-regulated exon at splice site 2 (STREX-1) exhibit an additional PKA consensus motif at serine residue 3 within the STREX insert (8, 10). PKA inhibits NPo of STREX channels via phosphorylation of S3STREX, probably by preventing palmitoylation of the C terminus (24). Interestingly, the S3STREX is located within the RCK1 and RCK2 linker only a few amino acids upstream of Ser695. BK channels are assembled as tetramers of pore-forming α-subunits (14), each of which includes a PKC phosphorylation site. We found that phosphorylation of only one BK α-subunit either at Ser1151 or Ser695 is sufficient for channel inhibition. Thus, the PKC-dependent inhibition obeys a single subunit rule, which is similar to the inhibitory effect of PKA in STREX channels, but different from insertless BK channels where phosphorylation of all four α-subunits is required for channel activation by PKA (10). Whether prevention of palmitoylation of the C terminus also plays a role for PKC-dependent BK channel inhibition is presently unknown. Note, however, that the phosphorylation of S3STREX by PKA produced a ∼20-mV rightward shift of the voltage-dependent activation curve (25), whereas PKC reduced the maximal BK channel activity without affecting the apparent voltage sensitivity. A similar observation has been made earlier with PKC on BK channels in pituitary CH4C1 cells (26).
The unconditional and conditional phosphorylation of BK channels by PKC implies differential tasks in channel regulation. Besides the direct inhibition of channel activity, PKC phosphorylation apparently modulates PKA- and PKG-mediated effects. In accordance with previously published data (9, 27), the phosphorylation of Ser1151 alone switches BK channel activation from PKA to PKG, whereas the additional phosphorylation of Ser695 renders the BK channel completely insensitive to both cyclic nucleotide-dependent kinases. Such a coregulation is not unique to BK channels but has also been observed in other ion channels. In T lymphocytes the modulation of delayed rectifier potassium channels by PKA is enhanced by PKC phosphorylation (28). Similarly, voltage-gated sodium channels require PKC phosphorylation to allow modulation by PKA (29).
The inhibition of BK channels by phorbol esters and PKCc has been shown before in isolated smooth muscle cells from different arterial beds (30, 31). In the present study, we used mouse TSMCs to prove that the regulation of BK channel activity by PKC in primary smooth muscle cells is identical to that observed in the recombinant channels. We chose TSMCs for two reasons. First, BK channels in mouse TSMCs represent the zero variant corresponding to the bovine trachea isoform. Second, the muscarinic receptor agonist CCh inhibits BK channels in TSMCs by activating the PKC via M2 receptors (12). The data obtained in TSMCs are in complete accordance with the regulation observed with the recombinantly expressed BK channels. CCh decreased BK channel activity in TSMCs by ∼50% and abolished the stimulating effect of the PKG activator 8-pCPT-cGMP, which was effective in the absence of CCh. The broad-spectrum PKC inhibitor Ro-31-8220 inhibited the effects of CCh. Dephosphorylation of presumably Ser1151, by pretreating inside-out patches from TSMCs with ATP-free solution in the presence of the PKC pseudosubstrate inhibitor PKC19–31, resulted in a switch of channel regulation from PKG to PKA, resembling the switch in the S1151A mutant. The regulation of BK channels by closely associated phosphatases has been shown before (16–18). Therefore, the switch of channel regulation from PKG to PKA was likely due to the activity of a phosphatase that had dephosphorylated the channel at the PKC motif before the channel became responsive to PKA. Applying the selective protein phosphatase 1 peptide inhibitor PPI2 to inside-out patches of TSMCs resulted in a substantial decrease of NPo and prevented channel activation by either PKG or PKA. This effect was dependent on PKC, because Ro 31-8220 abolished the inhibitory effect of PPI2 and allowed PKG to enhance NPo. Inhibition of a protein phosphatase 2A by 3 nM ocadaic acid had no influence on BK channel activity. These findings, together with experiments performed in HEK293 cells expressing recombinant channels (Fig. S6), provide strong evidence that BK channels are intimately associated with protein phosphatase 1 and PKC, and that interfering with the balance between kinase and opposing phosphatase activities can lead to the conditional phosphorylation of Ser695 with the described consequences on channel regulation. The findings with PPI2 are intriguing because they disprove the concept of an indirect regulation of BK channels by protein kinases. It has been demonstrated that protein phosphatase inhibitors abolish or reverse the stimulatory effect of PKG (17) or of compounds that activate cGMP-dependent pathways, such as the natriuretic peptide ANP (32) or the β-amyloid-precursor protein β-APP (33), on BK channel activity. These observations had been taken as evidence that BK channels are stimulated by dephosphorylation of the channel protein due to the activation of an associated protein phosphatase. In light of the present findings, however, it is very likely that protein phosphatase inhibitors in TSMCs lead to the phosphorylation of Ser695 by an endogenous PKC, which inhibits BK channels and renders them insensitive to cyclic nucleotide-dependent protein kinases.
