Abstract
Objectives To estimate the therapeutic and adverse effects of addition of inhaled anticholinergics to β2 agonists in acute asthma in children and adolescents.
Design Systematic review of randomised controlled trials of children and adolescents taking β2 agonists for acute asthma with or without the addition of inhaled anticholinergics.
Main outcome measures Hospital admission, pulmonary function tests, number of nebulised treatments, relapse, and adverse effects.
Results Of 37 identified trials, 10 were relevant and six of these were of high quality. The addition of a single dose of anticholinergic to β2 agonist did not reduce hospital admission (relative risk 0.93, 95% confidence interval 0.65 to 1.32). However, significant group differences in lung function supporting the combination treatment were observed 60 minutes (standardised mean difference −0.57, −0.93 to −0.21) and 120 minutes (−0.53, −0.90 to −0.17) after the dose of anticholinergic. In contrast, the addition of multiple doses of anticholinergics to β2 agonists, mainly in children and adolescents with severe exacerbations, reduced the risk of hospital admission by 30% (relative risk 0.72, 0.53 to 0.99). Eleven (95% confidence interval 5 to 250) children would need to be treated to avoid one admission. A parallel improvement in lung function (standardised mean difference −0.66, −0.95 to −0.37) was noted 60 minutes after the last combined inhalation. In the single study where anticholinergics were systematically added to every β2 agonist inhalation, irrespective of asthma severity, no group differences were observed for the few available outcomes. There was no increase in the amount of nausea, vomiting, or tremor in patients treated with anticholinergics.
Conclusions Adding multiple doses of anticholinergics to β2 agonists seems safe, improves lung function, and may avoid hospital admission in 1 of 11 such treated patients. Although multiple doses should be preferred to single doses of anticholinergics, the available evidence only supports their use in school aged children and adolescents with severe asthma exacerbation.
Key messages
The addition of multiple doses of anticholinergics to β2 agonist inhalations seems indicated in the initial management of children and adolescents with severe exacerbations of asthma (⩽55% of predicted FEV1)
For the larger group of children and adolescents with mild to moderate asthma exacerbations, there is no apparent benefit from adding a dose of anticholinergics to β2 agonists
Little evidence exists to support the systematic addition of anticholinergics to every β2 agonist inhalation, irrespective of patients’ disease severity
Introduction
The initial management of acute asthma exacerbations in children and adolescents focuses on the rapid relief of bronchospasm with inhaled or nebulised bronchodilators.1–6 Young people who are not responsive to bronchodilators require the addition of glucocorticoids.7,8 β2 agonists are the most effective of the bronchodilators owing to their rapid onset of action and the extent of achieved bronchodilation.9,10 Anticholinergic agents, such as ipratropium bromide and atropine sulphate, have a slower onset of action and weaker bronchodilating effect than β2 agonists but may relieve cholinergic bronchomotor tone and decrease mucosal oedema and secretions.11–13 Thus the combination of inhaled anticholinergics with β2 agonists may yield enhanced and prolonged bronchodilation.
Several randomised controlled trials have examined the efficacy of the addition of anticholinergics to β2 agonists for treating acute asthma in children and adolescents.14–17 Conflicting results from these trials were attributed to differences in the severity of the asthma, intensity (number of doses) of anticholinergic treatment, cointervention with glucocorticoids, and study power. A systematic review of randomised controlled trials published up to 1992 concluded that there was a 12% greater improvement in percentage predicted forced expiratory volume in 1 second (FEV1) with anticholinergic use but no reduction in hospital admission.18 As several new trials have been completed since 1992, the conclusions of the original review may need revision.19–21 The pooling of a larger number of randomised controlled trials may provide not only greater power for detecting group differences in hospital admission but also better insight into the influence of patients’ characteristics and treatment modalities on efficacy of treatment.22–24
We aimed to determine whether the addition of inhaled anticholinergics to β2 agonists leads to clinical improvement and affects the incidence of adverse effects in children and adolescents with acute asthma exacerbations. We also wanted to determine whether the intensity of treatment, severity of the exacer- bation, and concomitant use of glucocorticoids influenced the extent of the effect attributable to inhaled anticholinergics.
