Abstract
Protein misfolding and formation of β-sheet-rich amyloid fibrils or aggregates is related to cellular toxicity and decay in various human disorders including Alzheimer’s and Parkinson’s disease. Recently, we demonstrated that the polyphenol (-)-epi-gallocatechine gallate (EGCG) inhibits α-synuclein and amyloid-β fibrillogenesis. It associates with natively unfolded polypeptides and promotes the self-assembly of unstructured oligomers of a new type. Whether EGCG disassembles preformed amyloid fibrils, however, remained unclear. Here, we show that EGCG has the ability to convert large, mature α-synuclein and amyloid-β fibrils into smaller, amorphous protein aggregates that are nontoxic to mammalian cells. Mechanistic studies revealed that the compound directly binds to β-sheet-rich aggregates and mediates the conformational change without their disassembly into monomers or small diffusible oligomers. These findings suggest that EGCG is a potent remodeling agent of mature amyloid fibrils.
Keywords: Alzheimer, Parkinson, catechine, misfolding, oligomer
Previous studies have shown that the polyphenol (-)-epi-gallocatechine gallate (EGCG), found in large amounts in green tea, has antiamyloidogenic properties and modulates the misfolding of disease proteins and prions (1–5). EGCG directly binds to unfolded polypeptide chains and inhibits β-sheet formation, an early event in the amyloid formation cascade (6). In the presence of EGCG, the assembly of a new type of unstructured, SDS-stable, nontoxic oligomer was observed, instead of the expected formation of β-sheet-rich aggregates. This suggested that the compound redirects aggregation prone polypeptides into off-pathway protein assemblies (6), as has since been confirmed for other flavonoids (7).
These findings raise the question of whether EGCG might also be able to disassemble preformed, β-sheet-rich structures as well as earlier intermediates of fibrillogenesis. Other small molecules such as curcumin or short β-sheet breaker peptides were described to have this ability; however, their mechanism of action has not been elucidated (8, 9). In the present study, we examined the ability of EGCG to alter the structure of mature amyloid fibrils with biochemical and biophysical as well as cell-based assays.
Results and Discussion
To study the effect of EGCG on preformed amyloid aggregates, we first produced α-synuclein (αS) fibrils by incubating natively unfolded monomers (100 μM) at 37 °C for 7 d in phosphate buffer. Then aggregates were characterized by EM, atomic force microscopy (AFM), Thioflavin T (ThT) binding assays, and CD spectroscopy (Fig. S1). We observed that the in vitro generated αS aggregates have a β-sheet structure and a fibrillar morphology. Moreover, they efficiently bind the dye ThT, supporting previously published results (10).
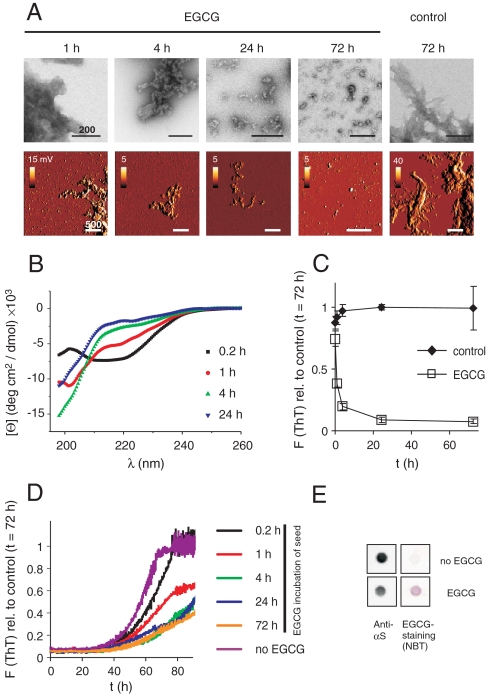
Next, we added an equimolar concentration of EGCG to the fibrils (50 μM αS monomer equivalent). The effect of the compound was monitored by time-resolved EM and AFM. We found that EGCG very efficiently remodels the ordered, fibrillar morphology of αS aggregates (Fig. 1A). After incubation of 24–72 h, smaller amorphous protein aggregates instead of long fibrils were readily detected, whereas shorter incubation (1–4 h) resulted in mixtures of both structures. This suggests that the compound binds to preformed αS aggregates and transforms their characteristic fibrillar structure.
Fig. 1.
