Abstract
Contralateral hemispheric representation of sensory inputs (the right visual hemifield in the left hemisphere and vice versa) is a fundamental feature of primate sensorimotor organization, in particular the visuomotor system. However, many higher-order cognitive functions in humans show an asymmetric hemispheric lateralization—e.g., right brain specialization for spatial processing—necessitating a convergence of information from both hemifields. Electrophysiological studies in monkeys and functional imaging in humans have investigated space and action representations at different stages of visuospatial processing, but the transition from contralateral to unified global spatial encoding and the relationship between these encoding schemes and functional lateralization are not fully understood. Moreover, the integration of data across monkeys and humans and elucidation of interspecies homologies is hindered, because divergent findings may reflect actual species differences or arise from discrepancies in techniques and measured signals (electrophysiology vs. imaging). Here, we directly compared spatial cue and memory representations for action planning in monkeys and humans using event-related functional MRI during a working-memory oculomotor task. In monkeys, cue and memory-delay period activity in the frontal, parietal, and temporal regions was strongly contralateral. In putative human functional homologs, the contralaterality was significantly weaker, and the asymmetry between the hemispheres was stronger. These results suggest an inverse relationship between contralaterality and lateralization and elucidate similarities and differences in human and macaque cortical circuits subserving spatial awareness and oculomotor goal-directed actions.
Keywords: BOLD signal, event-related functional MRI, brain evolution, delayed saccades, lateralization
In both human and nonhuman primates, left and right visual hemifields are initially processed separately by contralateral cerebral hemispheres: The left hemifield is represented in the right primary visual cortex (V1), and vice versa. Such contralateral architecture reflects a general cross-over pattern of sensory and motor organization in vertebrate organisms with bilateral symmetry and chiasmatic decussation (1). However, at the subsequent stages of cortical processing, inputs from the right and left hemifields become less segregated because of interhemispheric information transfer. For example, although a majority of neuronal response fields in macaque frontal and parietal visuomotor areas are tuned to the contralateral hemifield, some neurons have ipsilateral or bilateral response fields (2–5). The gradual convergence of information from both hemifields (at different stages of cortical processing) underlies a unified percept of visual space and is necessary for coordinating bihemispheric control of goal-directed actions such as saccadic eye movements and visually guided reaches. It enables continuous integration of sensory inputs and their internal representations across eye, head, and body movements and facilitates choice behavior when stimuli or response options span both hemifields. Most notably, split-brain studies have demonstrated that the resection of the corpus callosum (axon fibers connecting two cerebral hemispheres) disrupts this integrative processing, leading to profound perceptual and action deficits in tasks requiring interhemispheric transfer, e.g., when comparing stimuli in two opposite hemifields (6, 7). Characterizing the transition from strictly contralateral to more global bilateral space representations thus is important for understanding these basic functions.
A related but distinct organizational principle is the phenomenon of hemispheric specialization or lateralization—a condition of functional asymmetry between two sides of the brain, leading to a hemispheric dominance for a certain aspect of neural processing. For example, the right hemisphere in humans is thought to be more involved in the visuospatial domain, whereas the left hemisphere typically is dominant for language functions (7). Hemispheric lateralization has been long considered a uniquely defining feature of the human nervous system (8, 9). Recently an alternative view emerged that traces anatomical, behavioral, and functional asymmetries across many vertebrate species, providing an evolutionary framework for studying the development of lateralized brain functions (10, 11). The increased lateralization in humans may have been an emergent property accompanying the brain enlargement and growing cognitive repertoire in primate evolution (12). Notably, contralaterality and lateralization of a specific function are diametrical phenomena, because the dominance of one side precludes the balanced contralateral distribution of the processing between hemispheres. Therefore, understanding contralateral organization and hemispheric specialization in different species also is important in the phylogenetic context of cognition. This approach is of particular relevance for macaque monkeys, which serve as a standard animal model both for normal human brain functions and for disorders related to spatial awareness and neglect.
To investigate the spatial processing beyond early visual areas, monkey electrophysiology and recent human imaging studies have used delayed-response tasks that allow separating the visual, motor, and interleaving mnemonic/preparatory/attentional components of neural signals (13). Differences between results obtained in the two species have emerged. Macaque frontoparietal areas show a strong contralateral tuning of visual, memory-delay, and saccade responses (e.g., 2, 4), but human functional MRI (fMRI) studies report either no or weaker contralaterality of activations in the parietal and frontal cortex and no contralateral tuning for saccades (14, 15). These observations raise the question whether actual interspecies differences or methodological/signal factors account for these discrepancies. Moreover, various aspects of visuomotor cognitive representations in the human cortex exhibit profound hemispheric lateralization, and many studies suggest that the “dominant” right hemisphere can attend to both visual hemifields, whereas the left hemisphere preferentially represents the right hemifield (16), but no comparable findings in monkey electrophysiology have been reported.
To address these discrepancies, we provided a direct comparison between humans and monkeys with the same delayed-memory saccade task, using an event-related fMRI approach in monkeys as is done in human imaging and which is more comparable with monkey electrophysiological studies. In both species, we isolated spatial-specific cognitive signals reflecting spatial memory and planning (17). However, the contralaterality of visuomotor signals was significantly greater in monkeys than in humans, suggesting actual interspecies differences in the representation of space for goal-directed actions.
