Abstract
We investigated connections between the physiology of rat barrel cortex neurons and the sensation of vibration in humans. One set of experiments measured neuronal responses in anesthetized rats to trains of whisker deflections, each train characterized either by constant amplitude across all deflections or by variable amplitude (“amplitude noise”). Firing rate and firing synchrony were, on average, boosted by the presence of noise. However, neurons were not uniform in their responses to noise. Barrel cortex neurons have been categorized as regular-spiking units (putative excitatory neurons) and fast-spiking units (putative inhibitory neurons). Among regular-spiking units, amplitude noise caused a higher firing rate and increased cross-neuron synchrony. Among fast-spiking units, noise had the opposite effect: It led to a lower firing rate and decreased cross-neuron synchrony. This finding suggests that amplitude noise affects the interaction between inhibitory and excitatory neurons. From these physiological effects, we expected that noise would lead to an increase in the perceived intensity of a vibration. We tested this notion using psychophysical measurements in humans. As predicted, subjects overestimated the intensity of noisy vibrations. Thus the physiological mechanisms present in barrel cortex also appear to be at work in the human tactile system, where they affect vibration perception.
Keywords: psychophysics, somatosensory cortex, vibration, whisker, finger
An effective approach to uncover how neuronal activity leads to sensation is to vary stimulation parameters while correlating changes in firing with changes in the percept (1 –5). In the present work, we measured changes in neuronal activity in rat somatosensory (“barrel”) cortex induced by variation in vibration parameters and correlated these changes with perceptual reports from human subjects. The stimuli in the two cases were trains of whisker and skin vibration, respectively.
Isolated neurons respond more robustly to unpredictable (noisy) patterns of electrical stimulation than to repeating, periodic patterns (6) or constant inputs (7). Synaptic transmission also can be enhanced by noise (8). In a recent study of the effects of stimulus noise in rat barrel cortex, we found that whisker vibration trains containing temporal noise (irregularity in the sequence of interdeflection intervals) evoked a larger and more synchronous cortical response than did periodic vibration trains (9). In the present work, to verify that response amplification by noise is a general property of the sensory system, we explored another stimulus feature: We compared cortical responses to trains that had constant amplitude vs. trains that had varying amplitude. We then investigated the perceptual impact of noise using psychophysical experiments in humans. We predicted that noise, by enhancing neuronal firing rates and/or firing synchrony in somatosensory cortex, would produce a systematic bias in the perceived intensity of noisy vibrations compared with noise-free vibrations.
Results
Neuronal Responses in Barrel Cortex.
Stimulus trains consisted of 20 deflections (Fig. 1), with equal interdeflection intervals (IDIs). The noisy train included single deflections with amplitudes 50, 75, 100, 125, and 150% as large as the mean amplitude of the matched constant-amplitude train.
Fig. 1.
Stimuli in the electrophysiology and psychophysics experiments. (A) Upper trace: noisy-amplitude train consisting of 20 deflections with varying amplitudes. Lower trace: frequency-matched constant-amplitude train. A 7-s pause was inserted between trains. The inset shows the Gaussian shape of individual deflections. (B) Scheme of rat whisker stimulation and electrophysiological recording. Whiskers are shown full length but were cut after insertion into ladder.
Frequency Dependence and Amplitude Dependence of Neuronal Response and the Effect of Noise.
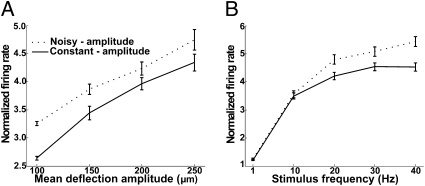
The magnitude of the barrel cortex response, averaged across neurons, was enhanced by the presence of amplitude noise. The frequency dependence and amplitude dependence of barrel cortex responses to constant and noisy stimuli is shown in Fig. 2. At each recording site, the whole-stimulus firing rate was calculated as the multiunit cluster's spike count (number of spikes between t0 and t20+50 ms, where t0 and t20 are the peak times of the first and 20th deflections, respectively) divided by elapsed time; the rate then was normalized to 1 for constant-amplitude stimuli at 1 Hz, 100 μm. Firing rates were averaged across 170 recording sites in nine rats. Fig. 2A plots responses as a function of mean deflection amplitude, with different frequencies pooled. The whole-stimulus firing rate increased as deflection amplitude increased; noise enhanced the response magnitude for all stimulus amplitudes. Fig. 2B plots the same data as a function of frequency, pooling different mean deflection amplitudes. Two additional findings emerge. First, the whole-stimulus firing rate increased as the train frequency increased. Second, the effect of noise depended on train frequency: Responses to constant-amplitude and noisy trains were equivalent at 1 and 10 Hz (paired t test, P > 0.38), but responses were significantly greater for noisy trains at 20, 30, and 40 Hz (P < 0.002 at each frequency).
Fig. 2.