Subjects and methods
Literature search and identification of trials
We used five search strategies to identify potentially relevant trials. Firstly, we searched Medline (1966-97), Embase (1980-97), and Cinahl (1982-97) databases using the following MeSH, full text, and keyword terms: [asthma, wheez*, or respiratory sounds] and [random*, trial*, placebo*, comparative study, controlled study, double-blind, single-blind] and [child* or infan* or adolescen* or pediatr* or paediatr*] and [emergenc* or acute*], and [ipratropium* or anticholinerg* or atropin*]. Secondly, we identified randomised controlled trials by hand searching medical journals identified through the Cochrane Collaboration, using the same terms. Thirdly, we checked bibliographies of all trials and review articles identified from the databases and medical journals to determine potentially relevant citations. Fourthly, we made inquiries to Boehringer Ingelheim, producer of ipratropium bromide, regarding other published or unpublished trials conducted worldwide and supported by the company or its subsidiaries. Finally, we contacted trialists working on childhood and adolescent asthma to identify potentially relevant trials.
Study selection
Criteria for considering trials included: (a) randomised controlled clinical trials conducted in an emergency department setting; (b) unprovoked asthma exacerbation in children aged 18 months to 17 years; (c) single or multiple doses of inhaled short acting anticholinergics combined with β2 agonists compared with β2 agonists alone; (d) admission to hospital as primary outcome, and change in pulmonary function tests, need for additional bronchodilator inhalations, relapse rate, and adverse effects as secondary outcome.
Each citation (title and abstract) identified through one of the search strategies was reviewed by one person and classed as a definite, possible, or clearly not randomised controlled trial. The complete article of all citations identified as definite or possible randomised controlled trials was obtained, irrespective of language of publication. These were assessed independently by two reviewers to determine if the study met the inclusion criteria and, if so, to evaluate methodological quality and extract data. Reviewers were masked to the authors’ names and affiliations, name of journal, date of publication, and sources of funding for the study.25 Disagreement between the reviewers was settled by consensus. When necessary, to verify study methodology and extracted information and to provide additional data, the reviewers contacted the first author, or all coauthors in cases of non-response, on at least three occasions by post, fax, or email.
Methodological quality
The methodological quality of each trial was assessed using Jadad’s instrument.25 This instrument evaluates the quality of randomisation and blinding and reasons for withdrawal on a score of 0 (worst) to 5 (best).
Statistical analyses
Treatment effects for dichotomous outcomes were reported as pooled relative risks with the fixed effect model26 or, in case of heterogeneity, the random effect model.27 The Dersimonian and Laird model was used to estimate the pooled absolute risk reduction and therefore estimate the number of patients needed to treat to prevent the adverse outcome of interest.27 For continuous outcomes, the weighted mean difference or the standardised weighted mean difference was used to estimate the pooled effect size.28 The weighted mean difference was reported for pulmonary function tests using the same unit of measure: the weighted sum of each trial’s difference between the mean of the experimental and the control group, reported on the same scale as the pulmonary function test.28 The standardised mean difference, reported in SD units, was used when the change in the same pulmonary function test was reported in different units (change in percentage predicted FEV1 and percentage change in FEV1): the weighted sum of each trial’s group mean difference divided by its pooled SD.29 The contribution of each trial to the pooled estimate is proportional to the inverse of the variance.30 Homogeneity of effect sizes were tested with the Dersimonian and Laird method with P=0.10 as the cut off point for significance27; heterogeneity was reported whenever identified. To detect possible biases, funnel plot symmetry was examined for trials contributing data to hospital admission.31 The pooled effect sizes are presented with the 95% confidence interval.
