Abstract
NY-ESO-1 is a “cancer-testis” antigen frequently expressed in epithelial ovarian cancer (EOC) and is among the most immunogenic tumor antigens defined to date. In an effort to understand in vivo tolerance mechanisms, we assessed the phenotype and function of NY-ESO-1–specific CD8+ T cells derived from peripheral blood lymphocytes (PBLs), tumor-infiltrating lymphocytes (TILs), and tumor-associated lymphocytes (TALs) of EOC patients with NY-ESO-1-expressing tumors, with or without humoral immunity to NY-ESO-1. Whereas NY-ESO-1–specific CD8+ T cells were readily detectable ex vivo with tetramers in TILs and TALs of seropositive patients, they were only detectable in PBLs following in vitro stimulation. Compared with PBLs, tumor-derived NY-ESO-1–specific CD8+ T cells demonstrated impaired effector function, preferential usage of dominant T-cell receptor, and enriched coexpression of inhibitory molecules LAG-3 and PD-1. Expression of LAG-3 and PD-1 on CD8+ T cells was up-regulated by IL-10, IL-6 (cytokines found in tumor ascites), and tumor-derived antigen-presenting cells. Functionally, CD8+LAG-3+PD-1+ T cells were more impaired in IFN-γ/TNF-α production compared with LAG-3+PD-1− or LAG-3−PD-1− subsets. Dual blockade of LAG-3 and PD-1 during T-cell priming efficiently augmented proliferation and cytokine production by NY-ESO-1–specific CD8+ T cells, indicating that antitumor function of NY-ESO-1-specific CD8+ T cells could potentially be improved by therapeutic targeting of these inhibitory receptors.
Keywords: tumor-infiltrating lymphocytes, IL-6, IL-10, T cell receptor
The presence of tumor-infiltrating lymphocytes within the tumor microenvironment is considered to be an indication of the host immune response to tumor antigens and is thought to reflect the dynamic process of “cancer immunoediting” (1). In epithelial ovarian cancer (EOC), support for the role of immune surveillance of tumors comes from observations indicating that the presence of intraepithelial CD8+-infiltrating T lymphocytes in tumors is associated with improved survival of patients with the disease (2). Although several lines of evidence have shown that spontaneous or vaccine-induced tumor-antigen-specific CD8+ T cells can recognize EOC targets (3), prolongation of survival in patients treated with immunization has only rarely been observed. This is probably a reflection of several in vivo immunosuppressive mechanisms in EOC-bearing hosts (4). Therefore, understanding factors that regulate the function(s) of tumor-antigen-specific CD8+ T cells is critical for effective control of tumor recurrence.
The NY-ESO-1 tumor antigen is a major target of CD8+ T cell recognition in EOC, eliciting both cellular and humoral immune responses in a proportion of patients with advanced NY-ESO-1-expressing tumors (5). However, similar to infectious disease models, chronic antigenic stimulation may result in exhaustion of antigen-specific CD8+ T cells (6) and loss of ability to produce key cytokines that are critical for the maintenance of CD8+ T cell memory (7). In this regard, programmed cell death-1 (PD-1, CD279), an immune inhibitory receptor belonging to the CD28:B7 family of costimulatory molecules, which is expressed on activated T cells, B cells, and myeloid cells (8), may play a role in down-regulating tumor immunity in EOC. Expression of the PD-1 ligand PD-L1 in ovarian cancer has been shown to inversely correlate with intraepithelial CD8+ T lymphocyte count (9), suggesting a pivotal role for the PD-1/PD-L1 pathway in initiating and maintaining tumor-antigen-specific T-cell hyporesponsiveness in the disease.
Another inhibitory molecule that has gained considerable recent attention is the lymphocyte activation gene-3 (LAG-3), located in the CD4 locus (10). LAG-3 is expressed on activated CD4+ and CD8+ T cells, negatively regulates T-cell expansion via inhibition of T-cell receptor (TCR) -induced calcium fluxes, and controls the size of the memory T-cell pool (11). Because LAG-3 and PD-1 are expressed on activated T cells, we predicted that both molecules could coordinately mediate the inability of tumor-antigen-specific T cells to efficiently control EOC. Here we report that whereas NY-ESO-1–specific CD8+ T cells were readily detectable ex vivo in tumor-infiltrating lymphocytes (TILs) and tumor-associated lymphocytes (TALs) of patients with spontaneous humoral immunity to NY-ESO-1, they were only detectable in peripheral blood lymphocytes (PBLs) following in vitro stimulation. However, tumor-derived NY-ESO-1–specific CD8+ T cells demonstrated impaired effector function, preferential usage of dominant TCR, and enriched coexpression of LAG-3 and PD-1. We demonstrate that LAG-3 and PD-1 on NY-ESO-1-specific and -nonspecific CD8+ T cells were significantly up-regulated by tumor-derived antigen-presenting cells (APCs) or by IL-6 and IL-10. Moreover, LAG-3 and PD-1 blockade enhanced effector function of NY-ESO-1–specific T cells. Together, our data point to a coordinate negative role of PD-1 and LAG-3 in regulating the functional properties of NY-ESO-1–specific CD8+ T cells in EOC. This understanding could ultimately lead to interventions to restore the effector function of NY-ESO-1–specific CD8+ T cells in human ovarian cancer.