Results
Two monkeys and 11 human subjects were scanned with blood oxygen level-dependent (BOLD)-sensitive fMRI sequences while they performed a delayed memory-guided saccade task under real-time behavioral control (Fig. 1A). In this task, subjects had to memorize the location of the visual cue and could prepare a specific movement in advance. A detailed account of the subjects’ training and performance is given in SI Text. We used an event-related design with long delays to separate contributions from different trial intervals and to dissociate randomly interleaved rightward and leftward trials. This approach allowed us to obtain event-based statistical activation maps and to extract BOLD signal time courses.
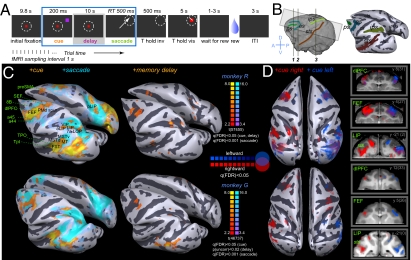
Fig. 1.
(A) Memory-guided saccade task. (B) Functional volume used in monkey studies, shown on the 3D reconstruction of the brain surface (lateral view) (Left) and inflated “white-gray matter boundary” surface (Right) with major sulci of interest colored. (C) Cortical areas activated by +cue, +memory-delay, and +saccade contrasts, shown on the inflated surface of each monkey brain. See Table S1 and the ROI definitions in Box S1 for the summary of activated areas and Fig. S1 for coronal sections. (D) Superimposed maps for the +cue right and +cue left contrasts, shown on (Left) the inflated surface (top view) and (Right) sample coronal sections (transparency scales with significance of activation). Coordinates are in the anterior commissure–posterior commissure (AC–PC) and stereotaxic (in parentheses) planes.
Spatial Distribution of Cue, Saccade, and Delay Activity in Monkeys.
Extensive and overlapping visual and oculomotor regions were activated by visual (cue) and visuomotor (saccade) task events, with the strongest bilateral peaks of activation located in the frontal cortex in the arcuate sulcus and the principal sulcus, in the parietal cortex along the intraparietal sulcus (ips), and in the superior temporal sulcus (sts) (Fig. 1 B and C, Fig. S1, and Table S1). The memory-delay activation maps were sparser, revealing only a few regions with significantly increased activity in the frontal eye field (FEF), lateral intraparietal (LIP), and middle temporal/temporal parietal occipital/temporo-parietal (MT/TPO/Tpt) areas (Fig. 1C). As discussed later, subsequent findings demonstrate that contralateral tuning of delay activity in monkeys rendered this contrast suboptimal.
With all target locations pooled together, most activation patterns were bilaterally symmetric, suggesting no hemispheric lateralization of functions involved in this task (Fig. S2). However, if BOLD responses reflect a neuronal population effect, left and right hemispheres should respond differentially in rightward vs. leftward trials, because large numbers of neurons in monkey oculomotor areas exhibit contralateral tuning (2–5). Separate maps for cue-right and cue-left contrasts demonstrated contralateral hemispheric preferences (Fig. 1D). To identify further loci with such spatial tuning, we generated maps for rightward vs. leftward contrast for each of the three epochs of interest: cue, memory-delay, and saccade response. Consistent with electrophysiology, areas that exhibited robust cue and memory activity—the dorsal lateral prefrontal cortex (dlPFC), FEF, LIP, plus several clusters in the sts—also showed contralateral preference in these epochs (Fig. S3). Saccade responses showed weaker contralateral tuning in these areas, and either contra- or ipsilateral saccade tuning was prominent in retinotopic visual areas (Fig. S4). In the next section we focus on the region-of-interest (ROI) analysis of spatial specificity using BOLD time courses extracted from areas defined by the statistical mapping as active in at least one of the three main task epochs in conjunction with known anatomical landmarks (SI Materials and Methods).
Time-Course of BOLD Activity and Contralaterality in Monkeys.
To examine BOLD signal time courses, we computed peritrial event-related averages (ERA) for the rightward and leftward target directions for each ROI. Trials started with a period of initial fixation, followed by the spatial cue, the delay period, saccade, target fixation, and reward (Fig. 1A). A typical ERA time course for a bilateral oculomotor area with delay-period activity (FEF) is shown in Fig. 2 A and B. The high BOLD signal during initial fixation is caused by visual/oculomotor activity resulting from eye movements in the preceding intertrial interval. As the trial advanced while central fixation was maintained, the signal gradually returned to baseline. The cue evoked a time-locked response lasting up to 5 s, which often was separated by a trough from the reminder of delay-period activity (15–20 s). A subsequent saccade response caused another time-locked peak of high magnitude. This three-component (cue-delay-saccade) response was characteristic across several oculomotor areas recruited in the task (Fig. 2C), but other areas had only a two-component (cue-saccade) or only a saccade response (Fig. S5).
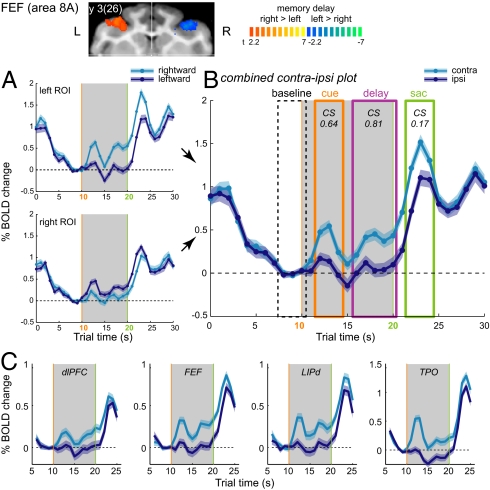
Fig. 2.