Response magnitude of barrel cortex neurons to constant- and noisy-amplitude trains. In both A and B, firing rates were normalized to 1 for constant-amplitude stimuli at 1 Hz, 100 μm. (A) Whole-train firing rates for constant- and noisy-amplitude stimuli, measured from train onset until 50 ms after final deflection, with the data associated with different deflection frequencies pooled. (B) Whole-train firing rates for constant- and noisy-amplitude trains, measured from train onset until 50 ms after final deflection, with the data associated with different deflection amplitudes pooled. Error bars in A and B indicate SEM across electrodes.
Fig. 3.
Response to individual deflections within constant- and noisy-amplitude trains. Neuronal response was taken as the normalized number of spikes within 20 ms after each whisker deflection for 20-, 30-, and 40-Hz stimuli. The upward shift means that the response of neurons to a given deflection amplitude was larger when the deflection was presented in the context of a noisy stimulus train. Responses were averaged across all neuronal clusters. Error bars indicate SEM across electrodes.
The phasic neuronal responses (spike count in a 20-ms window after each whisker deflection) also showed noise-induced enhancement (Fig. S1). Furthermore, the cross-correlograms among neuronal clusters reveal more synchronous firing within the population in the presence of noise (Fig. S2).
Effect of Noise on Neuronal Response to Individual Deflections.
Nonlinearity in the neuronal response as a function of deflection amplitude could contribute to the increased response to noisy trains. It would occur if the increase in response to deflections larger than the mean was of greater absolute magnitude than the decrease in response to deflections smaller than the mean (9). To determine whether this nonlinearity caused the heightened response to noisy trains, we compared the neuronal activity for deflections of a given amplitude when such deflections were embedded in noisy versus constant-amplitude trains (Fig. 3). In constant-amplitude trains, we obtained each point by computing the mean response across stimulus trains with one of the tested amplitudes (100, 150, 200, or 250 μm). In the noisy trains, we calculated responses by grouping together the deflections with amplitude matching each of the constant conditions. This analysis excluded data taken at 1 and 10 Hz, where the effect of noise was insignificant. Response was measured as the number of spikes within 20 ms after each whisker deflection, normalized to 1 for the 1-Hz, 100-μm constant-amplitude condition. We observed that the entire response curve shifted upward for noisy trains (Fig. 3). Thus, the response of barrel neurons to whisker deflections of a given amplitude was larger when those deflections were presented in a noisy context. The heightened response was triggered specifically by whisker motion and did not persist in the interval between whisker deflections (Fig. S3).
Opposite Behavior of Excitatory and Inhibitory Neurons.
One effect of noise might be to alter the balance between excitatory and inhibitory networks within cortex (10), an alteration previously detected in response to temporal noise (9). Neurons in barrel cortex can be categorized as regular-spiking units (RSUs) and fast-spiking units (FSUs), putative excitatory neurons and putative inhibitory neurons, respectively (11). Given that fewer than 15% of neurons in barrel cortex are GABAergic (12), the results presented until now reflect mainly the activity of excitatory neurons. To test whether amplitude noise acts differently on distinct classes of neurons, we isolated 21 FSUs and 76 RSUs. The criterion for classification was spike duration shorter than 0.65 ms for FSUs and longer than 0.75 ms for RSUs, consistent with previous studies (11, 13).
To quantify the consequences of noise, we used a firing-rate index IFR:
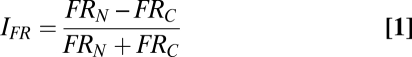 |
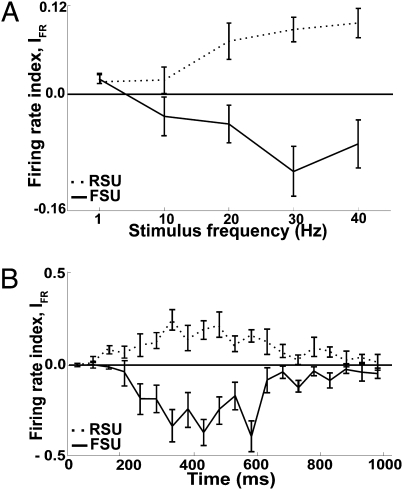
in which, for each recorded single unit, FRN and FRC were the spike counts per unit time (number of spikes occurring between t0 and t20+50 ms, divided by elapsed time); rate then was normalized to 1 for constant-amplitude trains at 1 Hz, 100 μm. Values of IFR > 0 indicate a greater response to noisy trains than to constant trains, whereas values < 0 indicate a greater response to constant trains. The average value of the index across all FSUs and RSUs, as vibration frequency increased, is given in Fig. 4A. At 1 and 10 Hz, the average indices for FSUs and RSUs were close to 0. At 20 Hz, the FSU and RSU indices both deviated from 0, but in opposite directions: RSUs showed larger responses during noisy trains (P = 0.001), and FSUs showed larger responses during constant trains (P = 0.02). At 30 and 40 Hz, the indices were further distanced from 0, still in a symmetrically opposed manner.
Fig. 4.
