Abstract
The planktonic larval stage is a critical component of life history in marine benthic species because it confers the ability to disperse, potentially connecting remote populations and leading to colonization of new sites. Larval-mediated connectivity is particularly intriguing in deep-sea hydrothermal vent communities, where the habitat is patchy, transient, and often separated by tens or hundreds of kilometers. A recent catastrophic eruption at vents near 9°50′N on the East Pacific Rise created a natural clearance experiment and provided an opportunity to study larval supply in the absence of local source populations. Previous field observations have suggested that established vent populations may retain larvae and be largely self-sustaining. If this hypothesis is correct, the removal of local populations should result in a dramatic change in the flux, and possibly species composition, of settling larvae. Fortuitously, monitoring of larval supply and colonization at the site had been established before the eruption and resumed shortly afterward. We detected a striking change in species composition of larvae and colonists after the eruption, most notably the appearance of the gastropod Ctenopelta porifera, an immigrant from possibly more than 300 km away, and the disappearance of a suite of species that formerly had been prominent. This switch demonstrates that larval supply can change markedly after removal of local source populations, enabling recolonization via immigrants from distant sites with different species composition. Population connectivity at this site appears to be temporally variable, depending not only on stochasticity in larval supply, but also on the presence of resident populations.
Keywords: larval dispersal, population connectivity, Ctenopelta, Lepetodrilus, East Pacific Rise
In marine benthic systems, dispersal in a planktonic larval stage influences the dynamics and spatial structure of populations and can be critical for regional persistence of species. It is informative to consider these systems in the framework of metapopulation theory (1) as a balance between extinction and dispersal-mediated colonization. The extent to which a local marine population is open (i.e., the proportion of recruits that come from other locales) may increase its resilience to perturbation (2, 3), but recruitment of progeny back into the natal site also contributes to persistence (4). Larval dispersal between deep-sea hydrothermal vent communities is an intriguing topic in this context because the habitat is spatially disjunct and populations are subject to local extinctions. A major challenge to solving questions of population openness (connectivity) in marine systems, however, is determining whether the source of each recruit is local or remote because the larvae are difficult to track. Consequently, fundamental questions about how vent populations persist and what physical and biological processes control their connectivity remain unresolved despite more than three decades of research (5).
Population genetic studies indicate that specific currents or topographic features may constitute barriers to dispersal between widely separated vents (6–9), but, on scales of tens to even a hundred kilometers, populations of many species show little genetic differentiation (8, 10, 11). On these small scales, the lack of significant increase in genetic differentiation with separation distance has been interpreted to mean that larvae are supplied in a well-mixed pool (12). Although larvae of some vent species have the potential to disperse long distances (13, 14), larval patchiness in the field (15, 16), enhanced larval supply directly downstream of source populations (17), and hydrodynamic analyses (18) suggest that larval retention may occur near natal sites. It is possible that these populations are largely self-sustaining on ecological time scales and maintain high apparent genetic connectivity through infrequent exchange of individuals over long periods.
A recent catastrophic eruption at vents near 9°50′N on the East Pacific Rise (EPR) created a natural clearance experiment and allowed us to study larval connectivity after the removal of local populations. Such perturbations are common along the fast-spreading EPR, where tectonic and magmatic events cause vents to open and close on decadal time scales (19). Since the discovery of vents at this site, researchers have detected two major eruptions, one in 1991 (20) and a second (the subject of the present study) in 2006 (21). The latter eruption introduced a major perturbation into local vent communities. New lava emerged between 9°46′ and 9°56′N and reached as far as 2 km off axis (22) (Fig. 1), paving over existing vent communities. The precise timing of lava extrusion is uncertain; estimates vary from late 2005 to January 2006 (21). Although the lava eradicated invertebrate communities, it did not plug all of the vents, and hydrothermal fluids (on which the communities depend) continued to flow from many of the orifices established before the eruption. One community survived at the southern margin of the eruption (V-vent at 9°47′N); the species composition there did not change detectably after the eruption (authors’ visual surveys) and was similar to pre-eruption faunas at the paved-over vents. To the north, a single colonized vent has been reported at 10°08′N (23), but its status at the time of the eruption is unknown. No other colonized vents are known between 9°56′N and the Clipperton Transform Fault (10°13′N).
Fig. 1.
Locations of vents and sample sites on the East Pacific Rise in the region of the eruption. Symbols designate vent sites (yellow circles), sediment traps (inverted triangles), and colonization experiments (squares) (blue = pre-eruption, red = post-eruption). Blue line outlines the extent of lava extruded in the 2005–2006 eruption (22). Bathymetry of ridge (53) is contoured at 10-m intervals. Map courtesy of S. A. Soule.