ERA BOLD trial time courses. (A) ERAs from left and right FEF (Inset: memory-delay right > left contrast) and (B) combined contraipsilateral time courses for bilateral ROIs, monkey R. Colored boxes denote time intervals (cue, delay, and saccade) used for estimating mean response amplitude (A) and calculating the contraversive selectivity: CS = (Acontra − Aipsi) / (|Acontra| + |Aipsi|). Shaded bands denote SEM across trials. (C) ERA time courses in selected frontal, posterior, parietal, and parieto-temporal areas that exhibited significant (per-sample t test, P < 0.05) contralateral memory-delay activity, averaged across two monkeys. (Individual data and other activated areas are shown in Fig. S5.)
Notice that contra- and ipsilateral trials diverge in the cue and especially in the memory periods, with the contralateral response being significantly higher than the ipsilateral response. This spatially specific activation reflects mnemonic and planning functions such as cue processing, spatial working memory, and saccade preparation. To quantify these patterns, we combined time courses from left and right ROIs (Fig. 2B) and calculated a contraversive selectivity index (CS) (SI Text) measuring the normalized amplitude difference between contralateral and ipsilateral responses for each of the three intervals of interest (cue, delay, saccade) across areas activated in the task. For each interval, we assessed the strength and the contralaterality of responses. The primary goal of this study is the monkey–human comparison of cue and subsequent mnemonic/planning signals that bridge the visual input and the motor output; therefore, we focus on the areas that exhibited consistent cue and memory-delay responses. In the frontal cortex, the dlPFC and FEF areas showed strongest contralateral cue and memory-delay activation (Fig. 2C). Other frontal ROIs [areas 44, 8B, and the dorsal premotor (PMd)] showed weaker cue and memory-delay response (Fig. S5A). In the posterior parietal cortex (PPC), the lower part of the dorsal LIP (LIPd) in the posterior third of the ips exhibited the strongest contralaterality in cue and delay intervals. These activation loci corresponded well to the histological verification of neuronal recording sites with sustained mnemonic/planning activity (18). Ventrally from the LIPd, the ventral lip (LIPv) showed weaker contralateral cue and memory-delay responses, and the ventral intraparietal area (VIP) in the fundus of the ips showed only saccade responses. Likewise, the anterior LIP (aLIP) had smaller cue and memory responses, and, caudal to LIP, the posterior LIP (pLIP) and lateral occipital parietal (LOP) areas showed weak cue but strong saccade responses (Fig. S5B). In the parieto-temporal areas in the sts, area MT showed contralateral cue and delay responses, whereas the adjacent ventral middle superior temporal (MSTv) and dorsal middle superior temporal (MSTd) areas had only cue and saccade responses (Fig. S5C). Polysensory TPO and Tpt areas, located more anterior in the dorsal bank, also showed strong contralateral cue and, unexpectedly, memory-delay activity (Fig. 2C).
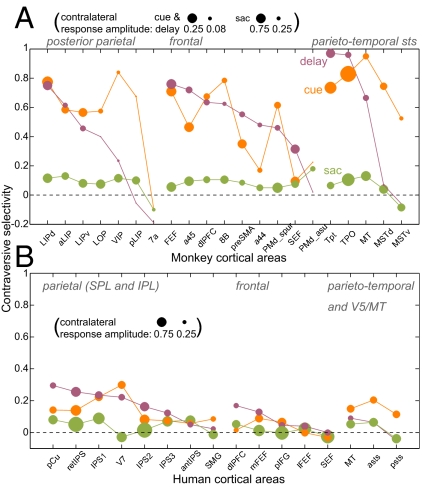
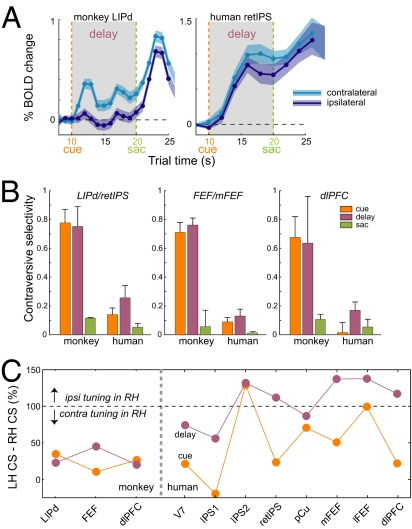
The CS and response amplitude in different intervals are summarized in Fig. 3A. Because CS is a relative and normalized measure, it does not reflect the variations in response level, so the contralateral response amplitude can be used as an indicator for the effect strength. Although several areas showed the contralateral tuning of cue and memory responses, only the LIP, dlPFC, FEF, MT, TPO, and Tpt areas had significant contralateral memory-delay activity in both monkeys.
Fig. 3.
Contraversive selectivity. (A) Monkeys (n = 2; individual data are given in Table S1). Areas in each group are sorted by CS for the memory delay. The size of the dot denotes the contralateral response amplitude Acontra for each interval [note difference (×3) in scales for cue and delay vs. saccades (sac)]. (B) Humans (n = 8). ERA time courses were extracted using individual GLM ROIs in each subject, similar to the analysis in monkeys. In monkeys, CSmem was strongly correlated with Amem_contra across areas (r = 0.84, P < 0.01), demonstrating that high memory-delay responses in monkeys also were contralateral. The correlation was weaker in humans because of several areas with untuned delay-period activity (r = 0.52, P < 0.05). In both species, cue and memory tuning covaried (r = 0.47 monkeys; r = 0.5 humans, P < 0.05). See Box S1 for ROI definitions.
