Abstract
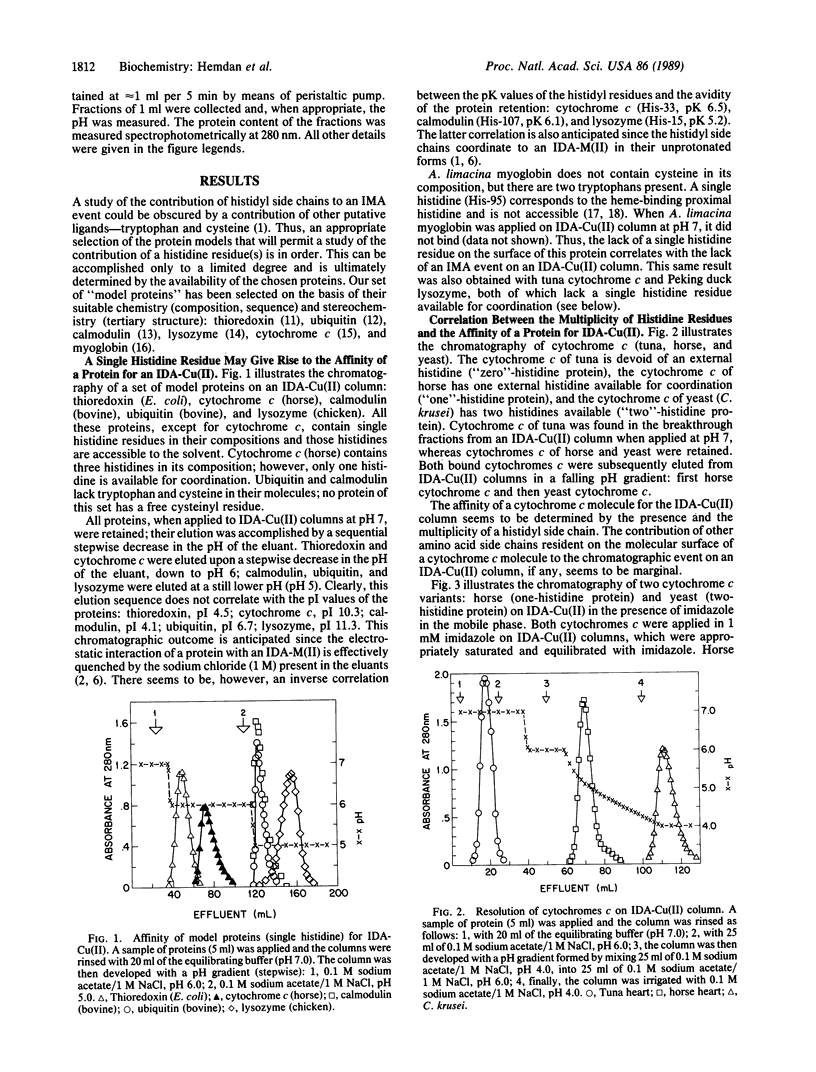
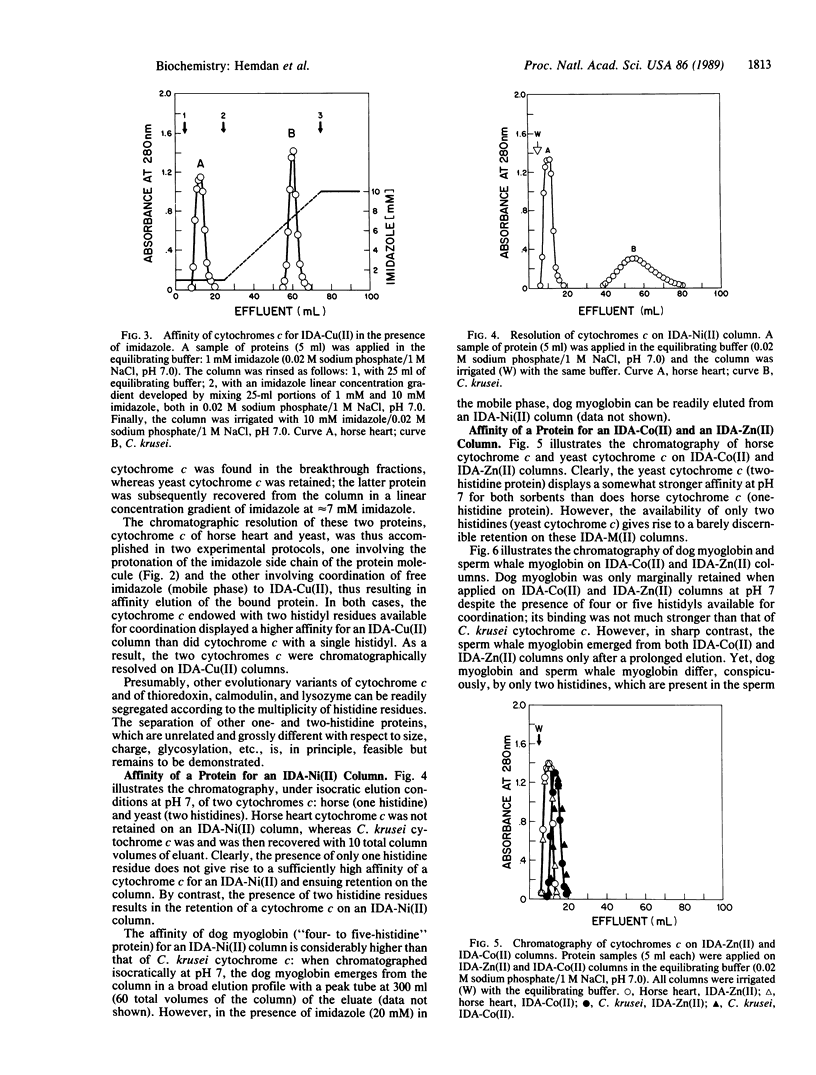
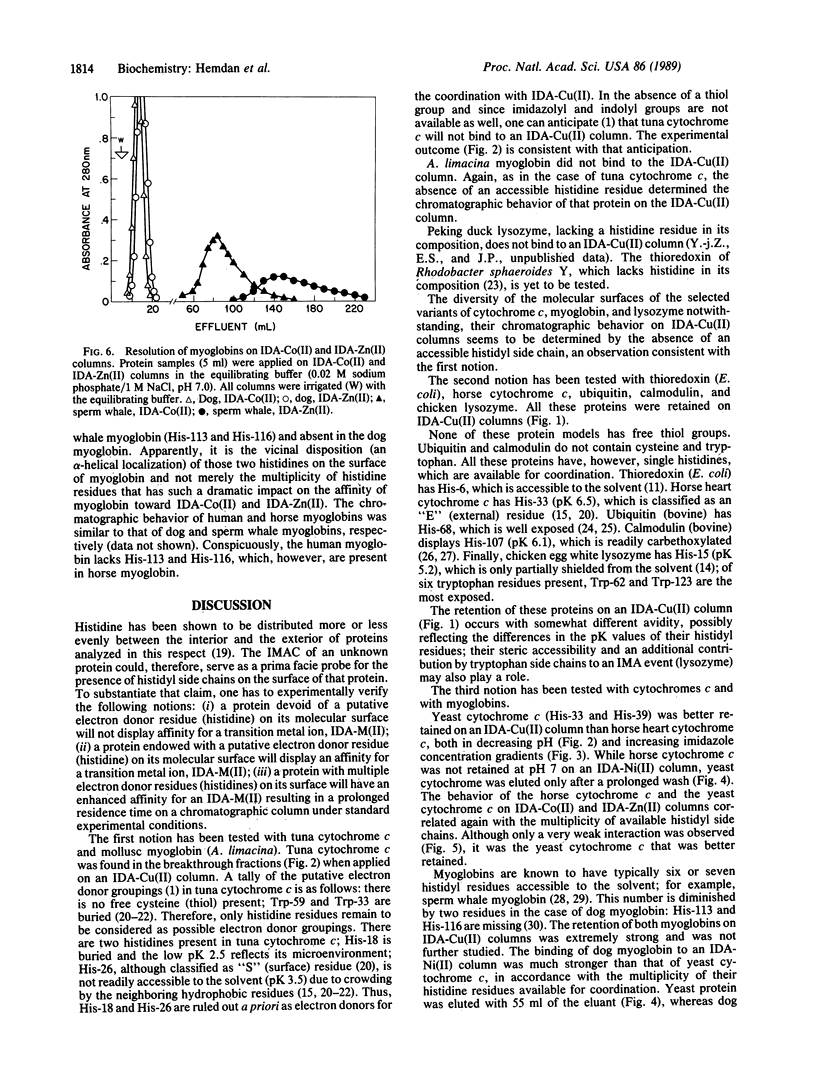
Immobilized metal ion affinity chromatography (IMAC) has been explored as a probe into the topography of histidyl residues of a protein molecule. An evaluation of the chromatographic behavior of selected model proteins--thioredoxin, ubiquitin, calmodulin, lysozyme, cytochrome c, and myoglobin on immobilized transition metal ions (Co2+, Ni2+, Cu2+, and Zn2+)--allows establishment of the following facets of the histidyl side chain distribution: (i) either interior or surface; (ii) when localized on the surface, accessible or unaccessible for coordination; (iii) single or multiple; (iv) when multiple, either distant or vicinal. Moreover, proteins displaying single histidyl side chains on their surfaces may, in some instances, be resolved by IMAC; apparently, the microenvironments of histidyl residues are sufficiently diverse to result in different affinities for the immobilized metal ions. IMAC, previously introduced as an approach to the fractionation of proteins, has become also, upon closer examination, a facile probe into the topography of histidyl residues. This is possible because of the inherent versatility of IMAC; an appropriate metal ion (M2+) can be selected to suit the analytical purpose and a particular chromatographic protocol can be applied (isocratic pH, falling pH, and imidazole elution).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L., Sulkowski E., Porath J. Facile resolution of alpha-fetoproteins and serum albumins by immobilized metal affinity chromatography. Cancer Res. 1987 Jul 15;47(14):3624–3626. [PubMed] [Google Scholar]
- Belew M., Yip T. T., Andersson L., Ehrnström R. High-performance analytical applications of immobilized metal ion affinity chromatography. Anal Biochem. 1987 Aug 1;164(2):457–465. doi: 10.1016/0003-2697(87)90519-7. [DOI] [PubMed] [Google Scholar]