In summary, we have shown that PKC via unconditional and conditional phosphorylation of the BK channel not only decreases channel open probability but also determines the regulation of the channel by other protein kinases such as PKG and PKA. By inhibiting BK channel activity and blunting the activating effects of PKG and PKA in TSMCs, PKC facilitates contraction and prevents relaxation via NO/cGMP- or cAMP-dependent pathways. The balance between phosphorylation by PKC and dephosphorylation by phosphatase 1 may vary in different cell types and may therefore explain the divergent results reported in the literature on BK channel regulation by PKG and PKA.
Materials and Methods
HEK293 cells were cultured for 24 h and then transiently transfected with the pcDNA3 plasmid containing the BK channel α-subunit BKA cloned from a bovine tracheal smooth muscle oligo dT-primed cDNA library (9) or with plasmids encoding the respective BKA mutant. The GenBank accession numbers for the BK channel isoform are AAK54352.1 (protein) and AY033472 (mRNA; BKA). Tracheal smooth muscle cells were isolated as described previously (12). Standard patch-clamp recording techniques were used to measure currents in the whole-cell, outside-out, or inside-out patch-clamp configuration. The membrane conductance (Gm) was calculated as Gm = I/(Em − Erev), where I is macroscopic current, Em is the test membrane potential, and Erev is the reversal potential for potassium. For normalization, Gm values were divided by Gmax, where Gmax was defined as the largest Gm value obtained in each experiment. Gm/Gmax voltage curves were fitted with a Boltzmann equation of the form G/Gmax = (1 + exp((V1/2 − Vm)/k)−1, where V1/2 is the membrane potential (Vm) required for half-maximal activation of the channels, and k is the logarithmic voltage sensitivity (i.e., ΔV required for an e-fold increase in activity). Further details of the experimental procedures are given in SI Materials and Methods.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912029107/DCSupplemental.
References
- 1.Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem. 2004;279:36746–36752. doi: 10.1074/jbc.M405621200. [DOI] [PubMed] [Google Scholar]
- 2.Sausbier M, et al. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci USA. 2004;101:9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rüttiger L, et al. Deletion of the Ca2+-activated potassium (BK) α-subunit but not the BKβ1-subunit leads to progressive hearing loss. Proc Natl Acad Sci USA. 2004;101:12922–12927. doi: 10.1073/pnas.0402660101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sausbier M, et al. Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel-deficient mice. Circulation. 2005;112:60–68. doi: 10.1161/01.CIR.0000156448.74296.FE. [DOI] [PubMed] [Google Scholar]
- 5.Werner ME, Zvara P, Meredith AL, Aldrich RW, Nelson MT. Erectile dysfunction in mice lacking the large-conductance calcium-activated potassium (BK) channel. J Physiol. 2005;567:545–556. doi: 10.1113/jphysiol.2005.093823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nara M, Dhulipala PDK, Wang Y-X, Kotlikoff MI. Reconstitution of β-adrenergic modulation of large conductance, calcium-activated potassium (maxi-K) channels in Xenopus oocytes. Identification of the cAMP-dependent protein kinase phosphorylation site. J Biol Chem. 1998;273:14920–14924. doi: 10.1074/jbc.273.24.14920. [DOI] [PubMed] [Google Scholar]
- 7.Fukao M, et al. Cyclic GMP-dependent protein kinase activates cloned BKCa channels expressed in mammalian cells by direct phosphorylation at serine 1072. J Biol Chem. 1999;274:10927–10935. doi: 10.1074/jbc.274.16.10927. [DOI] [PubMed] [Google Scholar]
- 8.Tian L, et al. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J Biol Chem. 2001;276:7717–7720. doi: 10.1074/jbc.C000741200. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X-B, et al. A molecular switch for specific stimulation of the BKCa channel by cGMP and cAMP kinase. J Biol Chem. 2001;276:43239–43245. doi: 10.1074/jbc.M104202200. [DOI] [PubMed] [Google Scholar]
- 10.Tian L, et al. Distinct stoichiometry of BKCa channel tetramer phosphorylation specifies channel activation and inhibition by cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 2004;101:11897–11902. doi: 10.1073/pnas.0402590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schubert R, Nelson MT. Protein kinases: Tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci. 2001;22:505–512. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- 12.Zhou X-B, et al. M2 muscarinic receptors induce airway smooth muscle activation via a dual, Gβγ-mediated inhibition of large conductance Ca2+-activated K+ channel activity. J Biol Chem. 2008;283:21036–21044. doi: 10.1074/jbc.M800447200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennelly PJ, Krebs EG. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991;266:15555–15558. [PubMed] [Google Scholar]