This large-scale removal of vent populations provided us with an opportunity to address questions about larval supply and recolonization at vents where initially there was no local larval source. This was possible only because we had been monitoring larval supply and colonization near 9°50′N before the eruption (24) and were able to mobilize quickly afterward to resume sampling. If larvae were typically supplied to these EPR vents in a well-mixed, time-invariant larval pool (12), we would expect little influence of the eruption on larval supply and early recolonization to be determined primarily by responses to conditions in the benthic environment. If, instead, local populations had been an important contributor to supply (17), we would expect a reduction in larval abundance after the eruption and distinct differences in species composition, depending on which remote source populations contributed immigrants. This altered pool of larval immigrants would constitute the pioneer colonists and potentially direct the trajectory of subsequent succession. Our specific objectives in this study were to determine whether larval supply changed significantly after the eruption and to explore the effects of this supply on recolonization.
Results
The species composition of larvae supplied to vents after the eruption differed markedly from that before (Fig. 2 and Table S1). Although supply of the abundant larval gastropod species varied substantially between cup intervals, all but one (Gorgoleptis emarginatus) arrived at a consistently different rate after the eruption than before (P < 0.05, MANOVA and ANOVA, Systat v. 11; Table S2). Supply of Cyathermia naticoides, Lepetodrilus spp., Gorgoleptis spiralis, and Bathymargarites symplector declined significantly after the eruption, despite the continued presence of potential source populations within 6 km to the south at V-vent. In contrast, Ctenopelta porifera, which had been virtually absent before the eruption [only a single individual in the 2004–2005 trap samples and one in a 2004 pump sample (25)] was supplied in significantly higher numbers afterward. During a few intervals of the larval sampling series, the change in supply of some species over several weeks was as high, or higher, than the difference in mean supply between pre- and post-eruption. When such a change occurs simultaneously across multiple species (e.g., the decrease in supply observed after pre-eruption interval 11, or after post-eruption interval 1; Fig. 2), it is likely associated with mesoscale hydrodynamic transport processes (24) that are unrelated to the eruption.
Fig. 2.
Larval supply measured in sediment traps. Traps sampled before the eruption (November 25, 2004, to April 21, 2005, at 7-day intervals; blue bars) near East Wall vent and after (July 1 to November 4, 2006; 6-day intervals; red bars) near P-vent. Supply (daily downward flux of larvae into 0.5-m2 trap opening) displayed for the six most abundant species/groups at either site that also were found as colonists: (A) Cyathermia naticoides, (B) Lepetodrilus spp; (C) Gorgoleptis spiralis, (D) Bathymargarites symplector, (E) Gorgoleptis emarginatus, (F) Ctenopelta porifera. Mean flux of each species except G. emarginatus changed significantly (P < 0.05, MANOVA and ANOVA; Table S2) after the eruption. Larval individuals of the five described lepetodrilid species in this region (Lepetodrilus elevatus, L. pustulosus, L. ovalis, L. cristatus, and L. tevnianus) were not distinguishable morphologically.
The post-eruption change was detectable in larvae of rare species as well; 14 of 27 larval gastropod taxa present at East Wall before the eruption were not found at P-vent afterward (Table S1). These differences in species composition are apparent in a nonmetric multidimensional scaling analysis (nMDS) (Systat v. 11) (Fig. 3A). Other groups also showed large changes in supply after the eruption (e.g., an increase in crabs Bythograea microps and Bythograea thermydron) (Table S1).
Fig. 3.
nMDS of species composition of larvae and colonists. The proximity of samples corresponds to the similarity in their species composition. Analysis conducted on Pearson correlation of fourth-root transformed abundance. (A) Larvae (excluding polychaetes) in sediment traps before (blue dots) and after (red dots) eruption; label designates site (E = East Wall, P = P-vent) and cup interval; Kruskal stress = 0.15. (B) Colonists (gastropods only) on blocks before the eruption (blue dots) and on sandwiches after (red dots); label designates site (WH = Worm Hole, TA = Tica; TY = Ty/Io, P = P-vent), environment (H = hot, W = warm), and replicate; Kruskal stress = 0.073. Mean species or species-group abundances listed in Table S3.
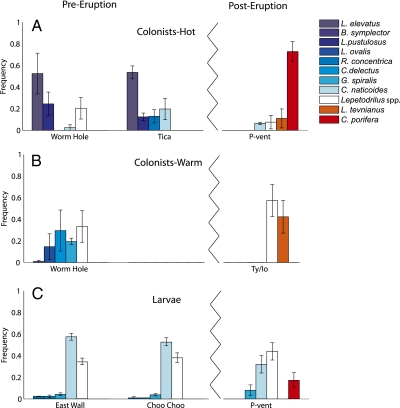
Species composition of colonists also changed distinctly after the eruption. Most surprising was the appearance of C. porifera (Fig. 4A) because it had never been reported before in the benthos from this segment of the EPR. The limpet Lepetodrilus tevnianus also was prominent in the hot environment after the eruption, whereas previously common species including Lepetodrilus elevatus, Lepetodrilus pustulosus, and Rhynchopelta concentrica were absent. The temperatures at P-vent (post-eruption) were similar to those at Tica (pre-eruption), so elevated temperatures do not appear to be responsible for these differences, although it is possible that chemistry differed. On surfaces in the warm environment, L. tevnianus was prominent after the eruption (Fig. 4B), whereas diverse species present before the eruption (Bathymargarites symplector, Lepetodrilus ovalis, L. pustulosus, Clypeosectus delectus, Gorgoleptis spiralis) had disappeared. The distinct differences in overall species composition, including rare species, before and after the eruption are apparent in nMDS analysis of gastropod colonists (Fig. 3B).
