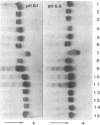
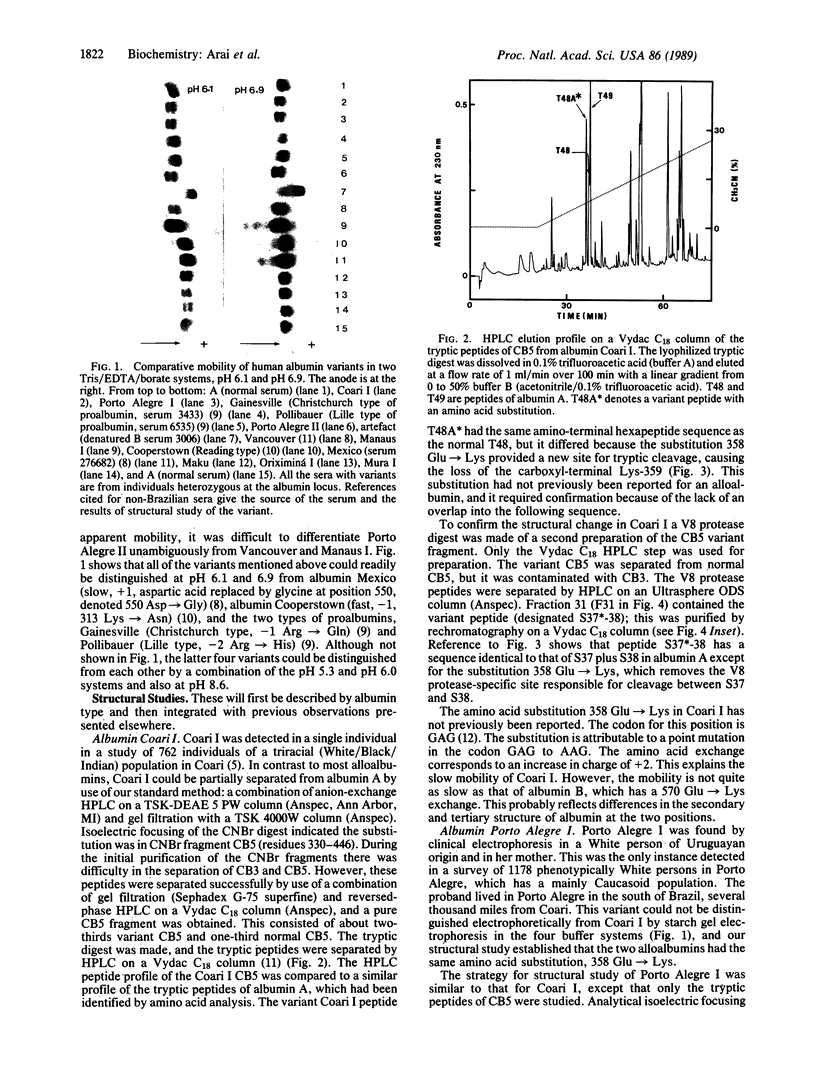
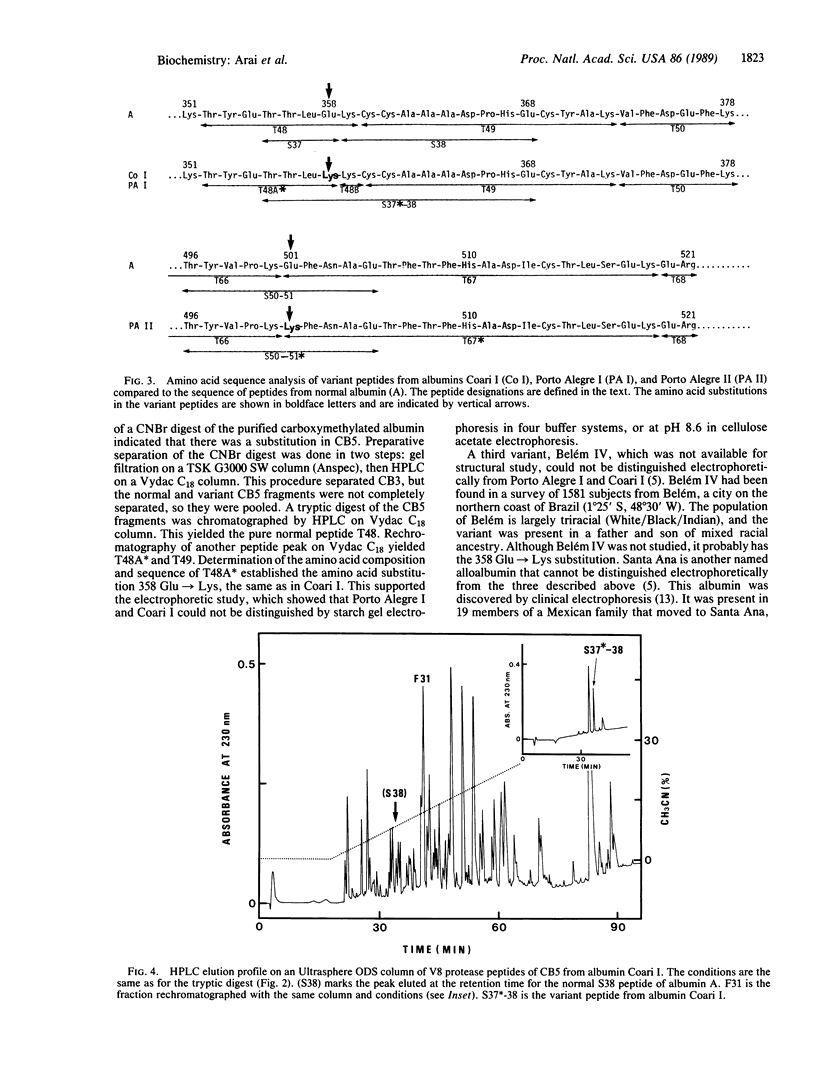
Abstract
Conventional horizontal starch-gel electrophoresis in four buffer systems and structural studies were performed on four albumin variants, and the findings were compared with similar previous data. Albumins Coari I and Porto Alegre I have a previously unreported amino acid substitution (glutamic acid replaced by lysine at position 358, denoted 358 Glu----Lys). The alteration in albumin Porto Alegre II (501 Glu----Lys) is the same as that found for three alloalbumins of Asiatic origin, designated Vancouver, Birmingham, and Adana. Albumin Oriximiná I has the same exchange as albumin Maku (541 Lys----Glu). Some of these findings can be explained only by the occurrence of independent mutations at the same site in the albumin gene. They also point to a third cluster of mutations in that gene, indicating hypermutability in some of its segments.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Ishioka N., Huss K., Madison J., Putnam F. W. Identical structural changes in inherited albumin variants from different populations. Proc Natl Acad Sci U S A. 1989 Jan;86(2):434–438. doi: 10.1073/pnas.86.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan S. O. The molecular abnormality of albumin Parklands: 365 Asp----His. Biochim Biophys Acta. 1985 Aug 23;830(3):320–324. doi: 10.1016/0167-4838(85)90289-4. [DOI] [PubMed] [Google Scholar]
- Helena M., Franco L. P., Salzano F. M. A comparative study of albumin variants found in Brazil. Hum Hered. 1985;35(1):34–38. doi: 10.1159/000153512. [DOI] [PubMed] [Google Scholar]
- Huss K., Madison J., Ishioka N., Takahashi N., Arai K., Putnam F. W. The same substitution, glutamic acid----lysine at position 501, occurs in three alloalbumins of Asiatic origin: albumins Vancouver, Birmingham, and Adana. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6692–6696. doi: 10.1073/pnas.85.18.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss K., Putnam F. W., Takahashi N., Takahashi Y., Weaver G. A., Peters T., Jr Albumin Cooperstown: a serum albumin variant with the same (313 Lys----Asn) mutation found in albumins in Italy and New Zealand. Clin Chem. 1988 Jan;34(1):183–187. [PubMed] [Google Scholar]
- Kueppers F., Holland P. V., Weitkamp L. R. Albumin Santa Ana: a new inherited variant. Hum Hered. 1969;19(4):378–384. doi: 10.1159/000152242. [DOI] [PubMed] [Google Scholar]
- Minghetti P. P., Ruffner D. E., Kuang W. J., Dennison O. E., Hawkins J. W., Beattie W. G., Dugaiczyk A. Molecular structure of the human albumin gene is revealed by nucleotide sequence within q11-22 of chromosome 4. J Biol Chem. 1986 May 25;261(15):6747–6757. [PubMed] [Google Scholar]
