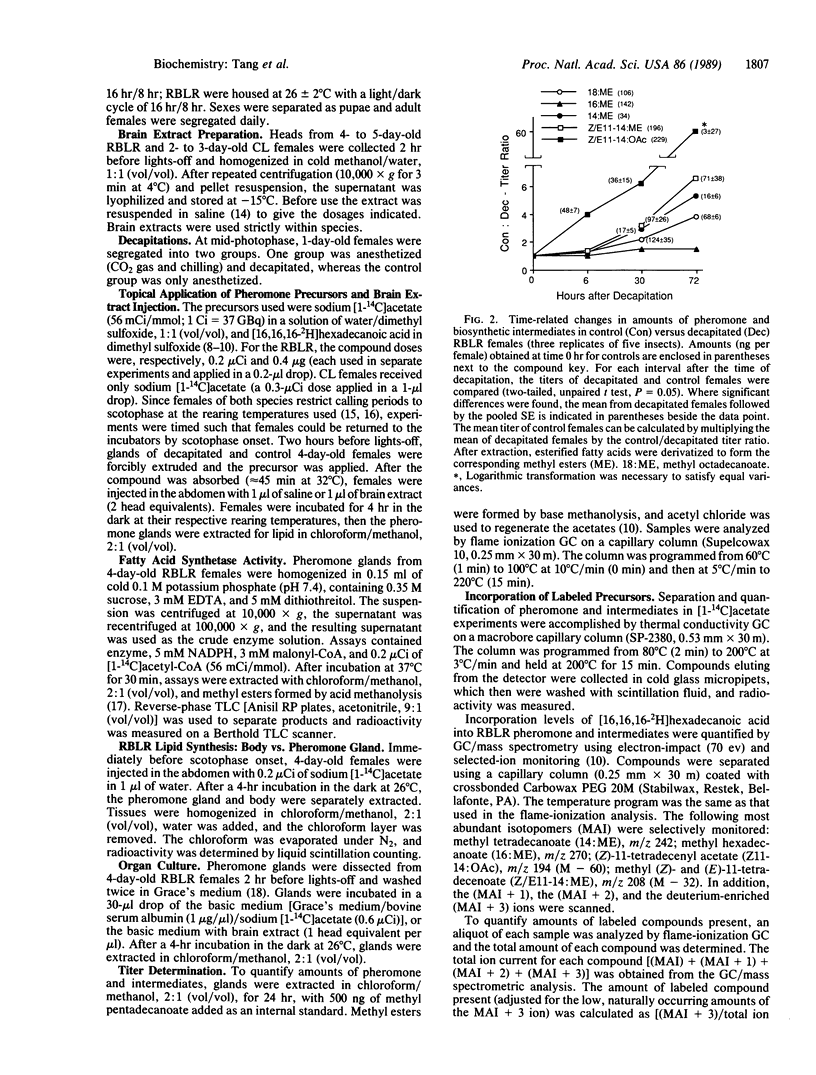
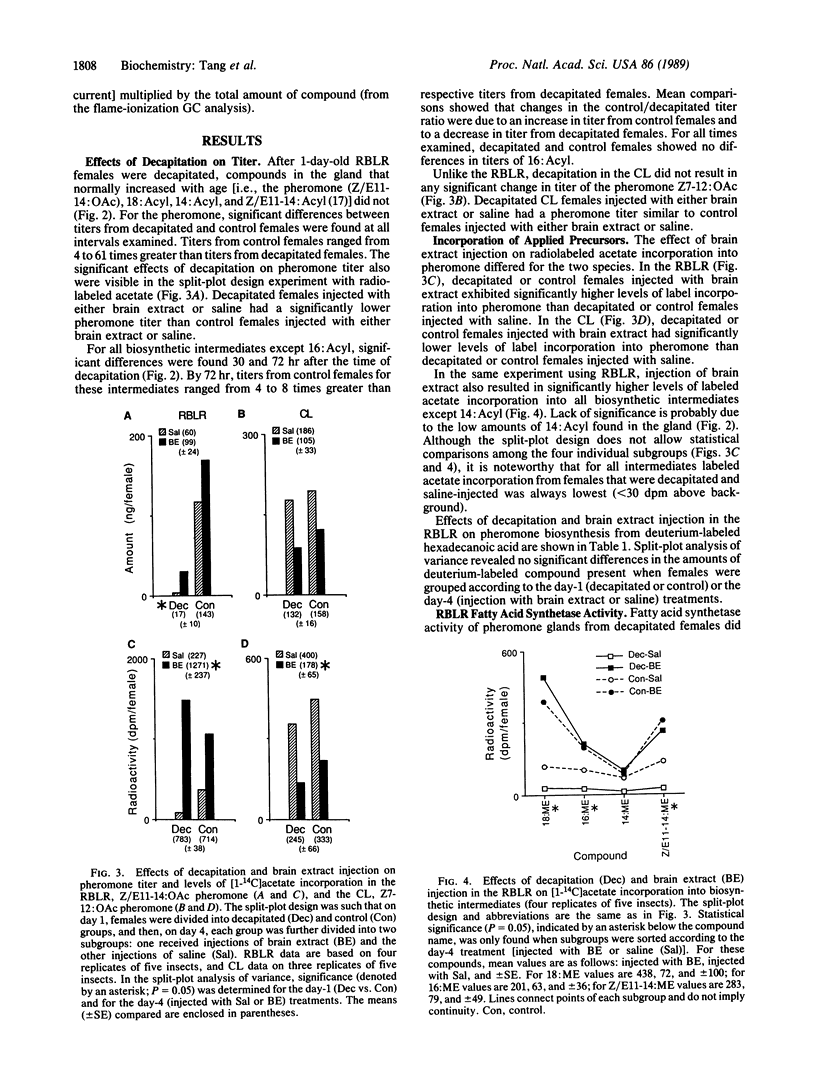
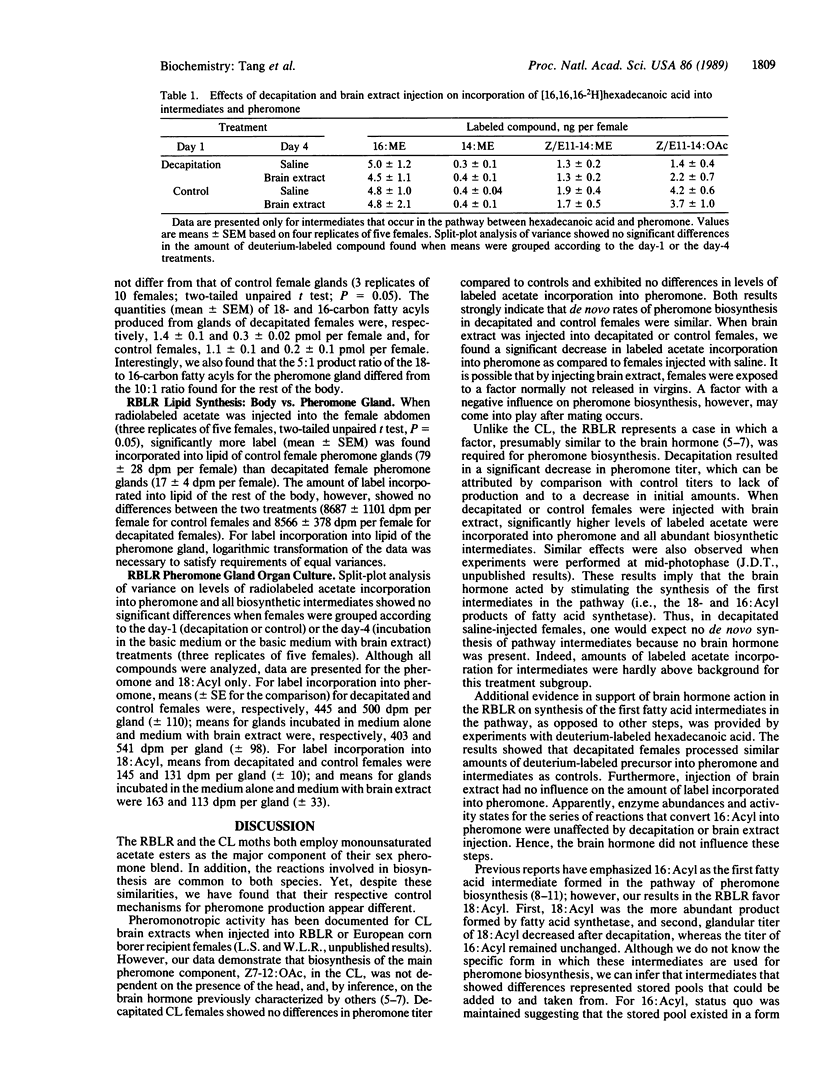
Abstract
Experiments were performed to characterize the action of a brain hormone on pheromone biosynthesis in female redbanded leafroller and cabbage looper moths. Results showed that the two species differed in their respective control mechanisms. In the cabbage looper, pheromone titer from decapitated females that received either saline or brain extract injections was not significantly different from control females, suggesting that pheromone biosynthesis was not dependent on the presence of the brain hormone. In contrast, with redbanded leafroller females, studies using radiolabeled acetate incorporation as well as incorporation of deuterium-labeled hexadecanoic acid showed that (i) the brain hormone was required for pheromone biosynthesis, (ii) the brain hormone regulated pheromone biosynthesis by activating synthesis of octadecanoyl and hexadecanoyl