Results
NY-ESO-1–Specific CD8+ T Cells Are Detectable ex Vivo at the Tumor Site of Seropositive Ovarian Cancer Patients.
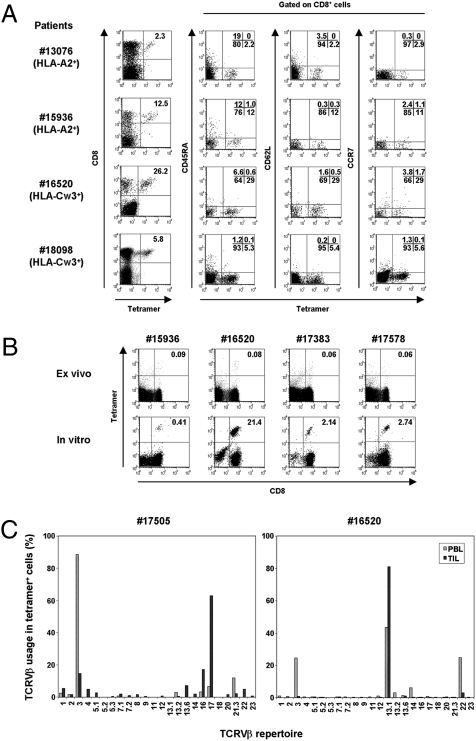
To assess antigen-specific immune responses at the ovarian tumor site of NY-ESO-1-seropositive EOC patients, TILs and TALs were isolated from EOC tissues and ascites, respectively, and the frequencies of NY-ESO-1–specific CD8+ T cells were determined directly ex vivo with HLA-matched (HLA-A*02-ESO157–165 or Cw*03-ESO92–100) tetramers. Even with tetramers for these limited epitopes, NY-ESO-1–specific CD8+ T cells were readily detectable in 10 out of 14 patients (71%) who were serum antibody-positive for NY-ESO-1 (Table S1 and Fig. 1A). The tumor-infiltrating tetramer+ cells showed CD45RAlowCD62LlowCCR7low effector memory phenotype (Fig. 1A). Moreover, less than 0.1% NY-ESO-1-specific tetramer+CD8+ T cells were detectable ex vivo in PBLs of the seropositive patients (Fig. 1B). As shown previously (3), in vitro stimulation once with NY-ESO-1 peptides was necessary to reveal NY-ESO-1-specific CD8+ T cells from PBLs of seropositive patients.
Fig. 1.
NY-ESO-1–specific CD8+ cells at the tumor site. (A) Ex vivo phenotypic analysis of NY-ESO-1 tetramer+ cells in TILs and TALs of NY-ESO-1-seropositive EOC patients. The four examples shown are representative of 15 individual samples. The HLA tetramers for individual patients are in parentheses. (B) The frequency of tetramer+ cells in PBLs before and after in vitro peptide presensitization. The number in the right upper quadrant indicates percentage of tetramer+CD8+ cells. (C) Comparison of TCR Vβ use of HLA-Cw*03-ESO92–100 tetramer+ cells derived from PBLs and TILs.
Because it has been reported that TCRs repertoire-specific for several peptide-MHC complexes are either diverse or highly conserved (12), we compared a TCR repertoire of NY-ESO-1–specific CD8+ T cells in PBLs and TILs by costaining with HLA-Cw*03-ESO92–100 tetramer and anti-Vβ monoclonal antibodies (mAbs). Tumor- and PBL-derived NY-ESO-1–specific CD8+ T cells used highly restricted TCRs, but their distributions were different, indicating a selective infiltration and/or expansion at tumor sites, assuming that in vitro stimulation did not alter the distribution of TCR usage (Fig. 1C). Moreover, these findings indicate that assessment of circulating NY-ESO-1-specific CD8+ T cells may not accurately reflect the phenotypic and functional properties of TILs in human ovarian cancer.
Expression of Coinhibitory Molecules Correlates with Impaired Effector Function of Tumor-Infiltrating NY-ESO-1–Specific CD8+ T Cells.
Next, we compared the effector function of NY-ESO-1-specific CD8+ T cells in TALs and TILs with that in PBLs. As shown in Fig. 2A, tetramer+ cells in PBLs exhibited high IFN-γ production in response to peptide antigen stimulation (range 31–84%; mean 60.8 ± 19.5%). Because of the limited number of available tetramers, we extended the functional analysis of NY-ESO-1-specific CD8+ T cells in PBLs to the use of overlapping peptides covering the entire length of NY-ESO-1. We found that 15 out of 23 PBLs (65%) derived from NY-ESO-1-seropositive EOC patients showed NY-ESO-1–specific CD8+ response (Table S2).
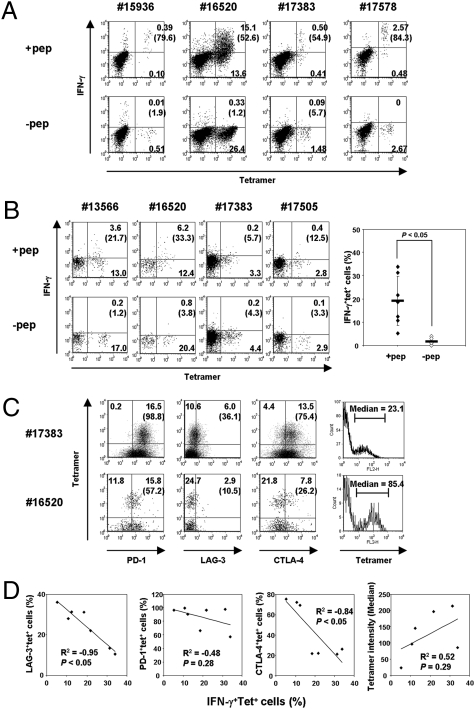
Fig. 2.