Five factors were a priori believed to potentially influence the extent or direction of the treatment response: (a) the intensity of anticholinergic treatment, (b) cointervention with glucocorticoids, (c) severity of exacerbation, (d) methodological quality, and (e) publication status. Randomised controlled trials were therefore grouped according to the intensity of anticholinergic protocol and, within each group, stratified on the presence or absence of systemic glucocorticoids. Whenever reported, the baseline percentage predicted FEV1 and hospital admission rate in the control groups were recorded as indicators of severity and examined for their potential interaction with treatment effect. Sensitivity analyses were performed to examine the effect on results of excluding unpublished trials and those with poor methodological quality. The meta-analysis was performed with Metaview 3.0.1 (Cochrane Review Manager, Cochrane Collaboration, Oxford).
Results
A total of 37 studies were reviewed in full text for possible inclusion; 34 were identified from the literature search and bibliographies, and three were identified by contact with trialists. Twenty seven studies were excluded for the following reasons: studies dealt with infants (n=1), adults (n=5), hospitalised patients (n=4), or non-acute asthma (n=12); non-randomised controlled trials (n=4); and anticholinergics alone were studied (n=1). A total of 10 randomised controlled trials were selected for inclusion.
Trials were grouped according to the intensity of the anticholinergic protocol: trials testing the addition of a single dose of anticholinergic to β2 agonist inhalations were grouped under single dose protocol, trials testing multiple doses in a predetermined fixed regimen were grouped under multiple dose fixed protocol, while a single trial testing the systematic addition of anticholinergics to every β2 agonist inhalation, leaving the number of inhalations determined by the patient’s needs, was named multiple dose flexible protocol (table 1). One trial, which tested two protocols, contributed to the first two strata.19 With one exception,14 the anticholinergic agent used was ipratropium bromide. Cointervention with glucocorticoids was infrequent even in trials focusing on children and adolescents with severe exacerbations. Most trials considered children and adolescents with severe exacerbations, one study considered children and adolescents with mild to moderate asthma,21 and the remainder failed to describe the baseline severity of their patients. Two of the largest trials, both of which reported no beneficial effect from the addition of anticholinergics to β2 agonists, have not yet been published in full text (R Peterson, personal communication).21 Methodology of six of the 10 trials was confirmed by the authors (Peterson, 1996)14,15,19–21; most were of high quality (Jadad’s quality score=5) (table 2). The main outcome variable was spirometric measurements in seven trials, all in school aged children; respiratory resistance measured by forced oscillation was used in the trial of children and adolescents aged 3-17 years21; clinical scores were used for the youngest patients in two trials.14,15 Not all trials considered each outcome. The reporting of adverse and side effects was variable. Adverse effects such as hypertension or tachycardia were reported so infrequently that they could not be considered in this review. Side effects such as nausea, vomiting, and tremor, which may interfere with patients’ compliance, were extracted whenever reported.
Table 1.