EGCG remodels αS fibrils into benign amorphous protein assemblies. (A) EGCG (50 μM) induced remodeling of fibrillar αS-His (50 μM) into spherical assemblies monitored by negative-stain EM and intermittent contact mode AFM. Scale bars = 200 nm (EM), 500 nm (AFM). 1st derivative (dz/dt) scans are shown for AFM to enhance contrast. (B) Loss of β-sheet structure of αS fibrils (50 μM) after incubation with EGCG. Fibrils were sonicated for 1 h before CD measurement. (C) Loss of ThT fluorescence after incubation of αS fibrils (50 μM) with EGCG (50 μM) under constant agitation at 37 °C was monitored for 72 h; error bars = SD, n = 3. (D) Time-dependent loss of seeding capacity of αS fibrils after incubation with EGCG. Fibrils (10% wt/wt) were preincubated with or without EGCG for the indicated times, sonicated for 1 h, and added to fresh monomeric αS (50 μM) in phosphate buffer. Seeded fibril formation was monitored by ThT fluorescence for 90 h. (E) Nondenaturing FRA of NP-40 resistant (total) aggregates after incubation of αS fibrils (50 μM) with EGCG (50 μM) for 10 min. EGCG-specific staining by NBT, and αS staining by anti-αS antibody shows rapid binding of EGCG to αS fibrils.
Using CD spectroscopy, we then examined whether EGCG-mediated remodeling influences the characteristic β-sheet structure of αS amyloid aggregates. Fibrils were analyzed after incubation with EGCG for 0.2, 1, 4, and 24 h (Fig. 1B). We observed that untreated αS fibrils have a negative peak shifted from 218 to approximately 220 nm (Fig. S1C) as has previously been reported for fibrillar aggregates of αS (11) and amyloid-β (Aβ) (12) possibly due to differential absorption flattening (13). Spectra remained unchanged by prolonged sonication and by incubation in the absence of EGCG (Fig. S1C). However, this peak disappeared in a time-dependent manner after EGCG treatment, indicating that the compound alters the β-sheet conformation of preformed αS fibrils (Fig. 1B). These results were confirmed with time-resolved ThT assays (14). As shown in Fig. 1C and Fig. S2A, incubation of preformed αS fibrils without EGCG did not significantly alter ThT binding, while in the presence of the compound ThT fluorescence decreased in a time-dependent manner. Incubation of fibrils with an equimolar concentration of EGCG-reduced ThT fluorescence to approximately 40% after 1 h and to approximately 10% after 72 h.
A characteristic feature of β-sheet-rich amyloid fibrils is that they are seeding-competent structures that can efficiently convert unpolymerized monomers from the soluble to the aggregated state (15). Therefore, we assessed whether EGCG-remodeled αS fibrils can seed the polymerization of natively unfolded monomers. Preformed αS fibrils were incubated with an equimolar concentration of EGCG for 0.2, 1, 4, 24, and 72 h. Then, aggregates were sonicated for 1 h and added to reactions containing an excess of αS monomers. Self-assembly of amyloid fibrils was monitored with the ThT fluorescence assay (14). Untreated amyloid structures accelerated αS polymerization (Fig. 1D and Fig. S1B), whereas EGCG-treated αS aggregates did not. This supports the results of the CD spectroscopy and ThT binding experiments (Fig. 1B and C) that the compound remodels preformed amyloid structures and alters their β-sheet conformation, which is required for aggregate seeding (15).
Using a nitroblue tetrazolium (NBT) staining assay (16), we next examined whether the conformational conversion is initiated by direct binding of EGCG to αS fibrils. Preformed aggregates were incubated for 10 min with EGCG and subsequently filtered through a cellulose acetate membrane (Fig. 1E and Fig. S2B). The membrane was then stained with NBT, a substance that specifically detects EGCG-bound polypeptide chains (6). We observed NBT-stained aggregates immediately after EGCG addition, indicating that the compound rapidly and directly associates with preformed amyloid fibrils.
Comparing the results from the staining experiments with those from time-resolved EM, ThT, and seeding assays, we found that appearance of the NBT signal is the event that occurs most rapidly (Fig. S2B). Loss of ThT binding, seeding competence, and fibrillar structure happen more slowly (Fig. 1A–D). This indicates that EGCG binding precedes structural change. Together, these data strongly suggest that direct binding of EGCG to fibrils drives amyloid remodeling.
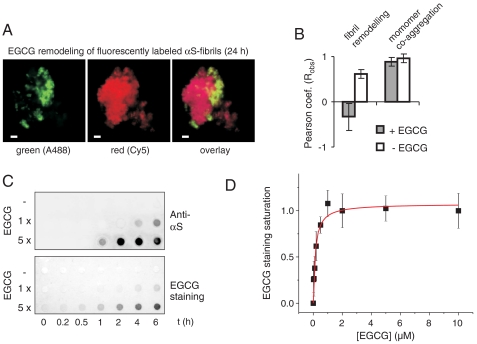
We then investigated the mechanistic details of this process. Does EGCG disassemble αS fibrils into smaller species that reaggregate into amorphous structures or does the remodeling process occur directly? To investigate these possibilities, red (Cy5) and green (Alexa 488) fluorescently labeled αS fibrils (5% wt/wt) were produced in vitro and their morphology was confirmed by AFM (Fig. S3A). To examine amyloid remodeling, labeled fibrils were then mixed and incubated with EGCG at an equimolar concentration (50 μM) for 1 or 24 h. Analysis by immunofluorescence (IF) microscopy revealed that the αS aggregates in the mix remained uniformly red or green after EGCG treatment (Fig. 2A and Fig. S3 B and C). This indicates that the compound mediates direct fibril restructuring and does not lead to a quantitative release of monomers or small oligomers that subsequently reassemble into larger protein aggregates. Quantitative analysis of red- and green-labeled aggregate mixtures shown in Fig. 2B, (negative Pearson coefficient; representative analyses are shown in Fig. S4), as well as monomer quantification by HPLC (Fig. S5A) confirmed this result. However, yellow αS aggregates were observed in control experiments by IF microscopy when red- and green-labeled αS monomers were coincubated with unlabeled protein for 24 h in the presence and absence of EGCG (high positive Pearson correlation coefficients; Fig. 2B and Fig. S3D), indicating fibril growth by addition of monomers carrying red or green labels (17).