Impaired effector function of CD8+ T cells at the ovarian tumor site correlates with the expression of coinhibitory molecules. (A and B) NY-ESO-1–specific IFN-γ production from PBLs following in vitro presensitization (A). Ex vivo IFN-γ production from TILs or TALs. The graph indicates the average and statistics of peptide-specific IFN-γ production from seven samples (B). The number in parentheses indicates percentage of IFN-γ-producing cells among NY-ESO-1–specific tetramer+ cells. (C) Expression of PD-1, LAG-3, or CTLA-4 molecules on NY-ESO-1–specific CD8+ cells at the tumor site. The number in parentheses indicates percentage among NY-ESO-1-specific tetramer+ cells. The corresponding median density of fluorescence intensity of NY-ESO-1+tetramer+ cells is also shown. (D) Correlation between NY-ESO-1–specific IFN-γ production and the expression of coinhibitory molecules or tetramer binding intensity from seven individual patient samples. The coefficient of correlation (R2) and P value are shown in each plot.
The existence of readily detectable NY-ESO-1–specific CD8+ T cells in ovarian tumors allowed us to investigate the function of tumor-antigen-specific CD8+ T cells infiltrating the tumor site ex vivo. We examined the effector function of tetramer+CD8+ T cells in response to antigenic stimulation with cognate NY-ESO-1 peptides by intracellular IFN-γ staining in seven samples that contained more than 1% NY-ESO-1 tetramer+CD8+ cells (Fig. 2B). Whereas tetramer+ cells did not produce IFN-γ without NY-ESO-1 peptide stimulation, peptide-specific IFN-γ production was detected in a variable proportion of tetramer+CD8+ cells (range 8–35%; P < 0.05). We also determined antigen-nonspecific CD8 function in PBLs, TALs, and TILs. The production of IFN-γ, TNF-α, and IL-2 by CD8+ T cells from these specimens was compared after stimulation with anti-CD3/anti-CD28 mAbs (Fig. S1). IFN-γ production was almost similar among the samples. However, we found that TNF-α and IL-2 from TALs and TILs were significantly lower than from autologous PBLs. These results indicate impaired effector function of CD8+ T cells at the ovarian tumor site.
To determine whether the difference in effector function of NY-ESO-1–specific CD8+ T cells in TILs and TALs is related to the expression of inhibitory receptors, we examined the expression of coinhibitory molecules on these cells using multiparameter flow cytometry. In the examples shown in Fig. 2C, tetramer+ cells from patient number 17383 TILs showed higher expression of PD-1, LAG-3, and CTLA-4 than cells from patient number 16520 TILs, which contained more IFN-γ-producing cells in response to peptide stimulation. Therefore, we explored the relationship between the expression of these coinhibitory molecules and IFN-γ production on tetramer+ cells (Fig. 2D). Because most tetramer+ cells expressed PD-1, we found a weak correlation between PD-1 expression and IFN-γ production. In contrast, LAG-3-expressing cells within tetramer+ cells ranged from 10 to 40%, and negatively correlated with the proportion of IFN-γ+ cells. The percentages of CTLA-4-expressing cells also negatively correlated with the proportion of NY-ESO-1-specific IFN-γ+ cells. Furthermore, because surface TCR expression level greatly influences antigenic sensitivity of T cells, we examined the relationship between fluorescence intensity of tetramer staining (HLA-Cw3+ samples) and IFN-γ+ production. As expected, there was a weak positive correlation between tetramer binding level and IFN-γ production. On the other hand, tumor-infiltrating Treg frequency did not correlate with IFN-g production (Fig. S2). These results indicate that whereas the phenotype of NY-ESO-1–specific CD8+ TILs and TALs is predominantly effector memory, the capacity for IFN-γ production is diminished in LAG-3+ and CTLA-4+ subsets of tumor-antigen-specific T cells. Moreover, TILs and TALs contained significantly more LAG-3+CD8+ T cells compared with PBLs from healthy individuals and EOC patients (Figs. S3 and S4).
LAG-3+CD8+ Cells Coexpress PD-1 at the Tumor Site.
To further analyze tumor-infiltrating LAG3+CD8+ T cells, we characterized these cells by staining with cell-surface molecules related to T-cell responsiveness (Figs. 3 and S5). In chronic infection, exhausted cells exhibit PD-1high and CD127low phenotype, and these cells are unable to produce IFN-γ to virus-antigen challenge (13). Because most NY-ESO-1 tetramer+ cells expressed PD-1 (Fig. 2 C and D), we reasoned that the expression level of PD-1 and CD127 could also be related to the effector function of tumor-infiltrating CD8+ T cells. We analyzed PD-1 and CD127 expression on whole CD8+ or LAG-3+CD8+ T cells (Fig. 3 A and B). Consistent with our findings for NY-ESO-1–specific CD8+ T cells, bulk CD8+ T cells from TALs and TILs exhibited higher frequencies of PD-1+CD8+ T cells as compared with PBLs from healthy donors or EOC patients. Interestingly, the subset of LAG-3+CD8+ T cells was remarkably enriched for PD-1+ cells in PBL, TILs, and TALs of ovarian cancer patients, suggesting coordinated regulation of LAG-3 and PD-1 expression.