Fig. 4.
Species composition of vent gastropod colonists and larvae. All gastropods were identified to species except larval and small juvenile Lepetodrilus, which could only be identified to genus. Jagged line separates pre- (Left) and post-eruption (Right) samples. (A) Colonists in hot vent environment before eruption at Worm Hole and Tica vents and after eruption at P-vent. Values are average relative abundances (±SE; n = 3) of seven most common species or species groups (those >2% of all vent gastropods at any of three sites). (B) Colonists in warm vent environment before eruption at Worm Hole and after eruption near Ty/Io vent. Values are average relative abundances (±SE; n = 3) of six most abundant species/groups (those >2% of all vent gastropods at either of two sites). (C) Larval supply in sediment traps before eruption at East Wall and Choo Choo vents and after eruption at P-vent. Values are average relative abundances (±SE; n = 21) of six most common species/groups that are also found as colonists.
The dramatic increase in larval supply of C. porifera after the eruption coincided with its appearance as a colonist (Fig. 4). Other species (e.g., B. symplector, C. naticoides, G. spiralis, R. concentrica) also showed a post-eruption change in larval supply that corresponded with change in colonist abundance (Tables S1 and S3). Unfortunately, we could not tell whether changes in larvae of individual lepetodrilid species (Lepetodrilus spp.) coincided with changes in the associated colonists because the larvae are not morphologically distinguishable at the species level. We suspect that larvae in this group predominantly represented predisturbance species (L. elevatus, L. pustulosus, L. ovalis) before the eruption and the postdisturbance pioneer (L. tevnianus) afterward, but molecular genetic identifications are needed for confirmation.
Discussion
The marked change in species composition of larval supply and colonists after the 2005–2006 eruption is consistent with a scenario in which pioneer species from remote populations were able to colonize successfully at 9°50′N only after the resident populations (and their larvae) had been eliminated. The observed changes are not consistent with a model of recolonization via a well-mixed larval pool, from which larval supply would be relatively unaffected by elimination of local populations. Clearly, the disturbance strongly affected larval supply in the local region. Surprisingly, the source of pioneer colonists did not appear to be the nearest established communities, such as V-vent (at 9°47′N) or other sites to the south whose faunal composition resembled the pre-eruption communities at 9°50′N. Instead, at least one prominent pioneer species, Ctenopelta porifera, arrived from possibly more than 300 km away, where vents at 13°N host the only known populations.
The post-eruption change in larvae and colonists could have developed through two different mechanisms, initiated either by altered larval supply or settlement responses to the altered environmental conditions. The first case might be expected if larval supply in the disturbed region declined precipitously directly after the eruption. This “larval vacuum,” caused by elimination of local populations, could open the sites to settlement of highly dispersive, but perhaps competitively inferior, immigrant species from remote regions that typically are not able to infiltrate established pre-eruption communities. The particular species of pioneers depends on which larvae are available in the interval shortly after the eruption, as influenced by time-variant transport processes (17), or spawning cycles (26). This type of postdisturbance colonization scenario, contingent on supply of new pioneers, has been observed in a variety of marine and terrestrial environments (27–29). Our larval flux measurements, initiated roughly 6 months after the main seismic event in January 2006, and possibly even longer after the main lava extrusion, did not measure supply in the first few months after the eruption. A pilot set of larval samples (Fig. S1) collected in the eruption region during May to June 2006 did reveal very low fluxes (averaging <1 day−1), but those results must be interpreted carefully because the collectors were located several hundred meters away from vents, where larval abundances are known to be reduced (16). Nevertheless, we think the altered supply scenario is a likely one, given prior evidence of local larval supply at this site (17), and the observations 6 months after the eruption of reduced fluxes of many predisturbance species.
An alternative possibility is that environmental conditions changed so drastically after the eruption that predisturbance species were not able to settle and survive, even if they were supplied as larvae. In this case, larval supply may or may not have declined directly after the eruption, but only species adapted to the new thermal or chemical conditions were able to colonize as pioneers. The chemical composition of hydrothermal fluids at some EPR vent sites are known to have changed after the eruption (30) and may have altered habitat suitability for select species. The structural habitat also changed, with Tevnia jerichonana replacing Riftia pachyptila as the main foundation tubeworm species, possibly facilitating establishment of associated gastropod species such as L. tevnianus. Environmental conditions at vents are known to correlate with distribution of some species (31, 32), and with faunal changes over time (33). Investigations of species’ tolerances to specific thermal and chemical habitats in the post-eruption sites are underway, but it is not yet known whether the gastropod species that were so prominent before the eruption are able to tolerate the post-eruption conditions. It is quite possible that altered larval supply and environmental tolerances both contributed to the faunal changes observed after the eruption.