Distinct effects of amplitude noise on putative inhibitory and excitatory neurons. (A) Average firing rate index, IFR, for the whole stimulus train. As stimulus frequency increased, FSUs showed an increasingly negative value of the index, whereas RSUs showed an increasingly positive value. (B) Average firing rate index, IFR, along the time course (950 ms) of a single 20-Hz stimulus train. Note the opposite behavior of the two types of neuron at a fine time scale. Error bars in A and B indicate SEM across neurons.
Symmetrically opposed behavior of RSUs and FSUs also was found across the course of single trains of vibrations. Fig. 4B shows the firing rate index in 50-ms bins for 20-Hz trains with a mean amplitude of 100 μm. For both RSUs and FSUs, the index deviated from 0 (but in opposite directions) about 200 ms after onset. Maximum deviation from 0 occurred at the same time, 200–600 ms after onset. The index returned to 0 at precisely the same time, as the train concluded. Noise also affected response synchrony between FSUs and between RSUs in opposite ways (Fig. S4).
The opposite effects of noise in putative excitatory and inhibitory neurons support the hypothesis that noise altered the balance between the two networks. The causal relationship could be as follows: The reduced response of inhibitory neurons to noisy inputs might release the remaining cortical population from suppression. More specifically, at frequencies equal to or above 20 Hz, the inhibitory network tended to fire less, and in a less synchronous manner, during noisy trains. This altered firing by the inhibitory network was accompanied by (and perhaps caused) an increased response magnitude and heightened coherence within the excitatory network.
Human Psychophysics.
Experiment 1: Thresholds for noise detection.
After observing the effect of temporal noise (9) and amplitude noise on neuronal responses (as discussed in the previous sections), we explored the perceptual impact of noise. Experiment 1 tested the ability of human participants (Fig. 5A) to detect different levels of amplitude noise. (SI Results and Fig. S5 describe analogous experiments on the temporal noise.) For trains of 500-ms duration, subjects could detect 50% amplitude noise significantly better than chance (P = 0.046) but showed less sensitivity in detecting 25% noise (P = 0.073) and performed at chance for lower levels of noise (P ≥ 0.25). The results of experiment 1 were used to select levels of noise to be applied in experiments 2 and 3. We believe it was important that the noise be subliminal in experiments 2 and 3 so that subjects’ decisions about the intensity of the vibrations were not confounded by their explicit perception of the vibrations’ regularity. Therefore we chose 10% amplitude noise, below the detection threshold.
Fig. 5.
Human subject performance in the intensity discrimination task. (A) (Upper) Stimulus presentation for human psychophysics. Subjects received stimulus pairs in which one train was noise-free and the other train contained amplitude noise. In the noise-detection experiment (experiment 1), the quantity of noise was 5%, 10%, 15%, 20%, 25%, and 50%. In experiments 2 and 3, the noisy train contained 10% amplitude noise. All stimuli had a mean frequency of 40 Hz. (Lower) Apparatus for human psychophysics. (B) The plot shows the percent of trials in which subjects reported the first vibration train as having higher intensity. Whether the two trains were of the same or different amplitudes, the presence of 10% amplitude noise in either the first or second train increased the likelihood of that train being judged as more intense. A1 and A2 are the amplitudes of the first and second trains of the stimulus pair, respectively. Error bars indicate SEM across subjects. (C) Although short noisy trains (0.5 s) induced an overestimation of the perceived intensity (consistent with experiment 2, as shown in B), long trains (1.5 s) did not show such an effect.
Experiment 2: Effect of noise on perceived intensity.
Fig. 5B illustrates the effect of 10% amplitude noise on subjects’ judgment regarding intensity. Train duration was 500 ms. When the first train contained amplitude noise, subjects were significantly more likely to judge that train as having the higher intensity than when the second train contained amplitude noise. Pooling all data from different amplitude differences, the effect of 10% amplitude noise was significant across subjects (P = 0.02). Similarly, subjects reported trains containing temporal noise as having higher intensity than regular trains (Fig. S5).
Experiment 3: Effect of train duration on noise effects.
In barrel cortex, the effect of noise disappeared by the end of the 1-s trial (Fig. 4B), leading to the prediction, tested in experiment 3, that human subjects would not perceive noisy trains as having higher intensity if duration of the vibration increased. Fig. 5C shows that when vibration trains were of 0.5-s duration, subjects perceived the noisy train as having higher intensity (replicating the result of experiment 2), but when vibration trains were extended to 1.5 s duration, subjects no longer perceived the noisy train as more intense.
Discussion
Physiological Effects of Noise.
The present results confirm the observation that neuronal firing rate measured over the whole stimulus train increases as either stimulus frequency or amplitude increases (9, 14 –21). The major physiological result is that, among RSUs, the magnitude and synchrony of responses increases when the amplitude within the train is irregular, provided that the frequency is above 10 Hz. The effects of amplitude noise are remarkably similar to earlier findings concerning temporal noise (9). Those experiments revealed that, at frequencies above 10 Hz, stimulus trains with an irregular sequence of IDIs evoked a larger and more synchronous barrel cortex response among RSUs than did frequency-matched periodic trains (9). Thus, the excitatory network of barrel cortex exhibits larger responses and enhanced response synchrony when stimuli contain unpredicted variations in deflection features, whether the noise is in the temporal or amplitude domain.