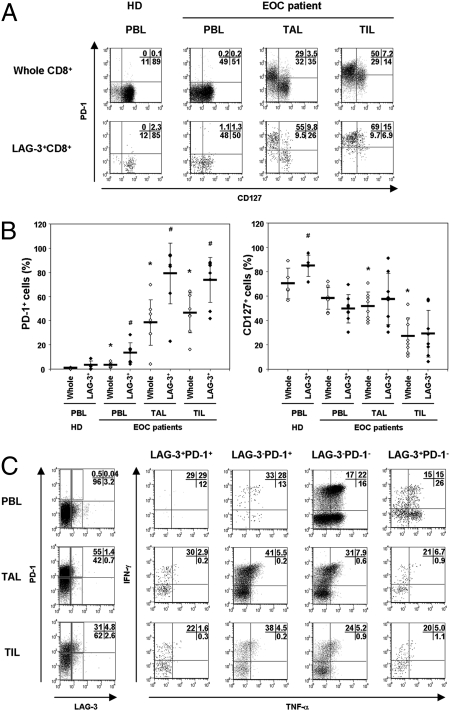
Fig. 3.
LAG-3+CD8+ T cells in TALs and TILs exhibit high PD-1 expression. (A and B) The expression of PD-1 and CD127 on whole CD8+ or LAG-3+CD8+ cells in PBLs, TALs, or TILs. *P < 0.05 compared with whole CD8+ cells of healthy donors’ (HD) PBLs. #P < 0.05 compared with whole CD8+ cells of each corresponding tissue. (C) IFN-γ and TNF-α production from each indicated population following stimulation with PMA/ionomycin. The result shown is representative of samples from three patients.
Because LAG-3 expression by NY-ESO-1-specific T cells negatively correlated with the proportion of IFN-γ+ cells (Fig. 2D), and the majority of LAG-3+CD8+ T cells coexpressed the PD-1 molecule, we examined the capacity for IFN-γ and TNF-α production from four CD8+ T cell subsets according to LAG-3 and PD-1 expression. PBLs, TALs, and TILs were stimulated with phorbol 12-myristate 13-acetate (PMA)/ionomycin, and the proportion of IFN-γ- and TNF-α-producing cells from each population was assessed by flow cytometry. CD8+ T cells in TALs and TILs produced almost the same amount of IFN-γ as PBLs, but TNF-α level in TALs and TILs was significantly lower than that in PBLs, which is consistent with the result shown in Fig. S1. We also found that the LAG-3+PD-1+ subset showed the fewest IFN-γ+/TNF-α+ double-positive cells among these populations in TILs and TALs (Fig. 3C). LAG-3−PD-1+ cells were capable of producing high levels of IFN-γ, but the frequency of dual-IFN-γ/TNF-α-producing cells was less than LAG-3−PD-1− cells. Together, these results indicate that LAG-3+PD-1+CD8+ cells in TALs and TILs have less capacity to produce IFN-γ and TNF-α, and suggest a role for LAG-3 in cooperating with PD-1 to reduce the effector function of CD8+ cells at the ovarian tumor site.
Dual Blockade of LAG-3 and PD-1 Pathway During Priming Restores Frequency and Effector Function of NY-ESO-1–Specific CD8+ Cells.
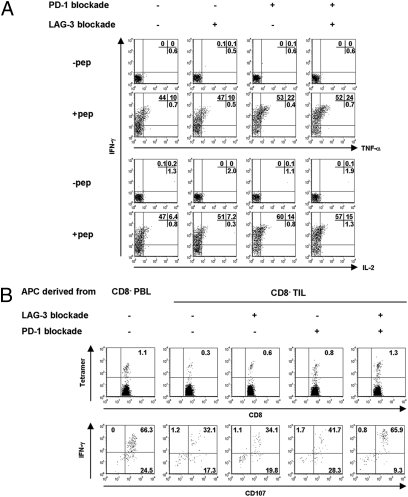
To test whether blockade of LAG-3 and/or PD-1 pathway could restore the effector function of NY-ESO-1–specific CD8+ T cells in TILs, whole TILs derived from NY-ESO-1-seropositive patients were cultured in the presence or absence of blocking antibodies to LAG-3 or PD-1 pathway for 2 days. PD-1 pathway blockade consisted of the combination of anti-PD-1 and anti-PD-L1 mAbs. The cells were then stimulated with NY-ESO-192–100 peptide for 6 h, and antigen-specific multicytokine production (IFN-γ, TNF-α, and IL-2) was determined by intracellular staining. Consistent with our data from Fig. 2B, NY-ESO-1-specific IFN-γ-producing cells were detectable upon peptide stimulation, and a small proportion were IFN-γ/TNF-α+ or IFN-γ/IL-2+ (Fig. 4A). LAG-3 blockade did not significantly increase cytokine production compared with T cells cultured without antibody. This is consistent with a previous report indicating that LAG-3 blockade did not modify the effector function of a CTL clone derived from TILs (14). In contrast, PD-1 pathway blockade significantly enhanced the total frequency of IFN-γ-producing cells, especially those that coexpressed TNF-α or IL-2. Based on our data showing impaired effector function of LAG-3+PD-1+CD8+ T cells, we hypothesized that dual, rather than individual, blockade of PD-1 and LAG-3 pathways could enhance effector function of the LAG-3+PD-1+CD8+ subset. Interestingly, we found that LAG-3 blockade did not enhance the effect of PD-1 blockade on the effector function of NY-ESO-1–specific CD8+ T cells. These findings indicate that whereas LAG-3 plays a role in the unresponsiveness of PD-1+CD8+ T cells, direct blockade of LAG-3 on T cells does not restore the function of tumor-antigen-specific CD8+ T cells in TILs or TALs.
