Abstract
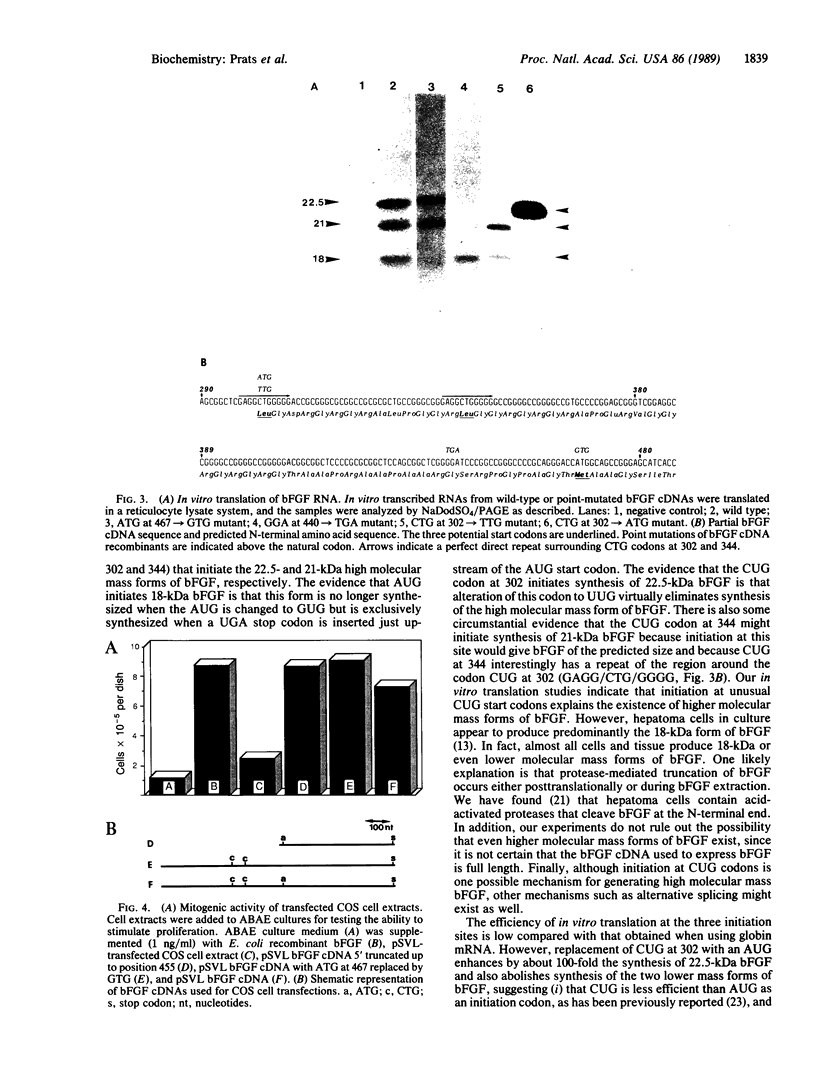
A 6.75-kilobase human hepatoma-derived basic fibroblast growth factor (bFGF) cDNA was cloned and sequenced. An amino-terminal sequence generated from a purified hepatoma bFGF was found to correspond to the nucleotide sequence and to begin 8 amino acids upstream from the putative methionine start codon thought to initiate a 154-amino acid bFGF translation product. This sequence suggests that a form of bFGF of at least 163 amino acids exists. The hepatoma cDNA was transcribed in vitro into RNA; in vitro translation of this RNA generated three forms of bFGF with molecular masses of 18, 21, and 22.5 kDa. By use of in vitro mutagenesis, it was found that the 22.5-kDa bFGF and possibly the 21-kDa form were initiated with CUG start codons. The 18-kDa bFGF was initiated with an AUG codon. By transfecting into COS cells human hepatoma bFGF cDNA and a construct from which the AUG initiator was eliminated, it was found that the higher molecular mass forms of bFGF were as biologically active as the 18-kDa form.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Abraham J. A., Whang J. L., Tumolo A., Mergia A., Friedman J., Gospodarowicz D., Fiddes J. C. Human basic fibroblast growth factor: nucleotide sequence and genomic organization. EMBO J. 1986 Oct;5(10):2523–2528. doi: 10.1002/j.1460-2075.1986.tb04530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra S. P., Rose J. A., Hardy M., Baroudy B. M., Anderson C. W. Direct mapping of adeno-associated virus capsid proteins B and C: a possible ACG initiation codon. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7919–7923. doi: 10.1073/pnas.82.23.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Clements J. M., Laz T. M., Sherman F. Efficiency of translation initiation by non-AUG codons in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Oct;8(10):4533–4536. doi: 10.1128/mcb.8.10.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J., Kolakofsky D. Ribosomal initiation from an ACG codon in the Sendai virus P/C mRNA. EMBO J. 1988 Jan;7(1):245–251. doi: 10.1002/j.1460-2075.1988.tb02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Bialecki H., Thakral T. K. The angiogenic activity of the fibroblast and epidermal growth factor. Exp Eye Res. 1979 May;28(5):501–514. doi: 10.1016/0014-4835(79)90038-1. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Neufeld G., Schweigerer L. Fibroblast growth factor. Mol Cell Endocrinol. 1986 Aug;46(3):187–204. doi: 10.1016/0303-7207(86)90001-8. [DOI] [PubMed] [Google Scholar]