The term “amplitude” varies according to stimulus design. In our experiments the duration of the pulse was fixed, so velocity and speed covary with amplitude: Amplitude noise also may be considered noise in velocity (or its absolute value, speed). Because of the robust encoding of stimulus velocity (15, 16) and the weaker encoding of pure amplitude (22), our view is that velocity (speed) is the stimulus feature through which noise exerts its effects.
The augmented magnitude and temporal precision in responses to noisy stimuli may reflect the tuning of the sensory system to the most commonly encountered stimulus statistics. Rats generate exploratory whisker motion (“whisking”) in the range of 5–15 Hz (23). Because noise has an effect only when the “carrier” frequency exceeds 10 Hz (Fig. 2B), the sensory system may record whisking motion equally well, no matter whether it is periodic or irregular 24). On the other hand, during object contact, whisker motion can be characterized as an irregular vibration containing frequencies up to a few hundred Hz (25 –32). The “noisiness” of such kinetic profiles would evoke a particularly robust and temporally precise cortical response. In addition, the fidelity of membrane potential variations to unpredictable whisker contacts in an awake mouse (33) might be comparable with the reliable spiking in response to the noisy stimulation in the present study. Enhanced response to unpredictable stimuli appears to generalize not only to different features within the vibrissal stimuli but also to other sensory modalities, as reviewed in ref. 9.
Mechanisms of Noise Sensitivity.
The interaction between inhibitory and excitatory networks seems to underlie noise effects. The preference of FSUs for constant-amplitude trains and the preference of RSUs for noisy-amplitude trains emerged at the same frequency (20 Hz) and at the same moment in the sequence of deflections (∼200 ms) (Fig. 4). RSUs were more synchronized to each other during noisy trains, whereas FSUs were more synchronized during constant-amplitude trains (Fig. S4). In summary, the behavior of the two neuron types was opposite in every way measured. We have argued previously (9) that periodic inputs might resonate with intrinsic oscillations of the membrane potential of inhibitory neurons (34, 35) more effectively than noisy inputs. Periodic inputs might drive the inhibitory network more, which would have the net effect of suppressing the excitatory network. An analogous argument can be made in the case of amplitude noise. Suppose that neurons in barrel cortex have sigmoidal input–output functions, where input is the strength of the sensory event (amplitude or speed of whisker movement), and output is spiking probability (figure 2b in ref. 16). In a noisy-amplitude train, some deflections would fall above and others below the threshold; the neurons would effectively “skip” some deflections, and the intrinsic resonance of inhibitory neurons would fail to become entrained. In contrast, a constant level of input above threshold would cause inhibitory neurons to spike for each deflection, allowing them to entrain over the course of the stimulus. By entraining inhibitory neurons, constant-amplitude trains would have the net effect of suppressing the excitatory network.
The intrinsic properties of regular-spiking neurons also could play a role. They appear to be particularly responsive to noisy membrane potential fluctuations in vitro (6, 7) and in vivo (36). The enhanced responses and temporal precision in the presence of noise could be favored by these intrinsic properties.
Correlated Perceptual and Physiological Effects of Noise.
Because, in primates, the animal's judgment of vibration frequencies is closely correlated with the spike count per trial in primary somatosensory cortex (37), we expected that the addition of noise would increase the perceived intensity of the vibrations presented to human subjects. We also predicted that the noise effects on perceived intensity would disappear with longer train duration, just as the noise effects on neuronal response disappear (Fig. 4B). Both predictions were verified (Fig. 5), allowing us to conclude that the facilitatory effects of noise generalize across species and have direct effects on perception. Noise also increased the synchrony of firing across neurons (Fig. S2), and response synchrony, in addition to response magnitude, might cause subjects to overestimate the intensity of noisy stimuli.
In summary, shifts in stimulus parameters had matching effects on barrel cortex neuronal responses and human vibration judgment. This finding supports previous evidence that temporal noise interferes with discriminations of sinusoidal vibration frequency in human subjects (38). However, the effects of noise could appear, at first glance, to be at odds with some earlier studies in primates. When monkeys were trained to indicate which of two successive vibrations had a higher frequency, the addition of temporal noise to the vibration altered neither the firing rates of primary somatosensory cortical neurons nor the judgments of stimulus intensity (37, 39, 40). To find an explanation for this discrepancy, we decided to examine experiment duration. This analysis shows that the sensitivity of barrel cortex neurons to both amplitude and temporal noise was strong at the outset of a lengthy recording session but vanished by the end of the session (Fig. S6). Thus, the sensory processing network altered its susceptibility to amplitude and temporal noise in a progressive manner during the course of the intensive stimulation regime. From this observation, we speculate that the cortex of monkeys after months of daily psychophysical testing might become stabilized in a noise-insensitive state (37, 39, 40). Subjects in both our current and previous psychophysics experiments (38), having never been exposed to the specific stimulus set, would be expected to show sensitivity to noise.