Fig. 4.
Dual blockade of LAG-3 and PD-1 pathways increases the frequency and enhances effector function of NY-ESO-1–specific T cells. (A) Whole TIL was cultured in the presence or absence of indicated blocking antibodies. After 2 days of culture, peptide-specific cytokine productions from NY-ESO-1 tetramer+ cells were analyzed. The result shown is representative of three independent experiments from two patients. (B) The frequency of tetramer+ cells and peptide-specific IFN-γ/CD107 expression following in vitro presensitization with or without LAG-3 and PD-1 blockade. Results shown are representative of two independent experiments from two patients.
Because LAG-3 signaling inhibits early events in primary activation of human T cells and LAG-3 blockade of primary resting T cells successfully enhances T-cell proliferation and activation (15), we reasoned that effective blockade of LAG-3 may depend on the context of the stimulatory conditions present during T-cell activation. To verify our hypothesis, we focused on LAG-3 and PD-1 pathway blockade during the early priming phase of NY-ESO-1–specific T cells. For this purpose, CD8+ T cells isolated from PBLs or TILs of NY-ESO-1-seropositive EOC patients were cocultured with NY-ESO-1 peptide-pulsed autologous CD8− cells (APCs) from PBLs or TILs. Expression of PD-1 and LAG-3 on NY-ESO-1–specific CD8+ T cells under these different stimulating conditions was determined. We found that NY-ESO-1-specific CD8+ T cells from PBLs cocultured with tumor-derived APCs exhibited a higher frequency of PD-1+ cells than when stimulated with PBL-derived APCs (Fig. S6A). Moreover, the NY-ESO-1-specific CD8+ TILs that exhibited high expression of PD-1 failed to expand when cocultured with peptide-pulsed APCs from TILs. However, these antigen-specific CD8+ TILs were able to proliferate when cocultured with PBL-derived APCs, suggesting that the tumor environment rendered PD-1+CD8+ T cells hyporesponsive. With regard to the potential role of LAG-3 in determining functional heterogeneity of PD-1+ T cells, we found no significant difference in the total frequency of LAG-3+ T cells under the different conditions, but found that the proportion of LAG-3+PD-1+ significantly increased when tumor-derived APCs were used for stimulation (Fig. S6B). In addition, tumor-derived APCs up-regulated PD-1 expression of CD8 clone (Fig. S7). These findings are relevant in vivo because tumor-derived APCs contribute to the tumor milieu that could facilitate the differentiation of LAG-3+PD-1+ cells.
To determine whether blockade of LAG-3 and PD-1 pathways during antigen stimulation would improve the quality of NY-ESO-1–specific T cells in the presence of tumor-derived APCs, CD8+ T cells from PBLs of a seropositive patient were stimulated with NY-ESO-192–100-pulsed tumor-derived APCs in the presence or absence of LAG-3 and/or PD-1 pathway blockade. When CD8+ cells were cocultured with tumor-derived APCs, the frequency of NY-ESO-1-specific tetramer+ cells and IFN-γ+/CD107+ cells significantly decreased compared with those stimulated with PBL-derived APCs (Fig. 4B). LAG-3 or PD-1 pathway blockade alone increased the frequency of NY-ESO-1 tetramer+ cells 2- to 3-fold, but there was no significant change in effector function of treated versus untreated cells. However, the combined blockade of LAG-3 and PD-1 pathways not only led to a marked increase in the frequency of NY-ESO-1+ tetramer+ cells but also to a significant increase in the proportion of dual-functional (IFN-γ+CD107+) tetramer+ cells compared with untreated cells or cells treated with a single antibody. Therefore, dual blockade of LAG-3 and PD-1 pathways during priming of tumor-antigen-specific T cells with tumor-derived APCs efficiently restores T-cell effector function to levels observed with peripheral blood-derived APCs.
LAG-3+PD-1+CD8+ Cells Induced by Ovarian Tumor Ascites Demonstrate Impaired Effector Function.