- Berg J. M. Proposed structure for the zinc-binding domains from transcription factor IIIA and related proteins. Proc Natl Acad Sci U S A. 1988 Jan;85(1):99–102. doi: 10.1073/pnas.85.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement-Metral J. D., Holmgren A., Cambillau C., Jörnvall H., Eklund H., Thomas D., Lederer F. Amino acid sequence determination and three-dimensional modelling of thioredoxin from the photosynthetic bacterium Rhodobacter sphaeroides Y. Eur J Biochem. 1988 Mar 1;172(2):413–419. doi: 10.1111/j.1432-1033.1988.tb13902.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. S., Hayes M. B. Nuclear magnetic resonance titration curves of histidine ring protons. V. Comparative study of cytochrome c from three species and the assignment of individual proton resonances. J Biol Chem. 1974 Sep 10;249(17):5472–5477. [PubMed] [Google Scholar]
- Dumur V., Dautrevaux M., Han K. The covalent structure of dog myoglobin. Biochim Biophys Acta. 1976 Feb 20;420(2):376–386. doi: 10.1016/0005-2795(76)90329-9. [DOI] [PubMed] [Google Scholar]
- Figueroa A., Corradini C., Feibush B., Karger B. L. High-performance immobilized-metal affinity chromatography of proteins on iminodiacetic acid silica-based bonded phases. J Chromatogr. 1986 Dec 26;371:335–352. doi: 10.1016/s0021-9673(01)94717-x. [DOI] [PubMed] [Google Scholar]
- Gitlin G., Bayer E. A., Wilchek M. Studies on the biotin-binding site of avidin. Tryptophan residues involved in the active site. Biochem J. 1988 Feb 15;250(1):291–294. doi: 10.1042/bj2500291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N. M. Avidin. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–364. doi: 10.1146/annurev.bi.51.070182.002003. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Hugli T. E., Gurd F. R. Carboxymethylation of sperm whale myoglobin in the dissolved state. J Biol Chem. 1970 Apr 25;245(8):1939–1946. [PubMed] [Google Scholar]