It is noteworthy that the effects of stimulus noise on perception were accounted for by the noise effects on excitatory neurons in rat cortex; effects of noise on inhibitory neurons were in the opposite direction to human perception. This finding suggests that the two classes of neurons have different contributions to sensory perception (41). We speculate that inhibitory neurons act locally, whereas excitatory neurons, which are more commonly projection cells, convey information to other regions which, one stage at a time, inform the subject's conscious decision about the stimulus (42). Perceptual judgments thus may be made on the basis of the output of the excitatory (projection) neuronal population.
Methods
Rat Electrophysiology.
Subjects, surgery, and data acquisition.
All experiments were in accordance with National Institutes of Health, international, and institutional standards for the care and use of animals in research. A summary of the experimental preparation is given below, and a detailed description is given in refs. 15 and 43. Nine adult (250–350 g) male Wistar rats were used. Anesthesia was induced by i.p. injection of urethane (1.5 g/kg). A 10 × 10 grid of electrodes 1.5 mm long with 400 μm tip-to-tip spacing (Cyberkinetics Inc) was inserted into the whisker region of the left somatosensory cortex (Fig. 1B), identified by vascular landmarks and stereotaxic coordinates (43). The depths of electrode penetrations were 700–1000 μm in all experiments, ensuring that recordings were made from the middle layers, presumably layer 4. Signals from all 100 electrodes were amplified simultaneously and filtered (gain 5,000; bandpass 250–7,500 Hz (Cyberkinetics Inc). A digital signal processor (Cyberkinetics Inc) extracted 1 ms of voltage (0.33 ms before and 0.67 ms after threshold crossing) and saved the waveform at 30,000 samples/s. The waveforms produced by a multiunit neuronal cluster at each channel were selected using spike-sorting programs, and their time stamps were saved for analysis. Earlier work showed that under these conditions each electrode captures the activity of a cluster of about three to five neurons (44). Single units were selected from clusters for Fig. 4 and Figs. S4 and S6 E and F.
Whisker deflections.
A five-rung “ladder” of thin glass micropipettes was constructed and attached to a piezoelectric wafer (Morgan Matroc). With a micromanipulator the ladder was positioned adjacent to the right side of the rat's snout so that the five rungs lay just below the corresponding five rows of whiskers; each whisker shaft rested lightly on the ladder about 5 mm from the skin (Fig. 1B). Whiskers were then cut 1 cm from the base.
Stimulus programming and data analysis were all performed in MATLAB (Mathworks) and LabView (National Instruments). Deflections had a truncated Gaussian form of 12-ms duration (Fig.1A). The signal, generated at 10,000 samples/s, passed through a digital-to-analog converter (National Instruments) and was sent to an amplifier (gain = 40) to drive the piezoelectric wafer. Wafer motion (recorded by placing one rung of the ladder in an optic sensor) reliably followed voltage input. In previous measurements (9), the power spectrum had negligible amplitude above 400 Hz, and thus the stimulus did not contain high-frequency components that induce wafer resonance. Under identical conditions, the displacement of the whisker shaft smoothly “followed” the wafer movement (15).
Trains of whisker deflections with constant amplitude and amplitude noise.
One trial consisted of a train of 20 deflections (Fig. 1A), giving 19 IDIs per trial. A pause of 7 s was inserted between consecutive trials to allow cortical neurons to recover from any effects of the preceding stimulus train (45).
The vibration frequency on each trial assumed a value of 1, 10, 20, 30, or 40 Hz, and mean amplitude assumed a value of 100, 150, 200, or 250 μm. Each train consisted either of deflections with constant amplitude or deflections with varying amplitude (“noisy-amplitude” trials) whose mean value was equal to that of a matching constant-amplitude train. Trains with amplitude noise were created by mixing values that included the mean amplitude but also included values 25% and 50% larger and smaller (Fig. 1A). For example, a noisy-amplitude train matched to a 100-μm constant-amplitude train contained deflection amplitudes of 50, 75, 100, 125, and 150 μm. One noisy train included four deflections of each of the five possible amplitudes. This condition, referred to as “50% noise,” was the only noise condition used in the physiological experiments (human psychophysics experiments used various conditions of noise). Every noisy trial had a pseudorandom sequence of deflection amplitudes, allowing each amplitude to be presented within different contexts. Because amplitude noise scaled with mean amplitude, the absolute amount of noise increased as mean amplitude increased.
The 40 different experimental conditions (five frequencies × four amplitudes × constant vs. noisy-amplitude) were presented in pseudorandom order with 30 trials per condition; a constant (or noisy) train with one frequency and amplitude was followed immediately by a noisy (or constant) train with the same frequency and amplitude.
Receptive field mapping.