The increase in larval supply of postdisturbance species after the eruption suggests that once the pioneers became established and reproductively mature they bolstered overall local larval supply to near pre-eruption levels. Although no broad survey of reproductive maturity was attempted, many of the gastropod colonists collected in November 2006 were larger than the minimum size of reproductive maturity, and mature gonads were observed in C. porifera and L. tevnianus.
Continued observations of the vent communities will show whether the post-eruption change in species composition persists and results in an ecological regime shift or is simply an early stage in succession that will transition back to the pre-eruption state. If local populations dominate larval supply, the established species may pre-empt occasional immigrants of other species from remote locales or from neighboring sites to the south with predisturbance faunas. Alternatively, if fluid chemical conditions revert to pre-eruption levels or if pioneers make the habitat more suitable for other species, the faunal communities may develop through a successional sequence as immigrants outcompete the initial colonists. Successional observations from the previous (1991) eruption cycle described a transition in the large, structure-forming species from the tubeworms T. jerichonana to R. pachyptila and eventually to the mussel Bathymodiolus thermophilus (33) that was attributed to changes in environmental conditions. Before the 2006 eruption, most 9°50′N vent sites hosted R. pachyptila, indicating a mid- to late stage of succession (16). Although changes in gastropod species composition also occur during succession at vents (34, 35), no clear sequence of species replacement has been observed, and the pioneer gastropod colonists after the 1991 eruption did not include C. porifera or L. tevnianus (36). Deterministic succession is found in terrestrial meadows and temperate forests (28, 37), both quite stable systems where disturbance time scales are long relative to species’ generation times. In contrast, the EPR vents are a system where disturbance occurs at intervals approaching species’ generation times. The inclusion of larval measurements through the 2006 eruption will allow future investigation of whether larval/propagule availability influences succession at vents, as it does in many other frequently disturbed marine and terrestrial communities (27, 38, 39). The extensive, coordinated history of observations in the 9°50′N EPR region and the ongoing monitoring there make it a truly unique site for studies of ecosystem response to disturbance.
Our results show that vent populations on the EPR are, as expected, connected by larval dispersal, but specific populations cannot be consistently characterized as open or closed. Once a catastrophic disturbance eliminates the community at a vent, the site becomes open to colonization via larvae from remote populations, possibly as far as 300 km away. After the site has become colonized, however, larvae from the local populations appear to dominate as potential recruits. This alternation between open and closed condition is quite different from the situation in more stable marine environments where interdisturbance period is long compared to species’ generation times, and connectivity is more likely to depend on population growth rates and transport processes than disturbance.
Although the magnitude of connectivity at these vents appears to depend strongly on the frequency of disturbance, the species composition of pioneer larvae is likely subject to temporally variable currents, as demonstrated for coastal habitats (40). One consequence of stochasticity in larval supply, and episodic opening of vent sites to new colonists, is the potential for occasional exchange between far distant sites, as was observed for C. porifera in the present study. Such an exchange could explain the high genetic connectivity reported for many vent species (12, 41), even in cases where most larvae are retained locally between disturbances.
Studies of immigration and succession at transient, geographically separated, vents on the EPR contribute to our understanding of vent systems in a metapopulation context (42) as they have in terrestrial volcanic systems (43, 44). In these highly disturbed systems, the important question may not be whether populations are open or closed (45) but instead how often they become open and for how long. This temporal variation in connectivity has important implications for predicting effects of natural perturbations, or anthropogenic impacts such as seafloor mineral mining, ecotourism, or bioprospecting.
Materials and Methods
Larvae were collected in McLane PARFLUX Mark 78H-21 time-series sediment traps with a 0.5-m2 collecting area. Particles dropped into collection cups filled with 20% dimethyl sulfoxide in saturated salt solution (46) as a preservative. Pre-eruption traps were positioned into the axial trough near vents by lowering on a wire from shipboard into a seafloor navigation network (17). These traps sampled at East Wall and Choo Choo vents (Fig. 1) on 7-day intervals between November 25, 2004, and April 21, 2005 (24). Following the eruption, a rapid-response cruise was launched in July 2006 aboard the R/V Atlantis. A trap was positioned near P-vent by submersible (Fig. 1), sampling on a 6-day interval between July 1 and November 4, 2006. P-vent was selected as a nearby alternative to East Wall, which was no longer venting vigorously, and Choo Choo, which had shut down. On recovery, samples from the traps were maintained at approximately 4 °C until the larvae (molluscs, polychaetes, and crustaceans) were sorted and identified morphologically (47) under a dissecting microscope to the lowest taxonomic level possible (e.g., species level for most gastropods). The traps collect larvae that are swimming or sinking downward, and flux into the trap is considered an indicator of larval supply to the benthos (25, 48).