Comparison with Human Imaging Data.
To compare results in monkeys directly with human imaging data, we conducted the same experiment in 11 human subjects. To separate better the cue response from subsequent memory-delay activity in slower and more sustained human BOLD signals (14), eight subjects were tested with variable-delay periods of 6, 10, 14, and 18 s. The results from the fixed-delay experiment in three control subjects corresponded to the results for the same delay in the variable-delay experiment; therefore, for brevity, we present only variable-delay data. (Fixed- and variable-delay experiments are discussed in SI Text.)
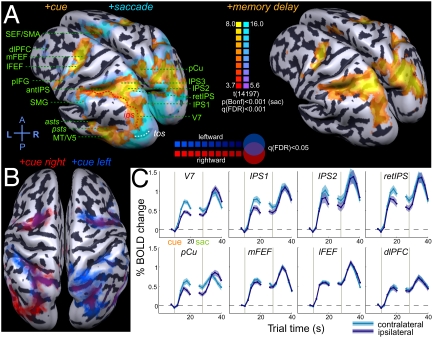
A plethora of areas reported in previous studies (e.g., 15, 19–21) were activated during cue, memory-delay, and saccade response periods. (ROI definitions and nomenclature are given in Box S1.) Here we focus on several areas in the PPC along the ips in the superior parietal lobule (SPL) and human FEF complex, which are considered plausible candidates for functional homology to monkey LIP and FEF. These dorsal frontoparietal regions showed robust cue, saccade, and sustained memory-delay activity (Fig. 4 and Fig. S6). Several other prefrontal areas [supplementary eye fields (SEF), dlPFC, posterior inferior frontal gyrus (pIFG)], inferior parietal areas [supramarginal gyrus (SMG)], and parieto-temporal areas [posterior superior temporal gyrus (STG), middle temporal gyrus (MTG), and sts] also exhibited robust cue and saccade activity and varying levels of delay activation.
Fig. 4.
Human imaging results. (A) Areas activated in the task (Table S2). Maps are superimposed on the inflated averaged cortical surface of eight subjects following cortex-based alignment. (Left) +Cue and +saccade contrasts. (Right) +Memory-delay contrast. (B) Superimposed maps for the +cue right and +cue left contrasts. (C) ERA plots for all delay periods, aligned to cue and to saccade events. Shaded bands denote intersubject SEM.
We searched for evidence of contralateral organization in the cue and memory responses but did not detect a level of contralaterality comparable with that observed in monkeys using the same techniques. Most human subjects did show a modest spatial tuning of cue and memory responses, but the effect was less robust than in monkeys (Fig. 4C and Fig. S6). In the PPC, the strongest contraversive selectivity was observed in the medial SPL in the anterior precuneus (pCu), in the putative retinotopic ips (retIPS) (22), in areas IPS1 and IPS2 (14), and in area V7. The SPL regions IPS3 and anterior IPS, and the SMG in the inferior parietal lobule (IPL) exhibited very little tuning. Frontal cortex showed even less contralaterality, with the medial/superior FEF slightly more tuned than the lateral/inferior FEF. The dlPFC showed some residual tuning, and the SMA/SEF and pIFG did not show any contralaterality, despite sustained memory responses. Parieto-temporal ROIs located in the posterior sts/STG/MTG (denoted “asts” and “psts”) and in the putative MT/V5 complex showed stronger contralaterality for the cue but not for sustained memory activity. These patterns are summarized in Fig. 3B. A side-by-side comparison of BOLD signal time courses in both species is shown in Fig. 5A, and Fig. 5B compares CS in monkeys and humans in selected ROIs (CS monkeys > CS humans, P < 0.05).
Fig. 5.
Monkey–human comparisons. (A) Time courses for 10-s delay period in two monkeys (LIPd) and eight humans (retIPS). Note the faster BOLD response and stronger contralaterality in monkeys. (B) CS in selected parietal and frontal ROIs (mean ± SEM across subjects). For all ROIs, CScue and CSmem were significantly larger in monkeys than in humans (P < 0.05, t test), but in all human ROIs CScue and CSmem were larger than zero (P < 0.05, t test), except for CScue in the dlPFC. (C) CS asymmetry between left hemisphere (LH) and right hemisphere (RH): %CSLH−RH =100(CSLH − CSRH) / CSLH in monkey and human parietal and frontal ROIs with strong delay-period activity.
Hemispheric and Visual Field Asymmetry in Monkeys and Humans.
An “ideal” contralateral organization dictates that both hemispheres respond in mirror-symmetrical fashion with equal contralateral tuning. To test this premise we separately calculated CS in left and right hemisphere ROIs. In both species the left hemisphere exhibited stronger contralaterality; this hemispheric asymmetry was modest in monkeys but was much more pronounced in humans (Fig. 5C and Fig. S2A). In monkey frontoparietal areas with strong memory-delay period activity (LIPd, FEF, dlPFC), the left hemisphere CS was higher than the right hemisphere CS by only 23 ± 12% and 29 ± 14% for cue and delay, respectively (mean ± SD), whereas in human frontoparietal areas [V7, IPS1/2, retIPS, pCu, lateral inferior FEF (lFEF), medial FEF (mFEF), and dlPFC], the difference was 49 ± 50% and 107 ± 31%, respectively (Fig. 5C and SI Text). In most human subjects, the contralaterality was present in the left brain but was weaker, not existent, or even reversed (i.e., ipsilateral > contralateral) in the right brain, across many recruited areas (Fig. S2A). For example, left hemisphere ROIs generated stronger right than left memory responses, but the right hemisphere demonstrated a similar pattern, albeit to a lesser degree. This observation is consistent with the hypothesis that the left hemisphere predominately encodes the right space, whereas the right hemisphere represents both hemifields (16).