Constant-amplitude 100-μm, 1-Hz deflections were applied to single whiskers to determine whether the neuronal cluster recorded at any given electrode had a statistically significant response to whisker movement and, if so, to identify the principal whisker and the response onset latency (44, 46). Clusters with statistically significant responses and a clear principal whisker were selected for analysis of responses to the full stimulus set. Response onset latencies were in the range of 5–9 ms, indicating that recordings were taken from a cortical layer with direct thalamic input, as expected from inspection of the electrode array insertion depth (700–1,000 μm) during the experiment.
Human Psychophysics.
First-year psychology students at the University of Sydney were previously unexposed to the stimulus set and were naïve as to the hypotheses tested. Eight, 14, and 8 subjects participated in experiments 1, 2, and 3 respectively.
Subjects sat in front of an apparatus and rested a hand on a platform with the pad of the right index finger touching a steel rod 5 mm in diameter (Fig. 5A). The rod was driven by a vibrotactile device (Type 4810 MiniShaker; Brüel & Kjaer). The timing and waveform of the vibrations were controlled using MATLAB and a National Instruments board. Each train contained 21 skin deflections, with each deflection having the shape of a truncated Gaussian waveform of 12-ms duration (a deflection shape identical to that used for the rat whiskers). The duration of the train of deflections (from the peak of the first deflection to the peak of the last deflection) always was 500 ms, giving a mean frequency of 40 Hz. The first train was presented 0.5 s after the subject responded to the previous trial, and the interval between trains within a trial was 0.5 s. Each trial contained one regular train (constant in both frequency and amplitude) and one train containing noise (Fig. 5A and Fig. S5A).
These stimulus conditions were used in three experiments, the first to ascertain subjects’ sensitivity in detecting noise, the second to ascertain the effect of noise on perceived stimulus intensity for 0.5-s trains, and the third to compare the effect of noise in 0.5-s versus 1.5-s trains. In experiment 1, at the end of the second train, subjects judged which of the two trains contained noise. Using this two-interval forced-choice paradigm, we measured each subject's performance for stimuli containing 5, 10, 15, 20, 25, and 50% amplitude or temporal noise using 20 trials per noise level. The definition of noise level refers to the largest quantity of jitter in that train, as was the case for the whisker stimuli.
Based on subjects’ thresholds for noise detection, experiment 2 used trains containing 10% amplitude noise (Fig. 5A) or 20% temporal noise (Fig. S5A). Unknown to the subjects, in two-thirds of the trials, the two trains had equal mean frequency and mean amplitude; the presence of amplitude or temporal noise in one train was the only difference between them. In one-third of trials there was a 20% difference in the mean amplitudes. For these trials, the train containing noise was equally likely to have the higher or lower mean amplitude. The order of the noisy and regular vibrations varied randomly. At the end of the second train, subjects judged which of the two trains was “more intense.” The trials that contained a real difference in stimulus intensity (20% difference in mean amplitude) were used as a control to confirm that subjects were carrying out the task correctly as an intensity judgment.
Experiment 3 tested the effect of amplitude noise in identical fashion to experiment 2, except that train duration was set to either 0.5 or 1.5 s.
Supplementary Material
Acknowledgments
We are grateful to the members of the laboratory and to various outside collaborators for valuable discussions. Erik Zorzin provided outstanding technical support. Marco Gigante drew Figs. 1B and 5A. This work was supported by Grant RG0041/2009-C from the Human Frontier Science Program, Contract BIOTACT-21590 from Eurpoean Community, by the Friuli Venezia Giulia region, and from the Italian Institute of Technology through the Brain Machine Interface Project (to M.E.D.), by Australian Research Council Contract DP0663086 (to J.A.H.), and by Australian Research Council Contract DP0987133 and a long-term fellowship from the Human Frontiers Science Program (to E.A.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914750107/DCSupplemental.