- Klee C. B., Vanaman T. C. Calmodulin. Adv Protein Chem. 1982;35:213–321. doi: 10.1016/s0065-3233(08)60470-2. [DOI] [PubMed] [Google Scholar]
- Moore G. R., Williams R. J. The solution structures of tuna and horse cytochromes c. Eur J Biochem. 1980 Feb;103(3):533–541. doi: 10.1111/j.1432-1033.1980.tb05977.x. [DOI] [PubMed] [Google Scholar]
- Peters T., Jr, Blumenstock F. A. Copper-binding properties of bovine serum albumin and its amino-terminal peptide fragment. J Biol Chem. 1967 Apr 10;242(7):1574–1578. [PubMed] [Google Scholar]
- Porath J., Carlsson J., Olsson I., Belfrage G. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature. 1975 Dec 18;258(5536):598–599. doi: 10.1038/258598a0. [DOI] [PubMed] [Google Scholar]
- Porath J., Olin B. Immobilized metal ion affinity adsorption and immobilized metal ion affinity chromatography of biomaterials. Serum protein affinities for gel-immobilized iron and nickel ions. Biochemistry. 1983 Mar 29;22(7):1621–1630. doi: 10.1021/bi00276a015. [DOI] [PubMed] [Google Scholar]
- ROSSIFANELLI A., ANTONINI E., CAPUTO A. HEMOGLOBIN AND MYOGLOBIN. Adv Protein Chem. 1964;19:73–222. doi: 10.1016/s0065-3233(08)60189-8. [DOI] [PubMed] [Google Scholar]
- Seamon K. B. Calcium- and magnesium-dependent conformational states of calmodulin as determined by nuclear magnetic resonance. Biochemistry. 1980 Jan 8;19(1):207–215. doi: 10.1021/bi00542a031. [DOI] [PubMed] [Google Scholar]
- Smith M. C., Furman T. C., Ingolia T. D., Pidgeon C. Chelating peptide-immobilized metal ion affinity chromatography. A new concept in affinity chromatography for recombinant proteins. J Biol Chem. 1988 May 25;263(15):7211–7215. [PubMed] [Google Scholar]