Characteristics of trials included in review. Age in years unless stated otherwise
| Trial | No of patients (age) | Baseline severity* | β2 agonist protocol
|
Anticholinergic protocol
|
Systemic steroids | Admission rate (%)† |
Reported outcomes
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Frequency | Drug | Frequency | Admission | PFT | ||||||
| Single dose protocol | |||||||||||
| Ducharme et al (unpublished) | 298 (3-17) | Mild-moderate | S | Every 30 minutes × 2-6 | IB | × 1 | ± | 20 | + | + | |
| Schuh et al19 | 80 (5-17) | <50% FEV1 | S | Every 20 minutes × 3 | IB | × 1 | − | 46 | + | + | |
| Beck et al32 | 25 (6-17) | <50% FEV1 | S | Every 20 minutes × 7 | IB | × 1 | NR | NR | − | + | |
| Phanichyakam et al33 | 20 (4-15) | NR | T | × 1 | IB | × 1 | NR | NR | − | + | |
| Cook et al15 | 30 (18 months-12) | Moderately severe | F | × 1 | IB | × 1 | − | NR | − | − | |
| Multiple dose (strict) protocol | |||||||||||
| Qureshi et al20 | 90 (6-18) | <50% FEV1 | S | Every 30 minutes × 3 | IB | Every 60 minutes × 2 | + | 31 | + | + | |
| Reisman et al16 | 24 (5-15) | ⩽55% FEV1 | S | Every 20 minutes × 7 | IB | Every 40 minutes × 3 | − | 23 | + | − | |
| Schuh et al19 | 81 (5-17) | <50% FEV1 | S | Every 20 minutes × 3 | IB | Every 20 minutes × 3 | − | 46 | + | + | |
| Watson et al17 | 31 (6-17) | 30-70% FEV1 | F | Every 60 minutes × 2 | IB | Every 60 minutes × 2 | − | NR | + | + | |
| Peterson et al (unpublished) | 163 (5-12) | <70% FEV1 | S | Every 15 minutes × 2-3 | IB | Every 45 minutes × 2-3 | ± | 30 | + | + | |
| Multiple dose (flexible) protocol | |||||||||||
| Guill et al14 | 31 (13 months-12) | NR | M | Every 20-30 minutes × 1-3 | AS | Every 20-30 minutes × 1-3 | − | NR | − | − | |
PFT=pulmonary function test; S=salbutamol; T=terbutaline; F=fenoterol; M=metaproterenol; IB=ipratropium bromide; AS=atropine sulphate, NR=not reported; ±=variable; +=yes; −=no.
FEV1 is percentage predicted forced expiratory volume in 1 second.
In control group.
Table 2.
Methodological quality of included trials. Numbers represent quality score values
|
Randomisation*
|
Blinding
|
Withdrawal/dropout
|
Quality score | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Method not described | Appropriate method | No blinding reported | Double blind; means not reported | Double blind; means appropriate | Not described | Described | ||||
| Single dose protocol | ||||||||||
| Ducharme et al (unpublished) | — | 2 | — | — | 2 | — | 1 | 5† | ||
| Schuh et al19 | — | 2 | — | — | 2 | — | 1 | 5† | ||
| Beck et al32 | 1 | — | — | — | 2 | 0 | — | 3 | ||
| Phanichyakam et al33 | 1 | — | 0 | — | — | 0 | — | 1 | ||
| Cook et al15 | — | 2 | — | — | 2 | — | 1 | 5† | ||
| Multiple dose strict protocol | ||||||||||
| Qureshi et al20 | — | 2 | — | — | 2 | — | 1 | 5† | ||
| Reisman et al16 | 1 | — | — | — | 2 | 0 | — | 3 | ||
| Schuh et al19 | — | 2 | — | — | 2 | — | 1 | 5† | ||
| Watson et al17 | 1 | — | — | 1 | — | — | 1 | 3 | ||
| Peterson et al (unpublished) | — | 2 | — | — | 2 | — | 1 | 5† | ||
| Multiple dose flexible protocol | ||||||||||
| Guill et al14 | — | 2 | — | — | 2 | — | 1 | 5† | ||
All trials scored 0 for non-reported randomisation.
Methodology confirmed by authors.