References
- 1.Hyvarinen J, Sakata H, Talbot WH, Mountcastle VB. Neuronal coding by cortical cells of the frequency of oscillating peripheral stimuli. Science. 1968;162:1130–1132. doi: 10.1126/science.162.3858.1130. [DOI] [PubMed] [Google Scholar]
- 2.Blake DT, Hsiao SS, Johnson KO. Neural coding mechanisms in tactile pattern recognition: The relative contributions of slowly and rapidly adapting mechanoreceptors to perceived roughness. J Neurosci. 1997;17:7480–7489. doi: 10.1523/JNEUROSCI.17-19-07480.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connor CE, Hsiao SS, Phillips JR, Johnson KO. Tactile roughness: Neural codes that account for psychophysical magnitude estimates. J Neurosci. 1990;10:3823–3836. doi: 10.1523/JNEUROSCI.10-12-03823.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connor CE, Johnson KO. Neural coding of tactile texture: Comparison of spatial and temporal mechanisms for roughness perception. J Neurosci. 1992;12:3414–3426. doi: 10.1523/JNEUROSCI.12-09-03414.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshioka T, Gibb B, Dorsch AK, Hsiao SS, Johnson KO. Neural coding mechanisms underlying perceived roughness of finely textured surfaces. J Neurosci. 2001;21:6905–6916. doi: 10.1523/JNEUROSCI.21-17-06905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carandini M, Mechler F, Leonard CS, Movshon JA. Spike train encoding by regular-spiking cells of the visual cortex. J Neurophysiol. 1996;76:3425–3441. doi: 10.1152/jn.1996.76.5.3425. [DOI] [PubMed] [Google Scholar]
- 7.Mainen ZF, Sejnowski TJ. Reliability of spike timing in neocortical neurons. Science. 1995;268:1503–1506. doi: 10.1126/science.7770778. [DOI] [PubMed] [Google Scholar]
- 8.Klyachko VA, Stevens CF. Excitatory and feed-forward inhibitory hippocampal synapses work synergistically as an adaptive filter of natural spike trains. PLoS Biol. 2006;4:e207. doi: 10.1371/journal.pbio.0040207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lak A, Arabzadeh E, Diamond ME. Enhanced response of neurons in rat somatosensory cortex to stimuli containing temporal noise. Cereb Cortex. 2008;18:1085–1093. doi: 10.1093/cercor/bhm144. [DOI] [PubMed] [Google Scholar]
- 10.Heiss JE, Katz Y, Ganmor E, Lampl I. Shift in the balance between excitation and inhibition during sensory adaptation of S1 neurons. J Neurosci. 2008;28:13320–13330. doi: 10.1523/JNEUROSCI.2646-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simons DJ. Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol. 1978;41:798–820. doi: 10.1152/jn.1978.41.3.798. [DOI] [PubMed] [Google Scholar]
- 12.Beaulieu C. Numerical data on neocortical neurons in adult rat, with special reference to the GABA population. Brain Res. 1993;23:284–292. doi: 10.1016/0006-8993(93)90884-p. [DOI] [PubMed] [Google Scholar]
- 13.Bruno RM, Simons DJ. Feedforward mechanisms of excitatory and inhibitory cortical receptive fields. J Neurosci. 2002;22:10966–10975. doi: 10.1523/JNEUROSCI.22-24-10966.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahissar E, Sosnik R, Bagdasarian K, Haidarliu S. Temporal frequency of whisker movement. II. Laminar organization of cortical representations. J Neurophysiol. 2001;86:354–367. doi: 10.1152/jn.2001.86.1.354. [DOI] [PubMed] [Google Scholar]
- 15.Arabzadeh E, Petersen RS, Diamond ME. Encoding of whisker vibration by rat barrel cortex neurons: Implications for texture discrimination. J Neurosci. 2003;23:9146–9154. doi: 10.1523/JNEUROSCI.23-27-09146.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arabzadeh E, Panzeri S, Diamond ME. Whisker vibration information carried by rat barrel cortex neurons. J Neurosci. 2004;24:6011–6020. doi: 10.1523/JNEUROSCI.1389-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castro-Alamancos MA. Absence of rapid sensory adaptation in neocortex during information processing states. Neuron. 2004;41:455–464. doi: 10.1016/s0896-6273(03)00853-5. [DOI] [PubMed] [Google Scholar]
- 18.Khatri V, Hartings JA, Simons DJ. Adaptation in thalamic barreloid and cortical barrel neurons to periodic whisker deflections varying in frequency and velocity. J Neurophysiol. 2004;92:3244–3254. doi: 10.1152/jn.00257.2004. [DOI] [PubMed] [Google Scholar]
- 19.Andermann ML, Ritt J, Neimark MA, Moore CI. Neural correlates of vibrissa resonance; band-pass and somatotopic representation of high-frequency stimuli. Neuron. 2004;42:451–463. doi: 10.1016/s0896-6273(04)00198-9. [DOI] [PubMed] [Google Scholar]
- 20.Melzer P, Champney GC, Maguire MJ, Ebner FF. Rate code and temporal code for frequency of whisker stimulation in rat primary and secondary somatic sensory cortex. Exp Brain Res. 2006a;172:370–386. doi: 10.1007/s00221-005-0334-1. [DOI] [PubMed] [Google Scholar]
- 21.Melzer P, Sachdev RN, Jenkinson N, Ebner FF. Stimulus frequency processing in awake rat barrel cortex. J Neurosci. 2006b;26:12198–12205. doi: 10.1523/JNEUROSCI.2620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinto DJ, Brumberg JC, Simons DJ. Circuit dynamics and coding strategies in rodent somatosensory cortex. J Neurophysiol. 2000;83:1158–1166. doi: 10.1152/jn.2000.83.3.1158. [DOI] [PubMed] [Google Scholar]