intermediates, and (iii) the brain hormone did not control other enzymes in the pathway. Regulation of fatty acid synthetase was unlikely since assays of the enzyme from decapitated and normal females showed no differences in the amount or distribution of the 18- and 16-carbon acyl end products. These results in conjunction with those from organ cultures of the pheromone gland suggest that the brain hormone acts by increasing the substrate supply for fatty acid synthesis.
Keywords: neuroendocrine regulation, pheromone biosynthesis, fatty acid synthesis, Argyrotaenia velutinana, Trichoplusia ni
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjostad L. B., Roelofs W. L. Sex Pheromone Biosynthesis in Trichoplusia ni: Key Steps Involve Delta-11 Desaturation and Chain-Shortening. Science. 1983 Jun 24;220(4604):1387–1389. doi: 10.1126/science.220.4604.1387. [DOI] [PubMed] [Google Scholar]
- Bjostad L. B., Roelofs W. L. Sex pheromone biosynthesis from radiolabeled fatty acids in the redbanded leafroller moth. J Biol Chem. 1981 Aug 10;256(15):7936–7940. [PubMed] [Google Scholar]
- Cardé R. T., Comeau A., Baker T. C., Roelofs W. L. Moth mating periodicity: temperature regulates the circadian gate. Experientia. 1975 Jan 15;31(1):46–48. doi: 10.1007/BF01924672. [DOI] [PubMed] [Google Scholar]
- Carrow G. M., Calabrese R. L., Williams C. M. Spontaneous and evoked release of prothoracicotropin from multiple neurohemal organs of the tobacco hornworm. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5866–5870. doi: 10.1073/pnas.78.9.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRACE T. D. Establishment of four strains of cells from insect tissues grown in vitro. Nature. 1962 Aug 25;195:788–789. doi: 10.1038/195788a0. [DOI] [PubMed] [Google Scholar]
- Raina A. K., Klun J. A. Brain factor control of sex pheromone production in the female corn earworm moth. Science. 1984 Aug 3;225(4661):531–533. doi: 10.1126/science.225.4661.531. [DOI] [PubMed] [Google Scholar]