Single dose protocols
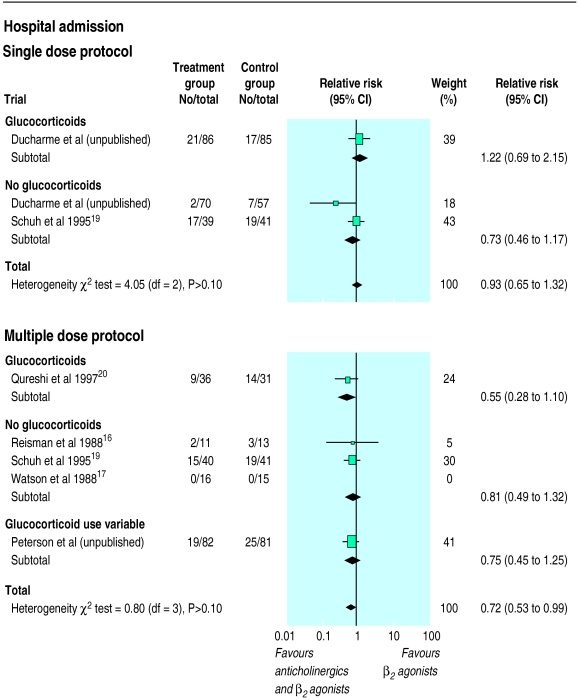
Five trials totalling 453 patients examined the efficacy of adding a single dose of 250 μg ipratropium bromide to β2 agonists. No reduction in hospital admission was observed when pooling the two trials reporting this outcome (relative risk 0.93, 95% confidence interval 0.65 to 1.32) (fig 1). As these two trials were of high methodological quality, this factor did not influence the results. Exclusion of the unpublished study, which focused exclusively on children and adolescents with mild to moderate exacerbations, did not alter the conclusion. There was no evidence of systematic bias identified by the measure of funnel plot asymmetry (intercept 1.5, −25 to 30).
Figure 1.
Pooled relative risk of hospital admission comparing addition of single and multiple doses of ipratropium bromide to β2 agonists in trials reporting data relative to hospital admission: one trial (Watson et al) not included in overall estimate of multiple dose protocol as absence of event in both groups prevented estimation of relative risk. Trials stratified according to concomitant administration of systemic corticosteroids. Width of horizontal line represents 95% confidence interval around point estimate (black square). Size of point estimate represents relative weight (% weight) of each trial in the pooled summary estimate (diamond). Vertical line is line of no effect (relative risk 1.0)
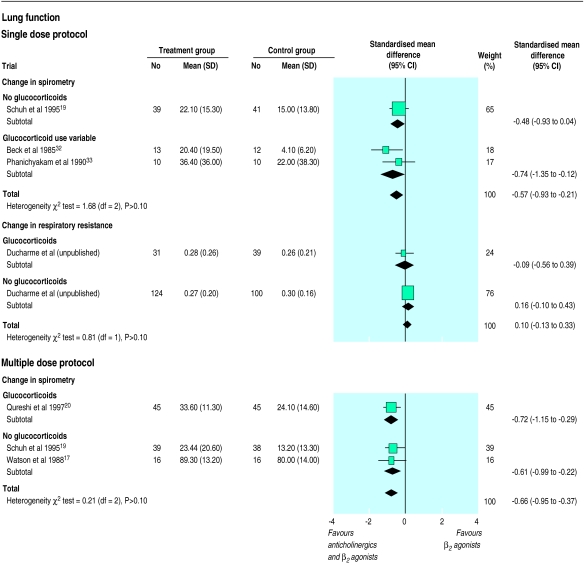
Four trials examined response to treatment using pulmonary function tests. In the two trials reporting the percentage change in FEV1, a difference of 16.10% (5.54% to 26.66%) between group means (weighted mean difference) was documented at 60 minutes and of 17.49% (4.46% to 30.53%) at 120 minutes after the inhalation of anticholinergics, both favouring anticholinergic use.32,33 When combining the three trials reporting change in lung function, either as change in percentage predicted FEV1 or percentage change from baseline FEV1, significant improvement, equivalent to half a SD in change, was still apparent at 60 minutes (standardised mean difference −0.57, −0.93 to −0.21) and at 120 minutes (−0.53, −0.90 to −0.17) after the dose of anticholinergics (fig 2).19,32,33 In the single trial examining the intervention in children with mild to moderate exacerbations, the absence of group difference observed at 60 minutes (0.10, −0.13 to 0.33) and at 120 minutes (0.02, −0.36 to 0.40) confidently ruled out any important change in respiratory resistance due to treatment (fig 2).21 No reduction in relapse rate (1.17, 0.56 to 2.45) was observed. The addition of a single dose of anticholinergics was not associated with increased vomiting (0.66, 0.30 to 1.44) or tremor (0.98, 0.88 to 1.10), but there was an apparent reduction in nausea (0.55, 0.33 to 0.91).