The East Wall, Choo Choo, and P-vent traps were located directly in the axial trough within 50 m of active vents. Three other traps were deployed after the eruption near Tica and Bio9 (starting May 16, 2006, at 2-day intervals) and Ty/Io (starting July 1, 2006, at 6-day intervals) (Fig. 1) but lacked precise navigation and were positioned out of the trough. Larvae from these traps were not used in primary analyses because larval abundance may be reduced outside the trough (16).
Colonists (larvae that had settled and metamorphosed) were collected on experimental surfaces deployed by the submersible Alvin in vent sites for 4- to 5-month durations. The surfaces were placed into two distinct environments in inhabited, diffuse-flow vents. The “hot” environment was characterized by the presence of tubeworms, vigorous flow, and maximum temperatures up to 30 °C. The “warm” environment lacked tubeworms and had moderate flow with temperatures less than 7 °C. These environments correspond respectively to the vestimentiferan and bivalve/suspension-feeder zones of previous studies at this site (35). In the 9°50′N region, species composition at different vents typically is similar within a zone, but varies substantially between zones (33, 35, 49). Surfaces used to quantify pre-eruption colonists were selected from a larger sample set from this region of the EPR (35, 49, 50) to match as closely as possible the deployment intervals and environmental and faunal characteristics of the post-eruption sites. They included surfaces placed at Worm Hole vent from November 1994 to April 1995 in hot (temperatures at surfaces of 1.9–10.9 °C) and warm (1.8–2.1 °C) environments and at Tica vent from December 1999 to May 2000 in hot (18.0–26.3 °C) environments (Fig. 1). Neither site supported abundant mussels, which were absent in post-eruption communities and are known to influence colonization (51). Species abundances in the different environments from these pre-eruption colonization samples are representative of those in the larger sample set. Post-eruption surfaces were deployed between July and November 2006 in the hot (23.2–26.7 °C) environment at P-vent and warm (2.2–6.5 °C) environment at a site near Ty/Io vent. These sites were selected because they were venting vigorously and were sufficiently large to accommodate replicate experimental surfaces; Worm Hole had shut down before the eruption and Tica was not visited on the eruption-response cruise due to time limitations. For all colonization surfaces, the thermal environment was measured with a temperature probe on deployment and recovery at the base of the surface. On recovery, they were placed in individual collection compartments for transport back to the ship. On shipboard, surfaces and their attached colonists were preserved in 80% ethanol, as were any detached individuals from the compartment retained on a 63-μm sieve. In the laboratory, each surface was examined under a dissecting microscope and all metazoan colonists (including detached individuals >1 mm) were enumerated and identified. Gastropods only were used in subsequent analyses because methods of identification and quantification were consistent across all samples for those species.
The colonization surfaces used before the eruption were basalt blocks, 10 cm on a side. Those used after the eruption were “sandwiches” of six Lexan plastic plates, each 0.64 cm thick and separated from each other by 0.95 cm to provide additional surface area within a 1,000-cm3 volume. Many vent species settle onto plastic surfaces (52), so we did not expect this change in surface composition to greatly alter species composition of colonists, especially over a period of months. To evaluate this assumption, we compared gastropod colonists between basalt blocks and Lexan sandwiches in a simultaneous, later deployment (November 2006 to January 2007) at Tica vent. nMDS analysis showed that dissimilarities based on species composition were no greater between surface types than within a type and that composition on both blocks and sandwiches was similar to that on sandwiches deployed earlier in comparable thermal habitat (Fig. S2). However, the surface area of sandwiches was more than twice the area of basalt blocks; because this difference potentially influences absolute abundance of colonists, we compare relative abundances between pre- and post-eruption deployments.
Larval supply was compared between pre- and post-eruption periods using MANOVA followed by univariate ANOVA (Systat v. 11; Table S2) for the six most abundant species at either site that were also found on colonization surfaces. Because sequential intervals in the time series were used as replicates, autocorrelation analysis (Matlab 7.1) was used to evaluate independence. Significant autocorrelation (P < 0.05) with a 1-step lag was detected in at least one record for three taxa (C. naticoides, G. spiralis, and G. emarginatus) and autocorrelation with a two-step lag was detected for Lepetodrilus spp. In each of these cases, when the ANOVA was repeated with subsampled records to avoid autocorrelation (every second or every third sample interval as appropriate) no changes in significance were found (P < 0.05 level). Differences in community composition were examined with nMDS of larval abundance in trap samples (Systat v. 11), using Pearson correlations of fourth root transformed values to emphasize rare taxa. Polychaetes were not included because their preservation was poor in some samples. For visual clarity, only the East Wall samples were plotted from the pre-eruption collections (when Choo Choo samples are added, they cluster near East Wall positions). Colonist community composition (gastropods only) was examined similarly with nMDS.