Fig. 2.
Direct remodeling of αS fibrils. (A) Red and green fluorescently labeled fibrils of αS formed for 7 d (total monomer concentration 50 μM, 2.5% αS-A488 or 2.5% αS-Cy5) were mixed and incubated with equimolar EGCG for 24 h. Fluorescence microscopy after incubation shows aggregates predominantly labeled by either the green or the red fluorophores, scale bar = 1 μm. (B) Pearson coefficients from colocalization studies of mixed green/red fibril remodeling and of green/red monomer coaggregation for 24 h (30 images, 10–50 aggregates/image). (C) EGCG binding precedes remodeling. Fibrillar αS (50 μM) was incubated with EGCG (50 μM, 250 μM) for 10 min in TBS. Aggregates were pelleted for 20 min at 200,000 × g, washed, resuspended in TBS and incubated at 37 °C for 10 min–6 h. SDS insoluble αS was quantified by anti-αS antibody (11) staining, aggregate-bound EGCG was detected by NBT staining after FRA. (D) Quantitative analysis of EGCG binding affinity. Fibrillar αS (100 nM) was incubated with EGCG (20 nM–10 μM) overnight at 37 °C in PBS. Aggregate-bound EGCG was detected by NBT staining after FRA and quantified densitometrically to determine EGCG binding affinities. Unspecific NBT membrane staining was subtracted and EGCG staining was normalized and fitted by a single binding site model; Kd = 100 ± 20 nM, mean ± SD, n = 4.
To investigate whether unbound EGCG is required for remodeling of preformed αS fibrils, aggregates were incubated with the compound for 10 min and then separated from solution by ultracentrifugation. Following a washing step remodeling of insoluble EGCG-treated protein aggregates was monitored using a filter retardation assay (FRA), which efficiently detects SDS-stable protein aggregates (18). We observed that compound treatment caused the appearance of SDS-resistant αS aggregates after an incubation period of 2–6 h, while such aggregates were not detected in the absence of EGCG (Fig. 2C). The conversion of SDS-unstable into SDS-stable αS aggregates in the presence of EGCG was also confirmed by SDS-PAGE and immunoblotting (Fig. S5B). Together, these results indicate that binding of EGCG to αS protein aggregates is necessary and sufficient for fibril remodeling, while unbound compound molecules are not required for this effect.
To confirm that EGCG is directly bound to αS aggregates, compound treated and untreated samples were also analyzed using NBT assays (2, 16). We found that the SDS-resistant αS aggregates retained on filter membranes stain purple after NBT staining (Fig. 2C), supporting the hypothesis that EGCG directly interacts with preformed aggregates and remodels their conformation in the solid state. To assess the affinity of EGCG binding to αS aggregates, the compound (20 nM - 10 μM) was incubated overnight at 37 °C with preformed fibrils (equivalent to a monomer concentration of 100 nM). Then, EGCG binding to aggregates was quantified by FRA and NBT staining assays (Fig. 2D and Fig. S6). Quantitative analysis of binding data resulted in an apparent dissociation constant of 100 ± 20 nM, indicating that the compound binds with high affinity to preformed amyloid fibrils.