In both species, no overall hemispheric lateralization was detected in this task: Most activations were bilateral with comparable extents and amplitudes when contra- and ipsilateral responses were combined (Fig. S2B). However, when responses were averaged across hemispheres, both species showed stronger responses to the targets in the right hemifield than in the left hemifield (P < 0.001; Wilcoxon matched pairs signed rank test across areas), reflecting predominantly smaller activations for left targets in the left hemisphere (Fig. S2C and SI Text).
Discussion
Contralaterality and Lateralization in Monkeys and Humans.
Using a time-resolved event-related fMRI study in monkeys, we found that the contralateral tuning of cue and memory-delay BOLD activity is far stronger in monkeys than in humans. The monkey fMRI data complement the electrophysiological evidence that a majority of neurons in the dlPFC, FEF, and LIP areas have contralateral response fields (2–5). Most human fMRI studies, including ours, show markedly less contralaterality. In phase-encoding (not event-resolved) experiments, the existence of spatial maps in the frontal and parietal cortex has been shown (23–27), but these findings cannot be attributed to a specific epoch of a task and provide no direct measure of tuning strength as compared with untuned activation. A few event-related studies using variants of the delayed saccade task in humans reported a contralateral specificity of cue and memory responses in frontal but not parietal (28) and in parietal (14, 22) cortex. Our data show weak contralateral preference in human frontoparietal areas, agreeing with the most recent studies (15) (SI Text). In all those human data, the differential contralateral > ipsilateral signal is a fraction of a larger untuned activation, in contrast with monkeys where the ipsilateral delay activity often stays near the baseline level. In both species, spatial tuning of the cue response and ensuing memory-delay activity was generally similar, suggesting the continuity of initial visual processing, memory retention, and saccade planning. The saccade response was marginally contralateral in monkeys but was not tuned in humans (14) (SI Text).
We emphasize that the issue of contralaterality is not a mere methodological matter of correspondence between neuronal and fMRI data. The contralateral tuning reflects a major organizational principle for perception and action in a primate visual world that is inherently separated into two hemifields by the current gaze axis. In both monkeys and humans, the feedforward inputs from the two hemifields are represented initially in a strict contralateral manner by the opposite hemispheres. The gradual progression from finely topographically organized early visual areas to a coarser, mainly contralateral, topography of parietal, frontal, and temporal areas (29) indicates a transformation from the “local” visual processing to a “global” representation of action space. The difference in the degree of contralateral organization among primate species might be related to the evolution of lateralization. Most anatomical and functional aspects of the macaque brain are fairly symmetrical, and lesions of the left or right hemispheres cause comparable contralateral deficits (30). The human brain, however, exhibits strong hemispheric lateralization of many cognitive functions, related most notably to verbal and emotional processing but also to the memorization, selection, preparation, and execution of actions. Right hemisphere lesions cause more severe, frequent, and persistent spatial neglect than left hemisphere lesions (16), and right, but not left, parietal damage is associated with right/left asymmetries in action planning (31). In particular, the human IPL shows profound hemispheric differences, with the right IPL implicated in allocating and sustaining spatial attention (32). Global (left and right) vs. local (left or right) allocation of attention increases activation in the right but not the left IPL (33), and the right SMG responds to both left and right stimuli, whereas the left IPL responds only to right stimuli (34). In our data, cue and delay activation was more extensive in the right SMG than in the left. In the SPL, a bihemispheric leftward bias caused by right hemispheric dominance in the visuospatial network during passive fixation has been reported (35). Khonsari et al. (36) found saccade preparatory signals (for left and right targets) only in the left PPC but found execution signals confined to the right PPC. The effects of transcranial magnetic stimulation over the human PPC during memory-guided pointing also revealed hemispheric asymmetry (37). Thus, it appears that the human cortex, confronted with new complex tasks and larger dimensions, has evolved to become more lateralized and specialized, losing the original functional symmetry (38). The increased lateralization may have led to a more uniform encoding of both ipsi- and contralateral fields in human frontoparietal areas, possibly as a result of a more abstract, less “visually driven” representation of space. Consequently, these areas respond almost equally to stimuli in either hemifield or even show a bilateral bias to one side of space (Fig. 5).
The same connectivity principles that may underlie the monkey–human differences in the contralateral organization are subjects of intense research on inter- and intrahemispheric communication. The increased human lateralization has been related to a diminished interhemispheric communication via the corpus callosum, resulting from evolutionary pressure to rely less on transfer of information across long fibers (7, 39). It may appear, following this reasoning, that human hemispheres should be more “isolated” and thus more contralateral, because bilateral and ipsilateral activations must be mediated by the excitatory interhemispheric connections. However, it is not known currently whether and which callosal connections are predominantly excitatory, inhibitory, or both (40). Furthermore, top-down and subcortical pathways may provide alternative conduits for interhemispheric integration (41, 42) and should be taken into the account.
Visuospatial Networks in Monkeys and Humans.