Supplementary Material
Acknowledgments
We are grateful for help at sea from K. Buckman, D. Fornari, A. Fusaro, I. Garcia Berdeal, B. Govenar, B. Hogue, R. Jackson, C. Strasser, T. Shank, S. Worrilow, and to the Captains and crew, Alvin group, and Chief Scientists (M. Lilley, C. Vetriani, K. Von Damm, and A. Thurnherr) during Atlantis cruises AT11-20, 11-26, 15-06, and 15-12, and the Captain, crew, and Chief Scientist (J. Cowen) of the New Horizon rapid response cruise. S. Bayer examined gastropod specimens for reproductive maturity, S. A. Soule provided a base topographic map of the eruption area, and two anonymous reviewers contributed useful comments. Principal investigators in the LADDER project (W. Lavelle, J. Ledwell, D. McGillicuddy, and A. Thurnherr) were instrumental in facilitating this project and provided input during numerous discussions. Support was provided by National Science Foundation Grants OCE-969105, OCE-9712233, and OCE-0424953, Woods Hole Oceanographic Institution grants from Deep Ocean Exploration Institute and the Ocean Venture Fund, a National Defense Science and Engineering Graduate Fellowship to D.A., and the Woods Hole Oceanographic Institution Jannasch Chair for Excellence in Oceanography to L.M.
Footnotes
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913187107/DCSupplemental.
References
- 1.Hanski I. Metapopulation Ecology. Oxford: Oxford University Press; 1999. p. 313. [Google Scholar]
- 2.Cowen RK, Sponaugle S. Larval dispersal and marine population connectivity. Ann Rev Mar Sci. 2009;1:443–466. doi: 10.1146/annurev.marine.010908.163757. [DOI] [PubMed] [Google Scholar]
- 3.Kritzer JP, Sale PF. Marine Metapopulations. Burlington: Elsevier Academic Press; 2006. [Google Scholar]
- 4.Hastings A, Botsford LW. Persistence of spatial populations depends on returning home. Proc Natl Acad Sci USA. 2006;103:6067–6072. doi: 10.1073/pnas.0506651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corliss JB, et al. Submarine thermal springs on the Galapagos Rift. Science. 1979;203:1073–1083. doi: 10.1126/science.203.4385.1073. [DOI] [PubMed] [Google Scholar]
- 6.Hurtado LA, Lutz RA, Vrijenhoek RC. Distinct patterns of genetic differentiation among annelids of eastern Pacific hydrothermal vents. Mol Ecol. 2004;13:2603–2615. doi: 10.1111/j.1365-294X.2004.02287.x. [DOI] [PubMed] [Google Scholar]
- 7.Johnson SB, Young CR, Jones WJ, Warén A, Vrijenhoek RC. Migration, isolation, and speciation of hydrothermal vent limpets (Gastropoda; Lepetodrilidae) across the Blanco Transform Fault. Biol Bull. 2006;210:140–157. doi: 10.2307/4134603. [DOI] [PubMed] [Google Scholar]
- 8.Won Y, Young CR, Lutz RA, Vrijenhoek RC. Dispersal barriers and isolation among deep-sea mussel populations (Mytilidae: Bathymodiolus) from eastern Pacific hydrothermal vents. Mol Ecol. 2003;12:169–184. doi: 10.1046/j.1365-294x.2003.01726.x. [DOI] [PubMed] [Google Scholar]
- 9.Young CR, Fujio S, Vrijenhoek RC. Directional dispersal between mid-ocean ridges: Deep-ocean circulation and gene flow in Ridgeia piscesae. Mol Ecol. 2008;17:1718–1731. doi: 10.1111/j.1365-294X.2008.03609.x. [DOI] [PubMed] [Google Scholar]
- 10.Craddock C, Lutz RA, Vrijenhoek RC. Patterns of dispersal and larval development of archaeogastropod limpets at hydrothermal vents in the eastern Pacific. J Exp Mar Biol Ecol. 1997;210:37–51. [Google Scholar]
- 11.Jollivet D, Chevaldonné P, Planque B. Hydrothermal-vent alvinellid polychaete dispersal in the eastern Pacific. 2. A metapopulation model based on habitat shifts. Evolution. 1999;53:1128–1142. doi: 10.1111/j.1558-5646.1999.tb04527.x. [DOI] [PubMed] [Google Scholar]
- 12.Vrijenhoek RC. Gene flow and genetic diversity in naturally fragmented metapopulations of deep-sea hydrothermal vent animals. J Hered. 1997;88:285–293. doi: 10.1093/oxfordjournals.jhered.a023106. [DOI] [PubMed] [Google Scholar]
- 13.Epifanio CE, Perovich G, Dittel AL, Cary SC. Development and behavior of megalopa larvae and juveniles of the hydrothermal vent crab Bythograea thermydron. Mar Ecol Prog Ser. 1999;185:147–154. [Google Scholar]