- 14.Shen K-Z, et al. Tetraethylammonium block of Slowpoke calcium-activated potassium channels expressed in Xenopus oocytes: Evidence for tetrameric channel formation. Pflugers Arch. 1994;426:440–445. doi: 10.1007/BF00388308. [DOI] [PubMed] [Google Scholar]
- 15.Niu X, Magleby KL. Stepwise contribution of each subunit to the cooperative activation of BK channels by Ca2+ Proc Natl Acad Sci USA. 2002;99:11441–11446. doi: 10.1073/pnas.172254699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinhart PH, Levitan IB. Kinase and phosphatase activities intimately associated with a reconstituted calcium-dependent potassium channel. J Neurosci. 1995;16:4572–4579. doi: 10.1523/JNEUROSCI.15-06-04572.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X-B, Ruth P, Schlossmann J, Hofmann F, Korth M. Protein phosphatase 2A is essential for the activation of Ca2+-activated K+ currents by cGMP-dependent protein kinase in tracheal smooth muscle and Chinese hamster ovary cells. J Biol Chem. 1996;271:19760–19767. doi: 10.1074/jbc.271.33.19760. [DOI] [PubMed] [Google Scholar]
- 18.Lin MT, Longo LD, Pearce WJ, Hessinger DA. Ca2+-activated K+ channel-associated phosphatase and kinase activities during development. Am J Physiol. 2005;289:H414–H425. doi: 10.1152/ajpheart.01079.2004. [DOI] [PubMed] [Google Scholar]
- 19.Wallner M, et al. Characterization of and modulation by a β-subunit of a human maxi KCa channel cloned from myometrium. Receptors Channels. 1995;3:185–199. [PubMed] [Google Scholar]
- 20.Tseng-Crank J, et al. Cloning, expression, and distribution of functionally distinct Ca2+-activated K+ channel isoforms from human brain. Neuron. 1994;13:1315–1330. doi: 10.1016/0896-6273(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y, et al. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002;417:515–522. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- 22.Xia X-M, Zeng X, Lingle CJ. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 2002;418:880–884. doi: 10.1038/nature00956. [DOI] [PubMed] [Google Scholar]
- 23.Yusifov T, Savalli N, Gandhi CS, Ottolia M, Olcese R. The RCK2 domain of the human BKCa channel is a calcium sensor. Proc Natl Acad Sci USA. 2008;105:376–381. doi: 10.1073/pnas.0705261105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian L, et al. Palmitoylation gates phosphorylation-dependent regulation of BK potassium channels. Proc Natl Acad Sci USA. 2008;105:21006–21011. doi: 10.1073/pnas.0806700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erxleben C, et al. Interacting effects of N-terminal variation and strex exon splicing on slo potassium channel regulation by calcium, phosphorylation, and oxidation. J Biol Chem. 2002;277:27045–27052. doi: 10.1074/jbc.M203087200. [DOI] [PubMed] [Google Scholar]
- 26.Hall SK, Armstrong DL. Conditional and unconditional inhibition of calcium-activated potassium channels by reversible protein phosphorylation. J Biol Chem. 2000;275:3749–3754. doi: 10.1074/jbc.275.6.3749. [DOI] [PubMed] [Google Scholar]
- 27.Widmer HA, Rowe ICM, Shipston MJ. Conditional protein phosphorylation regulates BK channel activity in rat cerebellar Purkinje neurons. J Physiol. 2003;552:379–391. doi: 10.1113/jphysiol.2003.046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Payet MD, Dupuis G. Dual regulation of the n type K+ channel in Jurkat T lymphocytes by protein kinases A and C. J Biol Chem. 1992;267:18270–18273. [PubMed] [Google Scholar]
- 29.Li M, et al. Convergent regulation of sodium channels by protein kinase C and cAMP-dependent protein kinase. Science. 1993;261:1439–1442. doi: 10.1126/science.8396273. [DOI] [PubMed] [Google Scholar]
- 30.Schubert R, Noack T, Serebryakov VN. Protein kinase C reduces the KCa current of rat tail artery smooth muscle cells. Am J Physiol. 1999;276:C648–C658. doi: 10.1152/ajpcell.1999.276.3.C648. [DOI] [PubMed] [Google Scholar]
- 31.Barman SA, Zhu S, White RE. Protein kinase C inhibits BKCa channel activity in pulmonary arterial smooth muscle. Am J Physiol. 2004;286:L149–L155. doi: 10.1152/ajplung.00207.2003. [DOI] [PubMed] [Google Scholar]
- 32.White RE, et al. Potassium channel stimulation by natriuretic peptides through cGMP-dependent dephosphorylation. Nature. 1993;361:263–266. doi: 10.1038/361263a0. [DOI] [PubMed] [Google Scholar]
- 33.Furukawa K, Barger SW, Blalock EM, Mattson MP. Activation of K+ channels and suppression of neuronal activity by secreted β-amyloid-precursor protein. Nature. 1996;379:74–78. doi: 10.1038/379074a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.