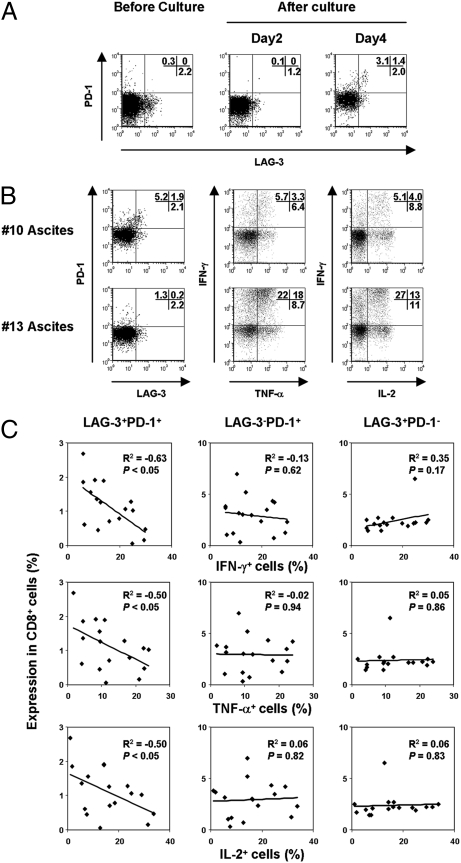
Because the majority of advanced ovarian cancer patients also have ascites, we next examined whether ovarian tumor ascites (without tumor-derived APCs) can also induce LAG-3+PD-1+ cells. Our results from 17 patients indicate that PD-1 and LAG-3 can be induced by tumor-derived ascites even without antigenic stimulation, and the resulting LAG-3+PD-1+ T cells were imprinted with diminished effector function (Fig. 5 A–C).
Fig. 5.
Ovarian tumor ascites induce PD-1 and LAG-3 expression on CD8+ T cells. (A) PBLs from healthy donor were cultured in tumor ascites. PD-1 and LAG-3 expression were analyzed on CD8+ cells. (B and C) At 4 days of culture in ascites, multicytokine production from CD8+ cells was evaluated following stimulation with PMA/ionomycin. The relationship between surface PD-1 and LAG-3 expression and multicytokine-producing CD8+ T cells cultured in ascites derived from 17 patients is represented.
IL-10 and IL-6, but Not TGF-β, Induce LAG-3+PD-1+ and LAG-3−PD-1+CD8+ T Cells.
Finally, to determine the factors involved in PD-1 and LAG-3 induction in human ovarian cancer, we measured cytokine levels in ascites. We found that IL-10, IL-6, TGF-β, and IFN-γ levels were detectable, but IL-2, IL-4, IL-17A, IL-1β, and TNF-α were not (Fig. S8A). Because IL-6, IL-10, and TGF-β are associated with immune suppression in ovarian cancer (16), we asked whether these cytokines are also involved in the induction of LAG-3+PD-1+ and LAG-3−PD-1+ cells. Treatment with IL-10 increased these populations in a dose-dependent manner, and IL-6 but not TGF-β slightly induced PD-1+ cells compared with control (Fig. S8B). In addition, we found that the combination of IL-10 and IL-6 enhanced PD-1 and LAG-3 expression (Fig. S8C). Together, these results indicate that the ovarian tumor milieu facilitates the generation of LAG-3+PD-1+ and LAG-3−PD-1+ cells in the presence or absence of tumor-derived cells, and blockade of these molecules enhances effector function of ovarian tumor-infiltrating antigen-specific CD8+ T cells.
Discussion
The identification of coinhibitory molecules that antagonize the function of tumor-antigen-specific T cells is crucial for effective cancer immunotherapy. In this study, we have focused on NY-ESO-1–specific CD8+ T cells detected directly ex vivo in human EOC specimens. We previously identified NY-ESO-1 as a prototypic tumor antigen target for specific immunotherapy of EOC (3, 5) and are currently assessing the immunogenicity of NY-ESO-1-based candidate vaccines in early-phase clinical trials under the sponsorship of the Cancer Vaccine Collaborative (http://www.cancerresearch.org). First, we found that NY-ESO-1–specific CD8+ T cells were readily detectable ex vivo in TILs and TALs of NY-ESO-1-seropositive EOC patients. In contrast, NY-ESO-1–specific CD8+ T cells were only readily detectable in PBLs of seropositive EOC patients following in vitro stimulation. Second, we demonstrated that tumor-infiltrating NY-ESO-1–specific CD8+ T cells are predominantly effector memory phenotype with wide variability in effector function, and include a subset with low responsiveness as determined by IFN-γ production. Third, tumor-derived NY-ESO-1–specific CD8+ T cells share highly restricted TCRs with their peripheral blood-derived counterparts, but demonstrate preferential usage of dominant TCR. This suggests that TCR structural diversity may partly account for functional differences between peripheral blood- and tumor-derived tumor-antigen-specific T cells. Fourth, whereas only a minority of peripheral blood-derived CD8+ T cells express PD-1 and/or LAG-3, the majority of tumor-derived CD8+ T cells including NY-ESO-1–specific CD8+ T cells express PD-1, and the LAG-3+PD-1+ T cell subset preferentially accumulates at the ovarian tumor site. Indeed, in the presence of tumor-derived APCs, antigen-specific peripheral blood-derived CD8+ T cells become endowed with the phenotypic and functional characteristics of TILs with respect to PD-1 and LAG-3 expression. Together, these results indicate that the fate of an antigen-experienced T cell upon TCR engagement is dictated by the environmental cues it receives from the ovarian tumor microenvironment.
The existence of NY-ESO-1-specific CD8+ T cells in TILs or TALs of ovarian cancer patients, detectable by direct ex vivo tetramer analysis, allowed us to demonstrate the wide variability in the proportion of IFN-γ-producing cells following antigen stimulation (range 5–34%). In previous studies, spontaneous NY-ESO-1-specific CD8+ T cell responses were detectable directly ex vivo in peripheral blood of melanoma patients who were seropositive for NY-ESO-1 (17). In a recent report by Milne et al. on a single NY-ESO-1-seropositive EOC patient, corresponding ascites and solid tumor specimen were shown to be enriched (up to 6%) for NY-ESO-1-tetramer-reactive CD8+ T cells (18). The levels of IFN-γ production by NY-ESO-1-reactive CD8+ T cells in ovarian cancer TILs are relatively high and correlated with tetramer fluorescence intensity. Although the link between reduced tetramer fluorescence intensity and effector function may reflect failure of TCR and CD8 colocalization on CD8+ TILs, leading to inability to produce IFN-γ in response to stimulation (19), it does not fully account for the functional heterogeneity of tumor-infiltrating antigen-specific T cells.