- 14.Marsh AG, Mullineaux LS, Young CM, Manahan DT. Larval dispersal potential of the tubeworm Riftia pachyptila at deep-sea hydrothermal vents. Nature. 2001;411:77–80. doi: 10.1038/35075063. [DOI] [PubMed] [Google Scholar]
- 15.Metaxas A. Spatial and temporal patterns in larval supply at hydrothermal vents in the northeast Pacific Ocean. Limnol Oceanogr. 2004;49:1949–1956. [Google Scholar]
- 16.Mullineaux LS, et al. Spatial structure and temporal variation in larval abundance at hydrothermal vents on the East Pacific Rise. Mar Ecol Prog Ser. 2005;293:1–16. [Google Scholar]
- 17.Adams DK, Mullineaux LS. Supply of gastropod larvae to hydrothermal vents reflects transport from local larval sources. Limnol Oceanogr. 2008;53:1945–1955. [Google Scholar]
- 18.Thomson R, et al. Constrained circulation at Endeavour ridge facilitates colonization by vent larvae. Nature. 2003;424:545–549. doi: 10.1038/nature01824. [DOI] [PubMed] [Google Scholar]
- 19.Perfit MR, Chadwick WW. Magmatism at mid-ocean ridges: Constraints from volcanological and geochemical investigations. In: Buck WR, Delaney P, Karson JA, editors. Faulting and Magmatism at Mid-Ocean Ridges, Geophys. Washington, DC: Amer Geophys Union; 1998. pp. 59–116. Monograph 116, [Google Scholar]
- 20.Haymon RM, et al. Volcanic eruption of the mid-ocean ridge along the East Pacific Rise crest at 9° 45-52'N: Direct submersible observations of sea-floor phenomena associated with an eruption event in April, 1991. Earth Planet Sci Lett. 1993;119:85–101. [Google Scholar]
- 21.Tolstoy M, et al. A sea-floor spreading event captured by seismometers. Science. 2006;314:1920–1922. doi: 10.1126/science.1133950. [DOI] [PubMed] [Google Scholar]
- 22.Soule SA, Fornari DJ, Perfit MR, Rubin KH. New insights into mid-ocean ridge volcanic processes from the 2005-06 eruption of the East Pacific Rise, 9° 46'-56'N. Geology. 2007;35:1079–1082. [Google Scholar]
- 23.Kastens KA, Ryan WBF, Fox PJ. Structural and Volcanic Expression of a Fast Slipping Ridge-Transform-Ridge-Plate Boundary: Sea MARC I and Photographic Surveys at the Clipperton Transform Fault. J Geophys Res. 1986;91(B3):3469–3488. [Google Scholar]
- 24.Adams DK. PhD dissertation. Cambridge, MA: MIT/WHOI; 2007. Influence of hydrodynamics on the larval supply to hydrothermal vents on the East Pacific Rise. [Google Scholar]
- 25.Beaulieu SE, Mullineaux LS, Adams DK, Mills SW. Comparison of a sediment trap and plankton pump for time-series sampling of larvae near deep-sea hydrothermal vents. Limnol Oceanogr Methods. 2009;7:235–248. [Google Scholar]
- 26.Tyler PA, Young CM. Reproduction and dispersal at vents and cold seeps. J Mar Biol Assn UK. 1999;79:193–208. [Google Scholar]
- 27.Berlow E. From canalization to contingency: Historical effects in a successional rocky intertidal community. Ecol Monogr. 1997;67:435–460. [Google Scholar]
- 28.McCook LJ. Understanding ecological community succession: Causal models and theories, a review. Vegetatio. 1994;110:115–147. [Google Scholar]
- 29.Sousa W. Natural disturbance and the dynamics of marine benthic communities. In: Bertness MD, Gaines SD, Hay ME, editors. Marine Community Ecology. Sunderland, MA: Sinauer Associates; 2001. pp. 85–130. [Google Scholar]
- 30.Nees HA, et al. Hydrothermal vent mussel habitat chemistry, pre- and post-eruption at 9 50' North on the East Pacific Rise. J Shellfish Res. 2008;27:169–175. [Google Scholar]
- 31.Luther GW, et al. Chemical speciation drives hydrothermal vent ecology. Nature. 2001;410:813–816. doi: 10.1038/35071069. [DOI] [PubMed] [Google Scholar]
- 32.Childress JJ, Fisher CR. The biology of hydrothermal vent animals: Physiology, biochemistry, and autotrophic symbioses. Oceanogr Mar Biol Ann Rev. 1992;30:61–104. [Google Scholar]
- 33.Shank TM, et al. Temporal and spatial patterns of biological community development at nascent deep-sea hydrothermal vents (9° 50'N, East Pacific Rise) Deep Sea Res Part II Top Stud Oceanogr. 1998;45:465–515. [Google Scholar]