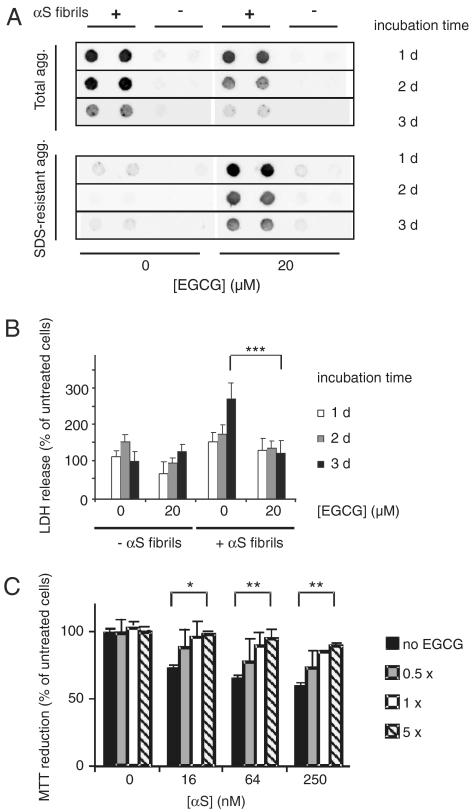
We next investigated whether EGCG is able to remodel insoluble αS aggregates in a cell model system. Preformed, insoluble αS amyloid fibrils were introduced into HEK-293 cells overexpressing αS (HEK-293 wt αS) using a well established transfection protocol (19). Six hours posttransfection of cells with aggregates, EGCG (20 μM) was added, and cultures were further incubated for 1–3 d at 37 °C. Cell lysates were prepared and the total amount of Nonidet P-40 (NP-40) insoluble αS aggregates was quantified by FRA (2). We found that EGCG treatment strongly reduced the amount of NP-40 resistant αS aggregates in HEK-293 cells in a time-dependent manner (Fig. 3A), indicating that the compound is both active in vitro and in vivo.
Fig. 3.
EGCG remodels and detoxifies cellular αS aggregates. (A) Fibrillar αS (1 μM) aggregates were introduced into HEK-293 cells expressing wild-type αS (HEK-293 wt αS). After 6 h cells were washed and incubated for 1–3 d in the absence or presence of 20 μM EGCG. NP-40 resistant (total) aggregates as well as SDS-resistant aggregates were quantified by FRA after cell lysis using anti-αS antibody. (B) Cytotoxicity of αS aggregates to HEK-293 wt αS cells was assayed by LDH-release. LDH signals were normalized to cells neither treated with αS aggregates nor treated with EGCG and incubated for 3 d. Values represent means ± SD, n = 8; *** P < 0.0005. (C) Reduction in metabolic activity of PC12 cells after addition of αS fibrils. Fibrils (100 μM) were incubated with different amounts of EGCG for 24 h at 37 °C and diluted into the cell culture media at the indicated concentrations. MTT reduction was normalized to cells neither treated with αS aggregates nor treated with EGCG. Values represent means ± SD (n = 3); * P < 0.01, ** P < 0.001.
In additional experiments we quantified the amount of SDS-stable αS protein aggregates in mammalian cells using a FRA (2). As shown in Fig. 3A, SDS-resistant αS aggregates were detected in compound-treated but not in untreated cells, supporting the in vitro results that EGCG converts SDS-labile αS structures into SDS-stable aggregates (Fig. 2C). Thus, our data indicate that the total amount of relatively unstable NP-40 resistant αS aggregates is decreased in EGCG-treated mammalian cells, while the relative amount of SDS-stable aggregates increases upon compound treatment.
Analysis of cell extracts by SDS-PAGE and immunoblotting confirmed the loss of SDS-labile aggregates. Incubation of cells with EGCG for 2 and 3 d significantly reduced the total amount of SDS-unstable αS aggregates, which dissociate to monomers in SDS gels (Fig. S7 A and B). We suggest that EGCG-induced remodeling increases the accessibility of αS aggregates to cellular clearance mechanisms such as autophagy.
A standardized lactate dehydrogenase (LDH) release assay (20) was utilized to investigate the toxicity of amyloid aggregates in mammalian cells. HEK-293 wt αS cells were transfected with preformed αS fibrils and after 1–3 d, LDH release was quantified (Fig. 3B). We observed that 3 d posttransfection, LDH activity was approximately 2.5-fold increased compared to nontransfected cells, indicating that uptake of amyloid structures is toxic for mammalian cells and over time significantly decreases membrane integrity (20). However, this effect was not detected when cells were treated with 20 μM EGCG, indicating that compound-mediated remodeling of αS amyloid aggregates correlates with reduced cellular toxicity. A similar effect was observed when cells not overexpressing αS were treated with both amyloid aggregates and EGCG (Fig. S7C).
Detoxification of αS aggregates by EGCG was confirmed using a pheochromocytoma (PC12) cell model and a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (6). Equal amounts of EGCG-treated and untreated αS fibrils were added to PC12 cells; their metabolic activity was measured after an incubation period of 24 h at 37 °C. We observed that untreated αS fibrils caused a significant inhibition of MTT reduction (approximately 40%, P < 0.001), while this effect was significantly diminished when fibrils were treated with EGCG (Fig. 3C). Thus, EGCG-mediated remodeling of β-sheet-rich amyloid structures reduces their cellular toxicity.
Our studies indicate that EGCG recognizes preformed αS amyloid fibrils and remodels their structure leading to the appearance of smaller amorphous protein aggregates with reduced cellular toxicity. This suggests that EGCG might also influence the conformation of other amyloid structures such as Aβ or tau, which are implicated in Alzheimer’s disease (AD) pathogenesis (21). For this reason, we examined the effect of EGCG and related compounds on preformed Aβ 1-42 (Aβ42) fibrils and oligomers using cell-free and cell-based assays.