Another interspecies difference was that the foci of the (strongly contralateral) memory-delay activation in monkeys were limited to a few regions in the dlPFC, FEF, LIP, MT, and TPO/Tpt, whereas in humans the (weakly tuned) delay activity was more widespread, especially in the PPC, demonstrating a more extensive visuospatial memory network (20). This outcome cannot be attributed to differences in statistical thresholds or similar reasons, because it was validated with the time courses extracted from areas involved in the task. Aside from potential technical considerations (i.e., more prolonged BOLD response to the cue in humans), this outcome may suggest a training effect (overtrained monkeys vs. almost naïve humans). However, it may also reflect the overall increase in complexity of the distributed network that evolved to solve difficult tasks but still is recruited even in simple spatial working memory paradigms.
The elucidation of putative homologies or functional correspondences between human and monkey cortical areas beyond the early visual system is far from resolved (43), especially in areas outside the “classical” dorsal frontoparietal network (44, 45). Although the comparisons are tempting, we cannot draw strong parallels between activations in the human IPL and parieto-temporal areas [SMG, temporo-parietal junction (TPJ), posterior STG, and sts] and the monkey sts. The data on the involvement of monkey sts regions in spatial awareness is inconclusive, but a few single-unit and lesion studies suggest that the superior temporal polysensory complex (including the TPO) and adjacent PGa are involved in the control of eye movements and visuospatial coordination (46, 47) and that the ablation of the STG/sts in monkeys may result in neglect-like syndromes (48, 49), similar to lesions in the human STG and IPL/TPJ (50–52). Not only are the homologies between human and macaque IPL and sts/STG unclear (in part because of strong hemispheric asymmetry in humans); the localization of human areas critically associated with the neglect and the involvement of the temporal lobe (mid STG) also are debated intensely (52). Our results showing the cue and delay activity in the monkey mid-to-posterior sts provide further motivation for cross-species investigations of parieto-temporal and IPL participation in the visuospatial processing for actions.
Comparison Between Techniques and Species.
The correspondence between fMRI activation patterns in monkeys and humans in previous studies (e.g., 53–58) and, partly, in our work is encouraging, although it does not necessarily imply that the underlying behavioral strategies and neuronal activity in the two species are the same. Nevertheless, monkey fMRI provides a crucial control for the common interpretation of human imaging and monkey electrophysiology data. Until now, comparative studies of monkey and human functional topography that used monkey fMRI have focused on spatial mapping and the delineation of homologous areas using block-design tasks. Significant progress has been made using this approach (reviewed in ref. 59). The event-related approach, now widely used in human fMRI, further facilitates the direct comparison between monkey and human imaging studies, on the one hand, and between imaging and electrophysiology studies, on the other, by dissociating different components of neural activity.
Besides contralaterality, other discrepancies between human fMRI and monkey electrophysiology have emerged. For example, single-neuron studies of antisaccades and prosaccades in macaque FEF do not match fMRI findings in human putative homologs, but the fMRI results in monkeys and humans agree (60). Similarly, perceptual suppression in the human V1 contrasts with negative findings in monkey V1 neurons, but Maier et al. (61) reported the perceptual modulation in monkey fMRI experiments. These and other studies suggest a complicated relationship between neuronal and BOLD activity and underscore differences between techniques (62). Our work presents an opposite example—the variation in contralateral tuning between human and macaque responses does not stem from the discrepancy between imaging and electrophysiology techniques and may be attributed to an actual difference between species.
In summary, by comparing event-related fMRI signals in monkeys and humans, we elucidated the differences in the way the two species process and retain spatial information for goal-directed actions. Further investigations of response selection and planning, combined with fMRI-guided electrophysiological recordings in the same monkeys, are needed to understand comprehensively interspecies similarities and differences and the relationship between fMRI and neuronal activity underlying these behaviors.
Materials and Methods
Monkeys and humans were scanned with BOLD-sensitive echo-planar imaging sequences in a 4.7-T scanner (Bruker) and a 3-T scanner (Siemens), respectively, while performing the delayed-memory saccade task (Fig. 1A). Functional images were motion-corrected, preprocessed, and analyzed with an event-related general linear model (GLM), and BOLD signal ERA time courses were extracted from ROIs defined by statistical activation maps and individual anatomical patterns. Trials affected by head, body, or limb motions were detected automatically and were eliminated from ERA analysis (Fig. S7). Details are given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank S. Wagner for help with scanning; M. Wilke for comments on the manuscript; H. Glidden for useful discussions; K. Pejsa, L. Martel, and N. Sammons for animal care; and V. Shcherbatyuk for computer support. This work was supported by Moore Foundation, National Eye Institute, and Boswell Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1002825107/DCSupplemental.