Figure 2.
Pooled standardised mean difference of change in lung function 60 minutes after addition of unique (single dose protocol) or last (multiple dose protocol) anticholinergic treatment to β2 agonists. Standardised mean difference, displayed on SD unit, represents difference in mean between groups relative to pooled SD of treatment and control groups. Studies reporting change in spirometry as percentage change in FEV1 or change in percentage predicted FEV1 were pooled while the trial reporting change in respiratory resistance is reported separately. Trials stratified according to concomitant administration of systemic corticosteroids. Width of horizontal line represents 95% confidence interval around point estimate (black square). Size of point estimate represents relative weight (% weight) of each trial in the pooled summary estimate (diamond). Vertical line is line of no effect (relative risk 0.0)
Multiple dose fixed protocols
Five trials, totalling 366 children, examined the effect of multiple doses of combined ipratropium bromide and β2 agonists in a fixed protocol. Pooling of the four trials contributing data to this outcome showed a 30% reduction in hospital admission rate in favour of the combination treatment (0.72, 0.53 to 0.99; fig 1) (R Peterson, personal communication).16,19,20 Eleven (95% confidence interval 5 to 250) patients would need to be treated with a multiple dose fixed protocol to prevent a single admission. Exclusion of the two smaller trials with lower quality scores16,17 did not affect the reduction in hospital admission rate attributable to combination treatment (0.72, 0.52 to 0.99) due to their small weights in the summary estimate. Similarly, exclusion of the unpublished trial did not affect the extent of reduction in hospital admission rate, although the significance level was affected (0.70, 0.47 to 1.05). There was no evidence of systematic bias identified by the funnel plot (intercept 0.22, −0.35 to 0.80). No group difference in relapse rate was observed (0.68, 0.31 to 1.51).
A difference in group means (weighted mean difference) of 9.66% (95% confidence interval 5.65% to 13.68%) was observed when improvement was reported as change in percentage predicted FEV1 (R Peterson, personal communication)17,19,20; a difference of 6.97% (1.60% to 12.34%) was observed in trials reporting the percentage change in FEV1.17,20 When combining all studies a significant improvement in spirometry, exceeding half a SD in change, favoured combination treatment (standardised mean difference −0.66, −0.95 to −0.37). The limited spectrum of baseline severity of patients enrolled in these five trials did not permit a meaningful analysis of tendency to examine the potential influence of baseline severity on extent of response. There was no significant group difference in the occurrence of side effects such as nausea (0.59, 0.30 to 1.14), vomiting (1.03, 0.37 to 2.87), and tremor (1.02, 0.63 to 1.64).
Multiple dose flexible protocol
Analysis of the single trial in which multiple inhalations of combined atropine sulphate and β2 agonist were given to 31 patients until a satisfactory clinical response was achieved did not show any significant difference in the few reported outcomes.14 There was no apparent impact on the number of inhalations required before discharge (one inhalation: relative risk 0.67, 0.27 to 1.66; two inhalations: 1.25, 0.57 to 2.75; three inhalations: 2.82, 0.12 to 64.39), although there was a tendency towards more inhalations in the intervention group. There was no group difference in the occurrence of tremor (0/15 versus 0/16).
Discussion
The intensity of anticholinergic treatment protocol influenced the extent of treatment response in terms of reduction in hospital admission. Whereas no group difference was observed in patients treated with a single dose of anticholinergics, a 30% reduction in hospital admission was observed in patients treated with multiple doses. However, important differences other than the intensity of anticholinergic treatment exist between trials to explain these apparent divergent conclusions. Therefore trials’ characteristics should be carefully considered before generalising results to all children and adolescents with acute asthma.