- 34.Mullineaux LS, Micheli F, Peterson CH, Lenihan HS, Markus N. Imprint of past environmental regimes on structure and succession of a deep-sea hydrothermal vent community. Oecologia. 2009;161:387–400. doi: 10.1007/s00442-009-1390-1. [DOI] [PubMed] [Google Scholar]
- 35.Mullineaux LS, Peterson CH, Micheli F, Mills SW. Successional mechanism varies along a gradient in hydrothermal fluid flux at deep-sea vents. Ecol Monogr. 2003;73:523–542. [Google Scholar]
- 36.Warén A, Bouchet P. Gastropoda and Monoplacophora from hydrothermal vents and seeps; new taxa and records. Veliger. 2001;44:116–231. [Google Scholar]
- 37.Clements FE. Plant succession and indicators. New York: The H. W. Wilson Company; 1928. [Google Scholar]
- 38.Paine RT. Controlled manipulations in the marine intertidal zone and their contributions to ecological theory. In: Goulden CE, editor. The Changing Scenes in Natural Sciences. Philadelphia, PA: Academy of Natural Sciences; 1977. pp. 245–270. [Google Scholar]
- 39.Huston M, Smith T. Plant succession: Life history and competition. Am Nat. 1987;130:168–198. [Google Scholar]
- 40.Planes S, Jones GP, Thorrold SR. Larval dispersal connects fish populations in a network of marine protected areas. Proc Natl Acad Sci USA. 2009;106:5693–5697. doi: 10.1073/pnas.0808007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jollivet D. Specific and genetic diversity at deep-sea hydrothermal vents: An overview. Biodivers Conserv. 1996;5:1619–1653. [Google Scholar]
- 42.Neubert MG, Mullineaux LS, Hill MF. A metapopulation approach to interpreting diversity at deep-sea hydrothermal vents. In: Kritzer JP, Sale PF, editors. Marine Metapopulations. London: Elsevier; 2006. pp. 321–350. [Google Scholar]
- 43.Carson HL, Lockwood JP, Craddock EM. Extinction and recolonization of local populations on a growing shield volcano. Proc Natl Acad Sci USA. 1990;87:7055–7057. doi: 10.1073/pnas.87.18.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards JS, Sugg PM. Arthropods as pioneers in the regeneration of life on the pyroclastic-flow deposits of Mount St. Helens. In: Dale VH, Swanson FJ, Crisafulli CM, editors. Ecological Responses to the 1980 Eruption of Mount St. Helens. New York: Springer; 2005. pp. 127–138. [Google Scholar]
- 45.Cowen RK, Lwiza KMM, Sponaugle S, Paris CB, Olson DB. Connectivity of marine populations: Open or closed? Science. 2000;287:857–859. doi: 10.1126/science.287.5454.857. [DOI] [PubMed] [Google Scholar]
- 46.Khripounoff A, Comtet T, Vangriesheim A, Crassous P. Near-bottom biological and mineral particle flux in the Lucky Strike hydrothermal vent area (Mid-Atlantic Ridge) J Mar Syst. 2000;25:101–118. [Google Scholar]
- 47.Mills SW, Beaulieu SE, Mullineaux LS. Photographic Identification Guide to Larvae at Hydrothermal Vents. Woods Hole Oceanog. Inst. Tech. Rept. Woods Hole, MA: Woods Hole Oceanographic Institution; 2009. WHOI-2009-05. [Google Scholar]
- 48.Todd CD, Phelan PJC, Weinmann BE. Improvements to a passive trap for quantifying barnacle larval supply to semi-exposed rocky shores. J Exp Mar Biol Ecol. 2006;332:135–150. [Google Scholar]
- 49.Mills SW, Mullineaux LS, Tyler PA. Habitat associations in gastropod species at East Pacific Rise hydrothermal vents (9°50’N) Biol Bull. 2007;212:185–194. doi: 10.2307/25066601. [DOI] [PubMed] [Google Scholar]
- 50.Hunt HL, Metaxas A, Jennings RM, Halanych K, Mullineaux LS. Testing biological control of colonization by vestimentiferan tubeworms at deep-sea hydrothermal vents (East Pacific Rise, 9°50'N) Deep-Sea Res I. 2004;51:225–234. [Google Scholar]
- 51.Lenihan HS, et al. Biotic interactions at hydrothermal vents: Recruitment inhibition by the mussel Bathymodiolus thermophilus. Deep Sea Res I. 2008;55:1707–1717. [Google Scholar]
- 52.Mullineaux LS, Mills SW, Goldman E. Recruitment variation during a pilot colonization study of hydrothermal vents (9° 50'N, East Pacific Rise) Deep Sea Res Part II Top Stud Oceanogr. 1998;45:441–464. [Google Scholar]
- 53.Soule SA, Escartin J, Fornari DJ. A record of eruption and intrusion at a fast-spreading ridge axis: Axial summit trough of the East Pacific Rise 9°-10°N. Geochem Geophys Geosyst. 2009;10:Q10T07. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.