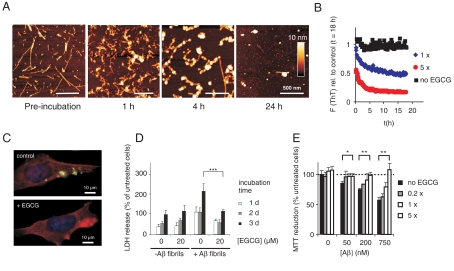
First, we produced insoluble amyloid aggregates by incubating Aβ42 peptide (15 μM) for 4 d at 37 °C. These structures were then analyzed by AFM. We observed that Aβ42 aggregates produced in vitro have a typical fibrillar morphology, confirming previous investigations with synthetic peptides (22). We then added an equimolar concentration of EGCG to the amyloid fibrils and investigated their morphology by AFM in a time-dependent manner. We found that compound treatment changes the shape of preformed Aβ42 amyloid fibrils, indicating that EGCG not only targets and remodels αS aggregates but also alters the structure of other amyloidogenic protein aggregates. Morphological changes were observed already 1–4 h after addition of EGCG to fibrils. Besides large aggregates with typical fibrillar morphology smaller oligomeric structures and amorphous protein aggregates were readily detected in EGCG-treated samples (Fig. 4A).
Fig. 4.
EGCG remodels Aβ42 fibrils and oligomers into benign SDS-resistant structures. (A) Fibrillar Aβ42 (15 μM) was incubated with EGCG (15 μM) for up to 24 h in PBS at 37 °C. EGCG-induced remodeling of Aβ42 fibrils into spherical assemblies was monitored by atomic force microscopy. (B) Fibrillar Aβ42 (equivalent 15 μM monomer) was incubated in the absence (Black) or presence of EGCG (15 μM, Blue; 75 μM Red) in PBS at 37 °C. Loss of ThT fluorescence was monitored at time intervals of 10 min, preceded by 5 s shaking each. (C) Immunofluorescence microscopy shows loss of intracellular Aβ aggregates (Green) in 7PA2 cells after ECGG (10 μM) treatment for 3 d. (phalloidin, Red; DAPI, Blue). (D) LDH release in 7PA2 cells after introduction of Aβ42 aggregates. The assay was performed as described in Fig. 3B. Values represent means ± SD, n = 8; *** P < 0.0005. (E) Aβ42 aggregates (15 μM) were incubated with the indicated amounts of EGCG for 24 h at 37 °C. Aggregates were then were diluted into the cell culture media at the indicated concentrations and added to PC12 cells. Metabolic activity was monitored after 3 d by MTT reduction. Values represent means ± SD (n = 3) normalized to cells neither treated with Aβ nor with EGCG; * P < 0.01, ** P < 0.001.
The effect of EGCG on preformed Aβ42 fibrils was confirmed by ThT and FRA assays (Fig. 4B and Fig. S8C). A significant reduction of ThT fluorescence was observed after an incubation period of 1–5 h, supporting the AFM results that EGCG-mediated remodeling is detectable after a short time. EGCG treatment also strongly increased the SDS stability of preformed Aβ42 aggregates, supporting the results obtained for αS that EGCG remodels amyloid fibrils in vitro.
We also assessed whether EGCG-related polyphenols such as C, EC, EGC, ECG, and GCG (structures in Fig. S8A) are able to remodel preformed Aβ42 fibrils. Aggregates were incubated with different concentrations of chemical compounds and samples were analyzed by ThT and FRA assays. Both revealed that the compounds ECG, GCG, and EGCG, which all contain a gallate group, are potent aggregate modulators, while the compounds C, EC, and EGC are not (Fig. S8 B and C). This indicates that the gallate moiety is critical for efficient amyloid remodeling.
To assess whether EGCG is active in vivo, preformed Aβ42 fibrils were introduced in APP overexpressing CHO cells (7PA2) (23) using the same procedure that was used for αS (19). Cells were then incubated for 3 d in the presence and absence of EGCG and disappearance of aggregates was investigated by indirect immunofluorescence microscopy (Fig. 4C and Fig. S9). We found that inclusion bodies with insoluble Aβ42 aggregates disappeared faster in EGCG-treated than in untreated cells, supporting the observations with αS aggregates in HEK-293 wt αS cells (Fig. 3A).
Analysis of EGCG-treated and untreated cells with FRA assays also confirmed these results (Fig. S10A). EGCG treatment reduced the total amount of NP-40 resistant Aβ42 aggregates in 7PA2 cells in a time-dependent manner, while the relative amount of SDS-stable Aβ42 aggregates increased after compound addition. No effect of EGCG on APP expression levels was observed under these experimental conditions (Fig. S10C). This result is similar to the one obtained with αS aggregates in the HEK-293 wt αS cell model (Fig. 3A), supporting the hypothesis that direct remodeling of aggregates is critical for the in vivo effect of the chemical compound. A similar effect of EGCG was observed when Aβ42 aggregates were introduced in CHO cells that do not overexpress APP (Figs. S10 B and D).