References
- 1.Capozzoli NJ. Why are vertebrate nervous systems crossed? Med Hypotheses. 1995;45:471–475. doi: 10.1016/0306-9877(95)90225-2. [DOI] [PubMed] [Google Scholar]
- 2.Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. II. Spatial properties. J Neurophysiol. 1991;66:1109–1124. doi: 10.1152/jn.1991.66.3.1109. [DOI] [PubMed] [Google Scholar]
- 3.Ben Hamed S, Duhamel JR, Bremmer F, Graf W. Representation of the visual field in the lateral intraparietal area of macaque monkeys: A quantitative receptive field analysis. Exp Brain Res. 2001;140:127–144. doi: 10.1007/s002210100785. [DOI] [PubMed] [Google Scholar]
- 4.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 5.Schall JD. Neuronal activity related to visually guided saccades in the frontal eye fields of rhesus monkeys: Comparison with supplementary eye fields. J Neurophysiol. 1991;66:559–579. doi: 10.1152/jn.1991.66.2.559. [DOI] [PubMed] [Google Scholar]
- 6.Gazzaniga MS, Bogen JE, Sperry RW. Some functional effects of sectioning the cerebral commissures in man. Proc Natl Acad Sci USA. 1962;48:1765–1769. doi: 10.1073/pnas.48.10.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazzaniga MS. Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition? Brain. 2000;123:1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- 8.Corballis MC. The Lopsided Ape: Evolution of the Generative Mind. New York: Oxford Univ Press; 1991. pp vii, 366 pp. [Google Scholar]
- 9.Warren JM. Handedness and laterality in humans and other animals. Physiological Psychology. 1980;8:351–359. [Google Scholar]
- 10.Corballis MC. Of mice and men—and lopsided birds. Cortex. 2008;44:3–7. doi: 10.1016/j.cortex.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Vallortigara G, Rogers LJ. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behav Brain Sci. 2005;28:575–589. doi: 10.1017/S0140525X05000105. discussion 589–633. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins WD, Rilling JK. A comparative MRI study of the relationship between neuroanatomical asymmetry and interhemispheric connectivity in primates: Implication for the evolution of functional asymmetries. Behav Neurosci. 2000;114:739–748. doi: 10.1037//0735-7044.114.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol. 1983;49:1268–1284. doi: 10.1152/jn.1983.49.5.1268. [DOI] [PubMed] [Google Scholar]
- 14.Schluppeck D, Curtis CE, Glimcher PW, Heeger DJ. Sustained activity in topographic areas of human posterior parietal cortex during memory-guided saccades. J Neurosci. 2006;26:5098–5108. doi: 10.1523/JNEUROSCI.5330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srimal R, Curtis CE. Persistent neural activity during the maintenance of spatial position in working memory. Neuroimage. 2008;39:455–468. doi: 10.1016/j.neuroimage.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mesulam MM. Spatial attention and neglect: Parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen RA, Cui H. Intention, action planning, and decision making in parietal-frontal circuits. Neuron. 2009;63:568–583. doi: 10.1016/j.neuron.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 18.Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res. 1988;70:216–220. doi: 10.1007/BF00271862. [DOI] [PubMed] [Google Scholar]
- 19.Astafiev SV, et al. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown MRG, et al. Comparison of memory- and visually guided saccades using event-related fMRI. J Neurophysiol. 2004;91:873–889. doi: 10.1152/jn.00382.2003. [DOI] [PubMed] [Google Scholar]
- 21.Curtis CE, Rao VY, D'Esposito M. Maintenance of spatial and motor codes during oculomotor delayed response tasks. J Neurosci. 2004;24:3944–3952. doi: 10.1523/JNEUROSCI.5640-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medendorp WP, Goltz HC, Vilis T. Directional selectivity of BOLD activity in human posterior parietal cortex for memory-guided double-step saccades. J Neurophysiol. 2006;95:1645–1655. doi: 10.1152/jn.00905.2005. [DOI] [PubMed] [Google Scholar]
- 23.Hagler DJ, Jr, Riecke L, Sereno MI. Parietal and superior frontal visuospatial maps activated by pointing and saccades. Neuroimage. 2007;35:1562–1577. doi: 10.1016/j.neuroimage.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kastner S, et al. Topographic maps in human frontal cortex revealed in memory-guided saccade and spatial working-memory tasks. J Neurophysiol. 2007;97:3494–3507. doi: 10.1152/jn.00010.2007. [DOI] [PubMed] [Google Scholar]
- 25.Schluppeck D, Glimcher P, Heeger DJ. Topographic organization for delayed saccades in human posterior parietal cortex. J Neurophysiol. 2005;94:1372–1384. doi: 10.1152/jn.01290.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294:1350–1354. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- 27.Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol. 2005;94:1358–1371. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtis CE, D'Esposito M. Selection and maintenance of saccade goals in the human frontal eye fields. J Neurophysiol. 2006;95:3923–3927. doi: 10.1152/jn.01120.2005. [DOI] [PubMed] [Google Scholar]
- 29.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 30.Gaffan D, Hornak J. Visual neglect in the monkey. Representation and disconnection. Brain. 1997;120:1647–1657. doi: 10.1093/brain/120.9.1647. [DOI] [PubMed] [Google Scholar]
- 31.Coulthard EJ, Nachev P, Husain M. Control over conflict during movement preparation: Role of posterior parietal cortex. Neuron. 2008;58:144–157. doi: 10.1016/j.neuron.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh-Curry V, Husain M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia. 2009;47:1434–1448. doi: 10.1016/j.neuropsychologia.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciçek M, Gitelman D, Hurley RS, Nobre A, Mesulam M. Anatomical physiology of spatial extinction. Cereb Cortex. 2007;17:2892–2898. doi: 10.1093/cercor/bhm014. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz S, et al. Attentional load and sensory competition in human vision: Modulation of fMRI responses by load at fixation during task-irrelevant stimulation in the peripheral visual field. Cereb Cortex. 2005;15:770–786. doi: 10.1093/cercor/bhh178. [DOI] [PubMed] [Google Scholar]