- Hann S. R., King M. W., Bentley D. L., Anderson C. W., Eisenman R. N. A non-AUG translational initiation in c-myc exon 1 generates an N-terminally distinct protein whose synthesis is disrupted in Burkitt's lymphomas. Cell. 1988 Jan 29;52(2):185–195. doi: 10.1016/0092-8674(88)90507-7. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Sasse J., Sullivan R., Smith J. A. Human tumor cells synthesize an endothelial cell growth factor that is structurally related to basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2448–2452. doi: 10.1073/pnas.83.8.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M., Smith S., Sullivan R., Shing Y., Davidson S., Smith J. A., Sasse J. Multiple forms of basic fibroblast growth factor: amino-terminal cleavages by tumor cell- and brain cell-derived acid proteinases. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1839–1843. doi: 10.1073/pnas.84.7.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Bifunctional messenger RNAs in eukaryotes. Cell. 1986 Nov 21;47(4):481–483. doi: 10.1016/0092-8674(86)90609-4. [DOI] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Kurokawa T., Sasada R., Iwane M., Igarashi K. Cloning and expression of cDNA encoding human basic fibroblast growth factor. FEBS Lett. 1987 Mar 9;213(1):189–194. doi: 10.1016/0014-5793(87)81489-8. [DOI] [PubMed] [Google Scholar]
- Montesano R., Vassalli J. D., Baird A., Guillemin R., Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7297–7301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D., Joseph-Silverstein J., Manejias R., Rifkin D. B. Mr 25,000 heparin-binding protein from guinea pig brain is a high molecular weight form of basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5778–5782. doi: 10.1073/pnas.84.16.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A., Brewer M. T., Thompson R. C., Moscatelli D., Presta M., Rifkin D. B. A form of human basic fibroblast growth factor with an extended amino terminus. Biochem Biophys Res Commun. 1987 Apr 29;144(2):543–550. doi: 10.1016/s0006-291x(87)80001-3. [DOI] [PubMed] [Google Scholar]
- Story M. T., Esch F., Shimasaki S., Sasse J., Jacobs S. C., Lawson R. K. Amino-terminal sequence of a large form of basic fibroblast growth factor isolated from human benign prostatic hyperplastic tissue. Biochem Biophys Res Commun. 1987 Feb 13;142(3):702–709. doi: 10.1016/0006-291x(87)91471-9. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis using M13-derived vectors: an efficient and general procedure for the production of point mutations in any fragment of DNA. Nucleic Acids Res. 1982 Oct 25;10(20):6487–6500. doi: 10.1093/nar/10.20.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]