The LDH release assay was utilized to examine the toxicity of Aβ42 aggregates in 7PA2 cells. Similar to the HEK-293 wt αS cell model, 7PA2 cells containing insoluble Aβ42 aggregates showed increased LDH activity after an incubation period of 3 d (Fig. 4D). However, this activity was reduced significantly in EGCG-treated cells. A beneficial effect was also observed when EGCG-treated and untreated Aβ42 aggregates were added to PC12 cells and toxicity was analyzed using an MTT assay (Fig. 4E). Thus, EGCG-mediated remodeling of Aβ42 fibrils reduces their toxicity in cell-based assays.
Recent studies indicate that small Aβ42 aggregates such as oligomers or protofibrils rather than long mature amyloid fibrils cause memory dysfunction in AD (24). We therefore investigated the effect of EGCG on preformed Aβ42 oligomers using cell-free and cell-based assays (Fig. S11). First, a relatively homogenous population of Aβ42 oligomers was prepared using a standardized protocol (25). These structures were then incubated in the presence and absence of EGCG for 24 h at 37 °C and the resulting aggregation products were analyzed by AFM. Untreated Aβ42 oligomers efficiently convert into protofibrillar aggregates, while such an effect was not detected in the presence of EGCG (Fig. S11A). This indicates that EGCG directly targets amyloid oligomers and influences their propensity to assemble into larger fibrillar structures.
To examine whether EGCG indeed remodels the structure of preformed Aβ42 oligomers, compound-treated and untreated samples were analyzed by FRA. We found that EGCG treatment considerably increases the amount of SDS-stable structures, indicating that the compound not only remodels amyloid fibrils but also prefibrillar assemblies such as oligomers (Fig. S11B).
Finally, we assessed whether EGCG treatment influences the toxicity of Aβ42 oligomers. Preformed oligomers (15 μM) incubated for 1 h with an equimolar concentration of EGCG (15 μM) were added to PC12 cells. Toxicity was quantified after 3 d using a standardized MTT assay. We observed that compound treatment significantly reduced the toxicity of preformed Aβ42 oligomers (Fig. S11C), supporting our thesis that EGCG-mediated remodeling of amyloid aggregates is beneficial for mammalian cells.
Together, our data indicate that EGCG binds to preformed amyloid fibrils and oligomers and directly alters their morphology in vitro and in vivo. We have obtained experimental evidence that EGCG binding precedes the structural rearrangement of amyloid fibrils and does not stimulate the release of soluble monomers or oligomers. We suggest that a compound-mediated reorientation of bonds between ordered protein molecules in the polymer might be responsible for amyloid remodeling and the appearance of unordered, amorphous protein aggregates.
It seems reasonable to speculate that the compound causes amyloid remodeling by altering the existing equilibrium between insoluble amyloid aggregates and soluble monomers (15). Our fluorescence correlation (Fig. 2B) and monomer quantification data can be interpreted as supporting this view. They suggest that in the absence of EGCG small amounts of soluble αS monomers dissociate from preformed amyloid fibrils in solution, whereas EGCG treatment inhibits this dissociation of protein molecules (Fig. S5A). An immediate recruitment of EGCG-bound dissociating monomers might lead to the accumulation of amorphous protein aggregates that initially are connected to amyloid fibrils. In our cell-free Aβ42 assays we observed spherical amorphous protein aggregates that are attached to amyloid fibrils after short incubation periods with EGCG (Fig 4A), supporting this idea. Accordingly, one would also expect that breakage of preformed fibrils by agitation or sonication should accelerate EGCG-mediated amyloid remodeling. We indeed observed that increased agitation of samples with preformed aggregates and EGCG stimulates the remodeling process (Fig. 1C and Fig. S2A). Thus, we suggest that EGCG binds to amyloid fibrils and either remodels protein–protein interactions in the solid phase or leads to immediate reaggregation of monomers at the fibril ends, in either case inhibiting the exchanges between protein monomers in the fibril and in solution.
The removal of toxic amyloid deposits is a central therapeutic aim in protein misfolding diseases (9, 26). EGCG was shown to exert antiamyloid activity by influencing cellular signal transduction pathways and reactive oxygen species (27). However, EGCG also directly binds to hydrophobic protein sequences both by hydrophobic interactions and by hydrogen bonding (28) and has been demonstrated to directly inhibit fibril formation of a wide range of amyloidogenic proteins (2–6). In the past, various small molecules have been shown to inhibit or reverse amyloid formation in vitro (9, 26). However, disassembly of large fibrils was suggested to increase the population of oligomeric amyloid species (24), and to exacerbate amyloid toxicity. Here, we demonstrate a different mechanism. EGCG treatment of amyloid fibrils does not reverse the amyloid formation process but directly converts fibrillar species into benign protein aggregates. EGCG or related compounds that decrease the deposition of amyloid protein aggregates (29) without increasing the load of toxic intermediates may thus be considered to be model substances for potential therapeutics for systemic and neurodegenerative amyloid diseases.