- 35.Siman-Tov T, et al. Bihemispheric leftward bias in a visuospatial attention-related network. J Neurosci. 2007;27:11271–11278. doi: 10.1523/JNEUROSCI.0599-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khonsari RH, et al. Lateralized parietal activity during decision and preparation of saccades. Neuroreport. 2007;18:1797–1800. doi: 10.1097/WNR.0b013e3282f1a986. [DOI] [PubMed] [Google Scholar]
- 37.Vesia M, Monteon JA, Sergio LE, Crawford JD. Hemispheric asymmetry in memory-guided pointing during single-pulse transcranial magnetic stimulation of human parietal cortex. J Neurophysiol. 2006;96:3016–3027. doi: 10.1152/jn.00411.2006. [DOI] [PubMed] [Google Scholar]
- 38.Doron KW, Gazzaniga MS. Neuroimaging techniques offer new perspectives on callosal transfer and interhemispheric communication. Cortex. 2008;44:1023–1029. doi: 10.1016/j.cortex.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Ringo JL, Doty RW, Demeter S, Simard PY. Time is of the essence: A conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb Cortex. 1994;4:331–343. doi: 10.1093/cercor/4.4.331. [DOI] [PubMed] [Google Scholar]
- 40.Bloom JS, Hynd GW. The role of the corpus callosum in interhemispheric transfer of information: Excitation or inhibition? Neuropsychol Rev. 2005;15:59–71. doi: 10.1007/s11065-005-6252-y. [DOI] [PubMed] [Google Scholar]
- 41.Colby CL, Berman RA, Heiser LM, Saunders RC. Corollary discharge and spatial updating: when the brain is split, is space still unified? Prog Brain Res. 2005;149:187–205. doi: 10.1016/S0079-6123(05)49014-7. [DOI] [PubMed] [Google Scholar]
- 42.Corballis MC. Visual integration in the split brain. Neuropsychologia. 1995;33:937–959. doi: 10.1016/0028-3932(95)00032-x. [DOI] [PubMed] [Google Scholar]
- 43.Orban GA, et al. Mapping the parietal cortex of human and non-human primates. Neuropsychologia. 2006;44:2647–2667. doi: 10.1016/j.neuropsychologia.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Galletti C, Battaglini PP, Fattori P. The posterior parietal cortex in humans and monkeys. News Physiol Sci. 1997;12:166–171. [Google Scholar]
- 45.Husain M, Nachev P. Space and the parietal cortex. Trends Cogn Sci. 2007;11:30–36. doi: 10.1016/j.tics.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruce C, Desimone R, Gross CG. Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. J Neurophysiol. 1981;46:369–384. doi: 10.1152/jn.1981.46.2.369. [DOI] [PubMed] [Google Scholar]
- 47.Scalaidhe SP, Albright TD, Rodman HR, Gross CG. Effects of superior temporal polysensory area lesions on eye movements in the macaque monkey. J Neurophysiol. 1995;73:1–19. doi: 10.1152/jn.1995.73.1.1. [DOI] [PubMed] [Google Scholar]
- 48.Luh KE, Butter CM, Buchtel HA. Impairments in orienting to visual stimuli in monkeys following unilateral lesions of the superior sulcal polysensory cortex. Neuropsychologia. 1986;24:461–470. doi: 10.1016/0028-3932(86)90091-6. [DOI] [PubMed] [Google Scholar]
- 49.Watson RT, Valenstein E, Day A, Heilman KM. Posterior neocortical systems subserving awareness and neglect. Neglect associated with superior temporal sulcus but not area 7 lesions. Arch Neurol. 1994;51:1014–1021. doi: 10.1001/archneur.1994.00540220060015. [DOI] [PubMed] [Google Scholar]
- 50.Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8:1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- 51.Karnath HO, Ferber S, Himmelbach M. Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature. 2001;411:950–953. doi: 10.1038/35082075. [DOI] [PubMed] [Google Scholar]
- 52.Mort DJ, et al. The anatomy of visual neglect. Brain. 2003;126:1986–1997. doi: 10.1093/brain/awg200. [DOI] [PubMed] [Google Scholar]
- 53.Koyama M, et al. Functional magnetic resonance imaging of macaque monkeys performing visually guided saccade tasks: Comparison of cortical eye fields with humans. Neuron. 2004;41:795–807. doi: 10.1016/s0896-6273(04)00047-9. [DOI] [PubMed] [Google Scholar]
- 54.Nakahara K, Hayashi T, Konishi S, Miyashita Y. Functional MRI of macaque monkeys performing a cognitive set-shifting task. Science. 2002;295:1532–1536. doi: 10.1126/science.1067653. [DOI] [PubMed] [Google Scholar]
- 55.Sawamura H, Georgieva S, Vogels R, Vanduffel W, Orban GA. Using functional magnetic resonance imaging to assess adaptation and size invariance of shape processing by humans and monkeys. J Neurosci. 2005;25:4294–4306. doi: 10.1523/JNEUROSCI.0377-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsao DY, Moeller S, Freiwald WA. Comparing face patch systems in macaques and humans. Proc Natl Acad Sci USA. 2008;105:19514–19519. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsao DY, et al. Stereopsis activates V3A and caudal intraparietal areas in macaques and humans. Neuron. 2003;39:555–568. doi: 10.1016/s0896-6273(03)00459-8. [DOI] [PubMed] [Google Scholar]
- 58.Vanduffel W, et al. Extracting 3D from motion: Differences in human and monkey intraparietal cortex. Science. 2002;298:413–415. doi: 10.1126/science.1073574. [DOI] [PubMed] [Google Scholar]
- 59.Orban GA, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn Sci. 2004;8:315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Ford KA, Gati JS, Menon RS, Everling S. BOLD fMRI activation for anti-saccades in nonhuman primates. Neuroimage. 2009;45:470–476. doi: 10.1016/j.neuroimage.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 61.Maier A, et al. Divergence of fMRI and neural signals in V1 during perceptual suppression in the awake monkey. Nat Neurosci. 2008;11:1193–1200. doi: 10.1038/nn.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.