- Neel J. V. Rare variants, private polymorphisms, and locus heterozygosity in Amerindian populations. Am J Hum Genet. 1978 Sep;30(5):465–490. [PMC free article] [PubMed] [Google Scholar]
- Neel J. V., Satoh C., Goriki K., Asakawa J., Fujita M., Takahashi N., Kageoka T., Hazama R. Search for mutations altering protein charge and/or function in children of atomic bomb survivors: final report. Am J Hum Genet. 1988 May;42(5):663–676. [PMC free article] [PubMed] [Google Scholar]
- Neel J. V., Satoh C., Hamilton H. B., Otake M., Goriki K., Kageoka T., Fujita M., Neriishi S., Asakawa J. Search for mutations affecting protein structure in children of atomic bomb survivors: preliminary report. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4221–4225. doi: 10.1073/pnas.77.7.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzano F. M., Helena M., Franco L. P., Ayres M. Alloalbuminemia in two Brazilian populations: a possible new variant. Am J Hum Genet. 1974 Jan;26(1):54–58. [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Blumberg B. S., Putnam F. W. Amino acid substitutions in genetic variants of human serum albumin and in sequences inferred from molecular cloning. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4413–4417. doi: 10.1073/pnas.84.13.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Ishioka N., Blumberg B. S., Putnam F. W. Application of an automated tandem high-performance liquid chromatographic system to peptide mapping of genetic variants of human serum albumin. J Chromatogr. 1986 May 30;359:181–191. doi: 10.1016/0021-9673(86)80072-3. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Isobe T., Putnam F. W., Fujita M., Satoh C., Neel J. V. Amino acid substitutions in inherited albumin variants from Amerindian and Japanese populations. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8001–8005. doi: 10.1073/pnas.84.22.8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Putnam F. W. Structural changes and metal binding by proalbumins and other amino-terminal genetic variants of human serum albumin. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7403–7407. doi: 10.1073/pnas.84.21.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitkamp L. R., Chagnon N. A. Albumin Máku: a new variant of human serum albumin. Nature. 1968 Feb 24;217(5130):759–760. doi: 10.1038/217759a0. [DOI] [PubMed] [Google Scholar]
- Weitkamp L. R., Salzano F. M., Neel J. V., Porta F., Geerdink R. A., Tárnoky A. L. Human serum albumin: twenty-three genetic variants and their population distribution. Ann Hum Genet. 1973 Apr;36(4):381–392. doi: 10.1111/j.1469-1809.1973.tb00602.x. [DOI] [PubMed] [Google Scholar]