Methods
Remodeling kinetics of αS fibrils (equivalent 50 μM monomer) were recorded in low-binding 96-well plates (#3651, Corning). EGCG (50 μM) was added to the protein solutions and triplicates of 100 μL were incubated at 37 °C under constant agitation (300 rpm) in Tris buffered saline (TBS, 150 mM NaCl, 20 mM Tris-HCl pH 7.2) for each time point. Samples were removed at the indicated time points (0.2, 1, 4, 24, and 72 h) and frozen in liquid nitrogen. After thawing, aliquots of 10 μL were removed for EM, AFM, and seeding assays. Protein samples were sonicated for 1 h and diluted 1∶4 in TBS to record CD spectra. Thioflavin (20 μM final concentration) was added and fluorescence (440 nm excitation, 485 nm emission, top read) was measured using a fluorescence plate reader (InfinitE M200, Tecan, Austria). In a reference experiment fibrils were incubated with EGCG under the same conditions in TBS (20 μM ThT) under intermittent agitation (5 s every 10 min). Aβ42 (15 μM) was incubated with EGCG (15 μM) in PBS under constant agitation at 37 °C and aliquots (10 μL) were removed after 1, 4, and 24 h and imaged by AFM. ThT remodeling kinetics were recorded at 37 °C in PBS (20 μM ThT) at 10 min intervals preceded by 5 s shaking using a fluorescence plate reader as above.
Additional methods are described in SI Text: αS preparation, in vitro aggregation and seeding experiments, CD spectroscopy, EM, AFM and fluorescence microscopy, filter retention, NBT binding assay, and monomer quantification, as well as generation of stable cell lines, cell culture, internalization of amyloid fibrils, LDH cell viability assay, MTT metabolic assay, and Western blotting.
Supplementary Material
Acknowledgements.
We thank G. Grelle and S. Kostka for technical assistance; S. Engelender for providing His-αS cDNA; A. Otto for MS measurements; R. Lurz (Max Planck Institute for Molecular Genetics) for recording EM images; C. Hänig for computer support; the department of M. Bienert, Leibnitz Institute for Molecular Pharmacology for the use of their CD spectrometer; and the lab of P. Selenko, Leibnitz Institute for Molecular Pharmacology for their help in expressing untagged αS. S. Schnögl and K. Hartmann critically read the manuscript and provided editorial support. AD and wt mouse brains were provided by B. Tachu, and 7PA2 cells were a gift of D. Walsh (University College Dublin). The project was funded by the Nationales Genomforschungsnetz Plus [01GS08170 NeuroNet (K.N. and E.E.W.) and 01GS08132 Alzheimer-IG (J.B., R.F., and E.E.W.)], the Initiative and Networking Fund of the Helmholtz Association [Helmholtz Alliance for Mental Health in an Aging Society (E.E.W.)], Förderinitiative Go-Bio (E.E.W), and the Deutsche Forschungsgemeinschaft [WA 1151/5-4 (E.E.W.), SFB 740 (E.E.W.), and SFB 618 (J.R. and E.E.W.)].
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910723107/DCSupplemental.