- Takagi T., Iida S., Matsuoka A., Shikama K. Aplysia myoglobins with an unusual amino acid sequence. J Mol Biol. 1984 Dec 25;180(4):1179–1184. doi: 10.1016/0022-2836(84)90277-8. [DOI] [PubMed] [Google Scholar]
- Takano T., Kallai O. B., Swanson R., Dickerson R. E. The structure of ferrocytochrome c at 2.45 A resolution. J Biol Chem. 1973 Aug 10;248(15):5234–5255. [PubMed] [Google Scholar]
- Tentori L., Vivaldi G., Carta S., Marinucci M., Massa A., Antonini E., Brunori M. The amino acid sequence of myoglobin from the mollusc Aplysia limacina. Int J Pept Protein Res. 1973;5(4):187–200. doi: 10.1111/j.1399-3011.1973.tb03452.x. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar S., Bugg C. E., Cook W. J. Structure of ubiquitin refined at 1.8 A resolution. J Mol Biol. 1987 Apr 5;194(3):531–544. doi: 10.1016/0022-2836(87)90679-6. [DOI] [PubMed] [Google Scholar]
- Walsh M., Stevens F. C. Chemical modification studies on the Ca2+-dependent protein modulator of cyclic nucleotide phosphodiesterase. Biochemistry. 1977 Jun 14;16(12):2742–2749. doi: 10.1021/bi00631a024. [DOI] [PubMed] [Google Scholar]
- Weber P. L., Brown S. C., Mueller L. Sequential 1H NMR assignments and secondary structure identification of human ubiquitin. Biochemistry. 1987 Nov 17;26(23):7282–7290. doi: 10.1021/bi00397a013. [DOI] [PubMed] [Google Scholar]
- Wüthrich K., Shulman R. G., Yamane T., Wyluda B. J., Hugli T. E., Gurd F. R. High resolution proton magnetic resonance studies of cyanoferrimyoglobins and alkylated derivatives from different species. J Biol Chem. 1970 Apr 25;245(8):1947–1953. [PubMed] [Google Scholar]
- el Rassi Z., Horváth C. Metal chelate-interaction chromatography of proteins with iminodiacetic acid-bonded stationary phases on silica support. J Chromatogr. 1986 May 30;359:241–253. doi: 10.1016/0021-9673(86)80078-4. [DOI] [PubMed] [Google Scholar]