Single dose protocols
Trials examining the effect of the addition of a single dose of anticholinergics to β2 agonists differed in several characteristics including age, severity of exacerbation, and cointervention with glucocorticoids. These differences may explain the apparent divergence of individual trial results regarding hospital admission. Furthermore, the incomplete reporting of hospital admission and the use of various pulmonary function tests reduced the effective sample size of patients that could be pooled. Interpretation of results obtained from pooling trials under the single dose protocol must therefore be made with caution and be limited to children and adolescents with characteristics similar to those enrolled in the trials. The single study which examined children and adolescents (age range 3 to 17 years) with mild to moderate asthma ruled out, with a narrow confidence interval, any meaningful improvement in respiratory resistance attributable to anticholinergic use.21 In contrast, trials that examined school aged children with severe asthma showed a significant improvement in FEV1 60 minutes after the combined inhalation; a change that persisted at 120 minutes.19,32 It is possible that differences in subjects’ age and baseline severity may be associated with various degrees of cholinergic induced bronchospasm. More trials in preschool children and children and adolescents with mild to moderate asthma are needed to confirm this hypothesis. Moreover, the sensitivity of response variables (change in percentage predicted FEV1, percentage change in FEV1, percentage change in respiratory resistance) to identify change as a result of treatment may vary. Finally, in contrast with current recommendations,1,3–6,34 none of the trials of children and adolescents with severe exacerbations systematically added glucocorticoids to the inhalation regimen. Whether this would have improved lung function remains to be proved.
Multiple dose fixed protocols
Trials testing the addition of multiple doses of anticholinergics to β2 agonists in a predetermined fixed regimen were more homogeneous, focusing on school aged children and adolescents (n=5) with severe exacerbations (n=3) and infrequent concomitant use of glucocorticoids (n=4). A 30% reduction in hospital admission rate was attributable to combination treatment. Eleven patients would need to be treated with such a protocol to prevent one admission. The pooling of similar patients, mostly school aged children and adolescents with severe asthma, certainly contributed to the identification of this benefit, as none of the individual trials had sufficient power to detect a group difference in admission rates. Yet, because of the relative homogeneity of the trials and the inability to obtain data stratified on severity, it is impossible to separate the effects of multiple dosing from that of baseline severity. Regarding lung function, significant group differences favouring the combination treatment were also observed, whether the response was expressed as change in percentage predicted FEV1 or as percentage change in FEV1. While modest, the extent of improvement in lung function is probably clinically meaningful as it was associated with a substantial reduction in hospital admissions. In a single study, glucocorticoids were systematically given to all enrolled patients.20 Although the trial reported the largest reduction in hospital admission, more trials are needed to confirm that the favourable effect of combined multiple doses of anticholinergics and β2 agonist will be sustained in the presence of glucocorticoids in children and adolescents with severe exacerbations.
Multiple dose flexible protocols
A single small trial examined the efficacy of systematically adding anticholinergics to every β2 agonist inhalation, tailoring the number of inhalations to patients’ response. This protocol did not seem to reduce the number of inhalations needed. No data were provided on hospital admission. Although this protocol most closely reflects physicians’ treatment preference when dealing with children and adolescents with mild to moderate asthma, little evidence exists at present to support its use. Further trials are therefore needed before any conclusion can be drawn about the protocol’s efficacy.
Side effects
No apparent increase in the occurrence of nausea, tremor, or vomiting was observed in subjects treated with either the single or multiple dose protocols. Clinically important adverse effects, such as tachycardia or hypertension, were reported too infrequently to permit any analyses.
Strengths and limitations
Like all systematic reviews this meta-analysis is limited by the quality of existing data.35 Fortunately, six out of 10 trials were of high quality. Exclusion of trials with lower reported methodological quality did not affect the conclusions. Interestingly, the two trials with the largest sample sizes and thus the greater power, failed to detect any group differences in lung function and hospital admission. Although they both had excellent quality scores, they remain unpublished. Clearly, publication bias exists in this area.36 Exclusion of these two unpublished trials, one of the single dose and one of the multiple dose fixed protocol, did not affect conclusions, only the significance level. Although somewhat insensitive when used for a small number of pooled trials, funnel plot statistics failed to identify any major differences between trials. With eight of the 10 trials originating from North America, the generalisability of study results to other countries should be considered, particularly regarding hospital admission. Although variations in hospital admission rates have been documented, these variations have been predominantly attributed to differences in asthma severity, daily prophylaxis, intensity of emergency treatment, and admission criteria.37,38 Clearly, important international variations in these factors could influence the anticipated response to treatment.