References
- 1.Zhu M, et al. The flavonoid baicalein inhibits fibrillation of alpha-synuclein and disaggregates existing fibrils. J Biol Chem. 2004;279(26):26846–26857. doi: 10.1074/jbc.M403129200. [DOI] [PubMed] [Google Scholar]
- 2.Ehrnhoefer DE, et al. Green tea (-)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington’s disease models. Hum Mol Genet. 2006;15(18):2743–2751. doi: 10.1093/hmg/ddl210. [DOI] [PubMed] [Google Scholar]
- 3.Rambold AS, et al. Green tea extracts interfere with the stress-protective activity of PrP and the formation of PrP. J Neurochem. 2008;107(1):218–229. doi: 10.1111/j.1471-4159.2008.05611.x. [DOI] [PubMed] [Google Scholar]
- 4.Hauber I, Hohenberg H, Holstermann B, Hunstein W, Hauber J. The main green tea polyphenol epigallocatechin-3-gallate counteracts semen-mediated enhancement of HIV infection. Proc Natl Acad Sci USA. 2009;106(22):9033–9038. doi: 10.1073/pnas.0811827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts BE, et al. A synergistic small-molecule combination directly eradicates diverse prion strain structures. Nat Chem Biol. 2009;5(12):936–946. doi: 10.1038/nchembio.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrnhoefer DE, et al. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat Struct Mol Biol. 2008;15(6):558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- 7.Meng X, Munishkina LA, Fink AL, Uversky VN. Molecular mechanisms underlying the flavonoid-induced inhibition of alpha-synuclein fibrillation. Biochemistry. 2009;48(34):8206–8224. doi: 10.1021/bi900506b. [DOI] [PubMed] [Google Scholar]
- 8.Yang F, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280(7):5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 9.Soto C, et al. Beta-sheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis: implications for Alzheimer’s therapy. Nat Med. 1998;4(7):822–826. doi: 10.1038/nm0798-822. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Uversky VN, Fink AL. Effect of familial Parkinson’s disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human alpha-synuclein. Biochemistry. 2001;40(38):11604–11613. doi: 10.1021/bi010616g. [DOI] [PubMed] [Google Scholar]
- 11.Antony T, et al. Cellular polyamines promote the aggregation of alpha-synuclein. J Biol Chem. 2003;278(5):3235–3240. doi: 10.1074/jbc.M208249200. [DOI] [PubMed] [Google Scholar]
- 12.Chiba T, et al. Amyloid fibril formation in the context of full-length protein: effects of proline mutations on the amyloid fibril formation of beta2-microglobulin. J Biol Chem. 2003;278(47):47016–47024. doi: 10.1074/jbc.M304473200. [DOI] [PubMed] [Google Scholar]
- 13.Castiglioni E, et al. Absorption flattening as one cause of distortion of circular dichroism spectra of Delta-RuPhen3. H2TPPS complex. Chirality. 2007;19(8):642–646. doi: 10.1002/chir.20436. [DOI] [PubMed] [Google Scholar]
- 14.LeVine H., 3rd Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 1999;309:274–284. doi: 10.1016/s0076-6879(99)09020-5. [DOI] [PubMed] [Google Scholar]
- 15.Jarrett JT, Lansbury PT., Jr. Seeding “one-dimensional crystallization” of amyloid: A pathogenic mechanism in Alzheimer’s disease and scrapie? Cell. 1993;73(6):1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 16.Paz MA, Fluckiger R, Boak A, Kagan HM, Gallop PM. Specific detection of quinoproteins by redox-cycling staining. J Biol Chem. 1991;266(2):689–692. [PubMed] [Google Scholar]
- 17.Collins SR, Douglass A, Vale RD, Weissman JS. Mechanism of prion propagation: Amyloid growth occurs by monomer addition. PLoS Biol. 2004;2(10):e321. doi: 10.1371/journal.pbio.0020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wanker EE, et al. Membrane filter assay for detection of amyloid-like polyglutamine-containing protein aggregates. Methods Enzymol. 1999;309:375–386. doi: 10.1016/s0076-6879(99)09026-6. [DOI] [PubMed] [Google Scholar]
- 19.Yang W, Dunlap JR, Andrews RB, Wetzel R. Aggregated polyglutamine peptides delivered to nuclei are toxic to mammalian cells. Hum Mol Genet. 2002;11(23):2905–2917. doi: 10.1093/hmg/11.23.2905. [DOI] [PubMed] [Google Scholar]
- 20.Abe K, Kimura H. Amyloid beta toxicity consists of a Ca(2 + )-independent early phase and a Ca(2 + )-dependent late phase. J Neurochem. 1996;67(5):2074–2078. [PubMed] [Google Scholar]
- 21.Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314(5800):777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt M, et al. Comparison of Alzheimer Abeta(1-40) and Abeta(1-42) amyloid fibrils reveals similar protofilament structures. Proc Natl Acad Sci USA. 2009;106(47):19813–19818. doi: 10.1073/pnas.0905007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh DM, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 24.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 25.Chafekar SM, Baas F, Scheper W. Oligomer-specific Abeta toxicity in cell models is mediated by selective uptake. Biochim Biophys Acta. 2008;1782(9):523–531. doi: 10.1016/j.bbadis.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Cohen FE, Kelly JW. Therapeutic approaches to protein-misfolding diseases. Nature. 2003;426(6968):905–909. doi: 10.1038/nature02265. [DOI] [PubMed] [Google Scholar]
- 27.Mandel SA, Amit T, Kalfon L, Reznichenko L, Youdim MB. Targeting multiple neurodegenerative diseases etiologies with multimodal-acting green tea catechins. J Nutr. 2008;138(8):1578S–1583S. doi: 10.1093/jn/138.8.1578S. [DOI] [PubMed] [Google Scholar]
- 28.Maiti TK, Ghosh KS, Dasgupta S. Interaction of (-)-epigallocatechin-3-gallate with human serum albumin: fluorescence, fourier transform infrared, circular dichroism, and docking studies. Proteins. 2006;64(2):355–362. doi: 10.1002/prot.20995. [DOI] [PubMed] [Google Scholar]
- 29.Hunstein W. Epigallocathechin-3-gallate in AL amyloidosis: A new therapeutic option? Blood. 2007;110(6):2216. doi: 10.1182/blood-2007-05-089243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.