- 23.Kleinfeld D, Ahissar E, Diamond ME. Active sensation: Insights from the rodent vibrissa sensorimotor system. Curr Opin Neurobiol. 2006;16:435–444. doi: 10.1016/j.conb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Curtis JC, Kleinfeld D. Phase-to-rate transformations encode touch in cortical neurons of a scanning sensorimotor system. Nat Neurosci. 2009;12:492–501. doi: 10.1038/nn.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arabzadeh E, Zorzin E, Diamond ME. Neuronal encoding of texture in the whisker sensory pathway. PLoS Biol. 2005;3:e17. doi: 10.1371/journal.pbio.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arabzadeh E, Panzeri S, Diamond ME. Deciphering the spike train of a sensory neuron: Counts and temporal patterns in the rat whisker pathway. J Neurosci. 2006;26:9216–9226. doi: 10.1523/JNEUROSCI.1491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hipp J, et al. Texture signals in whisker vibrations. J Neurophysiol. 2006;95:1792–1799. doi: 10.1152/jn.01104.2005. [DOI] [PubMed] [Google Scholar]
- 28.Ritt JT, Andermann ML, Moore CI. Embodied information processing: Vibrissa mechanics and texture features shape micromotions in actively sensing rats. Neuron. 2008;57:599–613. doi: 10.1016/j.neuron.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolfe J, et al. Texture coding in the rat whisker system: Slip-stick versus differential resonance. PLoS Biol. 2008;6:e215. doi: 10.1371/journal.pbio.0060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diamond ME, von Heimendahl M, Knutsen PM, Kleinfeld D, Ahissar E. ‘Where’ and ‘what’ in the whisker sensorimotor system. Nat Rev Neurosci. 2008a;9:601–612. doi: 10.1038/nrn2411. [DOI] [PubMed] [Google Scholar]
- 31.Diamond ME, von Heimendahl M, Arabzadeh E. Whisker-mediated texture discrimination. PLoS Biol. 2008b;6:e220. doi: 10.1371/journal.pbio.0060220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lottem E, Azouz R. Dynamic translation of surface coarseness into whisker vibrations. J Neurophysiol. 2008;100:2852–2865. doi: 10.1152/jn.90302.2008. [DOI] [PubMed] [Google Scholar]
- 33.Crochet S, Petersen CCH. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat Neurosci. 2006;9:608–610. doi: 10.1038/nn1690. [DOI] [PubMed] [Google Scholar]
- 34.Llinás RR, Grace AA, Yarom Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc Natl Acad Sci USA. 1991;88:897–901. doi: 10.1073/pnas.88.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fellous JM, et al. Frequency dependence of spike timing reliability in cortical pyramidal cells and interneurons. J Neurophysiol. 2001;85:1782–1787. doi: 10.1152/jn.2001.85.4.1782. [DOI] [PubMed] [Google Scholar]
- 36.Azouz R, Gray CM. Dynamic spike threshold reveals a mechanism for synaptic coincidence detection in cortical neurons in vivo. Proc Natl Acad Sci USA. 2000;97:8110–8115. doi: 10.1073/pnas.130200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luna R, Hernández A, Brody CD, Romo R. Neural codes for perceptual discrimination in primary somatosensory cortex. Nat Neurosci. 2005;8:1210–1219. doi: 10.1038/nn1513. [DOI] [PubMed] [Google Scholar]
- 38.Harris JA, Arabzadeh E, Fairhall AL, Benito C, Diamond ME. Factors affecting frequency discrimination of vibrotactile stimuli: Implications for cortical encoding. PLoS One. 2006;1:e100. doi: 10.1371/journal.pone.0000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romo R, Hernández A, Zainos A, Salinas E. Somatosensory discrimination based on cortical microstimulation. Nature. 1998;392:387–390. doi: 10.1038/32891. [DOI] [PubMed] [Google Scholar]
- 40.Salinas E, Hernandez A, Zainos A, Romo R. Periodicity and firing rate as candidate neural codes for the frequency of vibrotactile stimuli. J Neurosci. 2000;20:5503–5515. doi: 10.1523/JNEUROSCI.20-14-05503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cardin JA, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Lafuente V, Romo R. Neural correlate of subjective sensory experience gradually builds up across cortical areas. Proc Natl Acad Sci USA. 2006;103:14266–14271. doi: 10.1073/pnas.0605826103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rousche PJ, Petersen RS, Battiston S, Giannotta S, Diamond ME. Examination of the spatial and temporal distribution of sensory cortical activity using a 100-electrode array. J Neurosci Methods. 1999;90:57–66. doi: 10.1016/s0165-0270(99)00061-8. [DOI] [PubMed] [Google Scholar]
- 44.Petersen RS, Diamond ME. Spatial-temporal distribution of whisker-evoked activity in rat somatosensory cortex and the coding of stimulus location. J Neurosci. 2000;20:6135–6143. doi: 10.1523/JNEUROSCI.20-16-06135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung S, Li X, Nelson SB. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron. 2002;34:437–446. doi: 10.1016/s0896-6273(02)00659-1. [DOI] [PubMed] [Google Scholar]
- 46.Armstrong-James MA, Diamond ME, Ebner FF. An innocuous bias in whisker use in adult rats modifies receptive fields of barrel cortex neurons. J Neurosci. 1994;14:6978–6991. doi: 10.1523/JNEUROSCI.14-11-06978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.