Because the intrinsic variability of effector/memory CD8+ T cell subsets contributes to significant differences in in vivo function, we asked whether there are functionally distinct subsets of tumor-antigen-specific TILs based on the expression of LAG-3 and PD-1 inhibitory molecules. In an attempt to provide the basis for this functional heterogeneity, we showed that the majority of PD-1+CD8+ TILs lacked expression of CD127, and this phenotype is known to be associated with impaired effector-to-memory transition (20). We also demonstrated that LAG-3 expression inversely correlated with the effector function of NY-ESO-1–specific CD8+ TILs. In addition, most LAG-3+ cells from the tumor site coexpressed PD-1 and highly expressed CTLA-4, another inhibitory receptor of the CD28 family. The LAG-3+PD-1+ T cells exhibited the lowest IFN-γ/TNF-α production. Because cells that are PD-1+ but lack LAG-3 expression exhibit functional properties intermediate between LAG-3−PD-1− and LAG-3+PD-1+ cells, we propose that LAG-3 plays a critical role in reducing the effector function of PD-1+CD8+ cells at the ovarian tumor site.
In previous studies, blocking the PD-1/PD-L1 pathway in vivo incompletely restored frequency and function of lymphocytic choriomeningitis virus-specific CD8+ T cells from chronically infected mice (21). More recently, Fourcade et al. reported that PD-1 expression was up-regulated on NY-ESO-1–specific CD8+ T cells in PBLs of melanoma patients, and PD-L1 pathway blockade enhanced the number of antigen-specific cytokine-producing CD8+ T cells (17). Here we have focused on coinhibitory pathway blockade ex vivo under different stimulating conditions and differentiation states of CD8+ T cells and observed distinct outcomes. Whereas LAG-3 blockade enhanced the frequency of NY-ESO-1 tetramer+ cells and polyfunctional T cells only during priming in the presence of APCs, these effects were observed for PD-1 pathway blockade in the presence or absence of APCs. These findings are of considerable in vivo significance because expression of PD-L1, the ligand for PD-1, has been observed in ovarian and other human cancers (9, 22). With regard to LAG-3, the results are also consistent with a previous report indicating that LAG-3 blockade did not enhance T-cell function of a TIL-derived clone (14) and indicate that LAG-3 blockade is effective during early events of T-cell activation, probably via its binding to MHC class II.
Our data also indicated that dual blockade of PD-1 and LAG-3 pathways increased the frequency and effector function of NY-ESO-1–specific CD8+ T cells. This was most notable under stimulatory conditions where tumor-derived APCs were present, and less so in the absence of antigenic stimulation. In a murine model of chronic viral infection, Blackburn et al. (23) demonstrated that dual blockade of LAG-3 and PD-1 in vivo resulted in significant increases in antigen-specific CD8+ T cell numbers and function, as well as marked reductions in viral titer compared with untreated or single-antibody-treated mice. Because coculture of peripheral blood- and tumor-derived CD8+ T cells with tumor-derived APCs resulted in generation of PD-1+LAG-3+ cells with diminished effector function, the PD-1- and/or LAG-3-positive “hyporesponsive” phenotype appears to be acquired upon infiltration of tumor-antigen-specific T cells into the tumor site. Although this phenotype could be acquired by chronic antigen stimulation (21, 23), our data demonstrate a role for non-antigen-driven generation of this phenotype by IL-10 and IL-6, but not TGF-β.
In summary, our results have identified PD-1 and LAG-3 as important inhibitory molecules frequently expressed by ovarian tumor-infiltrating lymphocytes. LAG-3 plays a role in attenuating the effector function of PD-1+CD8+ T cells, wherein effector function is most impaired in antigen-specific LAG-3+PD-1+CD8+ TILs. These observations provide insights into the mechanisms of hyporesponsiveness to NY-ESO-1–specific T cells infiltrating human EOC. Because dual blockade of LAG-3 and PD-1 pathways acted additively to efficiently restore T-cell effector function, our findings indicate that antitumor T-cell function could potentially be improved by therapeutic targeting of these inhibitory receptors in human ovarian cancer.
Materials and Methods
PBLs, TILs, and TALs.
Tissue specimens, ascites fluids, and peripheral bloods were obtained from patients undergoing surgery for EOC at the Roswell Park Cancer Institute, Buffalo, NY, under an approved protocol from the Institutional Review Board.
Ex Vivo Cell Analysis of NY-ESO-1–Specific CD8+ Cells.
Phycoerythrin- or allophycocyanin-conjugated HLA-A*0201/ESO157–165 and HLA-Cw*03/ESO92–100 tetramers were obtained from the Lausanne branch of the Ludwig Institute for Cancer Research. Cells were stained with tetramers and mAbs against CD8 (BD Biosciences), PD-1 (eBioscience or BD Biosciences), and LAG-3 (Alexis Biochemicals or R&D Systems). Cytokine production from tetramer+ cells, TILs, or TALs was determined as described previously (3). NY-ESO-1–specific CD8+ T cells in PBLs were determined as described previously (3).
ELISA.
NY-ESO-1–specific antibodies were measured in the serum by ELISA analysis, as described previously (3, 5).
Blocking Antibody Treatment.
TILs were cultured in complete medium in the presence or absence of 10 μg/mL anti-PD-1 (J116) + 10 μg/mL anti-PD-L1 (M1H1) mAbs (eBioscience) (24), 20 μg/mL anti-LAG-3 (17B4) mAb (Alexis Biochemicals), or a combination of these mAbs. After 2 days of culture, multicytokine production from tetramer+ cells was determined by intracellular cytokine staining (ICS). Isolated CD8+ cells (5 × 105) were cocultured with NY-ESO-1 peptide-pulsed CD8− cells (5 × 105) derived from PBLs or TILs, and blocking antibodies were added to the culture at days 0 and 4. Ten to fifteen days after culture, the frequency of tetramer+ cells and the effector function against peptide were determined by ICS.
Culture of PBLs with Tumor Ascites.
Tumor ascites were filtered using a 0.22-μm sterile syringe to completely eliminate cells. PBLs from healthy donors were cultured in ascites fluid for 4 days. PD-1 and LAG-3 expression on CD8+ cells was determined; multicytokine production from CD8+ cells was assessed following 6-h PMA and ionomycin stimulation.
Statistical Analysis.
Comparison between paired and unpaired groups was performed using the appropriate Student’s t test. The correlation coefficient and P value between the graphs of two datasets were determined by Pearson product-moment correlation using SigmaStat 3.5 software (Systat). P < 0.05 was defined as statistically significant.
Supplementary Material
Acknowledgments
We thank Mr. Anthony Miliotto, Ms. Jianqun Liao, Ms. Cathy Grande, and Ms. Erika Ritter for their excellent technical support. This work was supported by the Cancer Research Institute Ovarian Cancer Working Group Grant, CRI/LICR Cancer Vaccine Collaborative Grant, Ovarian Cancer Research Fund, and a Hilton-Ludwig Cancer Metastasis Grant of the Ludwig Institute for Cancer Research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1003345107/DCSupplemental.
References
- 1.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Sato E, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odunsi K, et al. Vaccination with an NY-ESO-1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proc Natl Acad Sci USA. 2007;104:12837–12842. doi: 10.1073/pnas.0703342104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coukos G, Conejo-Garcia JR, Buckanovich R, Benencia F. Vascular leukocytes: A population with angiogenic and immunossuppressive properties highly represented in ovarian cancer. Adv Exp Med Biol. 2007;590:185–193. doi: 10.1007/978-0-387-34814-8_13. [DOI] [PubMed] [Google Scholar]
- 5.Odunsi K, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res. 2003;63:6076–6083. [PubMed] [Google Scholar]
- 6.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marrack P, Kappler J. Control of T cell viability. Annu Rev Immunol. 2004;22:765–787. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- 8.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 9.Hamanishi J, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruniquel D, Borie N, Triebel F. Genomic organization of the human LAG-3/CD4 locus. Immunogenetics. 1997;47:96–98. doi: 10.1007/s002510050332. [DOI] [PubMed] [Google Scholar]
- 11.Workman CJ, et al. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol. 2004;172:5450–5455. doi: 10.4049/jimmunol.172.9.5450. [DOI] [PubMed] [Google Scholar]
- 12.Valmori D, et al. Tetramer-guided analysis of TCR β-chain usage reveals a large repertoire of melan-A-specific CD8+ T cells in melanoma patients. J Immunol. 2000;165:533–538. doi: 10.4049/jimmunol.165.1.533. [DOI] [PubMed] [Google Scholar]
- 13.Radziewicz H, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demeure CE, Wolfers J, Martin-Garcia N, Gaulard P, Triebel F. T lymphocytes infiltrating various tumour types express the MHC class II ligand lymphocyte activation gene-3 (LAG-3): Role of LAG-3/MHC class II interactions in cell-cell contacts. Eur J Cancer. 2001;37:1709–1718. doi: 10.1016/s0959-8049(01)00184-8. [DOI] [PubMed] [Google Scholar]
- 15.Maçon-Lemaître L, Triebel F. The negative regulatory function of the lymphocyte-activation gene-3 co-receptor (CD223) on human T cells. Immunology. 2005;115:170–178. doi: 10.1111/j.1365-2567.2005.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kryczek I, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fourcade J, et al. PD-1 is a regulator of NY-ESO-1–specific CD8+ T cell expansion in melanoma patients. J Immunol. 2009;182:5240–5249. doi: 10.4049/jimmunol.0803245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milne K, et al. Tumor-infiltrating T cells correlate with NY-ESO-1–specific autoantibodies in ovarian cancer. PLoS One. 2008;3:e3409. doi: 10.1371/journal.pone.0003409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demotte N, et al. Restoring the association of the T cell receptor with CD8 reverses anergy in human tumor-infiltrating lymphocytes. Immunity. 2008;28:414–424. doi: 10.1016/j.immuni.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Kaech SM, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 21.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 22.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 23.Blackburn SD, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jurado JO, et al. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J Immunol. 2008;181:116–125. doi: 10.4049/jimmunol.181.1.116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.