Our review has attempted to summarise the best evidence available up to July 1998. Our review has identified six trials not included in the prior review18; two trials published before 1992,14,33 two studies published after 1995,19,20 and two unpublished trials (R Peterson, personal communication),21 totalling 718 more subjects. Our thorough systematic search for published and unpublished trials resulted in the identification of important trials, thus increasing the power and scope of the review.39 Our review is strengthened by the direct confirmation of methodology and extracted data with the authors of six of 10 trials. Nevertheless, the number and size of studies being pooled under each protocol remains small. Obviously, the current conclusions may be seriously modified by the results of larger trials.40
Design and reporting of trials
Trials should improve on three main aspects: interventions, choice of outcomes, and reporting of results. Firstly, because systemic glucocorticoids are now the standard treatment of children and adolescents with severe exacerbations they should be systematically given with β2 agonists in future trials. Conversely, in children and adolescents with mild to moderate exacerbations trials are needed to evaluate the potential benefit of systematically adding anticholinergics to every β2 agonist inhalation, titrating the number of inhalations to patients’ response. Secondly, as admission and relapse rates are relatively gross measures of efficacy, subject to practice variation, future trials should include more sensitive and reliable endpoints such as quality of life, duration of hospitalisation, and duration of symptoms following discharge. Finally, there is a need for consistent reporting of baseline severity, glucocorticoid use, and admission and relapse rates.
Acknowledgments
We thank the Cochrane Airways Review Group, namely Stephen Milan and Anna Tomato, for the literature search and ongoing support, and Dr Paul Jones and Dr Fred Wolf for their constructive comments. We also thank Dr Terry Klassen and Dr David McGillivray, the trials’ authors, namely Dr MF Guill, FA Qureshi, S Schuh, KP Dawson, and T Klassen, and Ms Judith Marshall for preparation of the manuscript.
Footnotes
Funding: None.
Conflict of interest: None.
References
- 1.British Medical Association. Asthma: a follow up statement from an international paediatric asthma consensus group. Arch Dis Child. 1992;67:240–248. doi: 10.1136/adc.67.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warner JO, Gotz M, Landau LI, Levison H, Milner AD, Pedersen S, et al. Management of asthma: a consensus statement. Arch Dis Child. 1989;64:1065–1079. doi: 10.1136/adc.64.7.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guidelines on the management of asthma. Thorax. 1993;48:1–24S. doi: 10.1136/thx.48.10.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rachelefsky GS, Warner JO. International consensus on the management of pediatric asthma: a summary statement. Pediatr Pulmonol. 1993;15:125–127. doi: 10.1002/ppul.1950150211. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell EA. Consensus on acute asthma management in children. Ad Hoc Paediatric Group. N Z Med J. 1992;105:353–355. [PubMed] [Google Scholar]
- 6.Ernst P, Fitzgerald JM, Spier S. Canadian asthma—consensus conference —summary of recommendations. Can Respir J. 1996;3:89–100. [Google Scholar]
- 7.Tal A, Levy N, Bearman JE. Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics. 1990;86:350–356. [PubMed] [Google Scholar]
- 8.Scarfone RJ, Fuchs SM, Nager AL, Shane SA. Controlled trial of oral prednisone in the emergency department treatment of children with acute asthma. Pediatrics. 1993;92:513–518. [PubMed] [Google Scholar]
- 9.Svedmyr N. Fenoterol: A beta2-adrenergic agonist for use in asthma pharmocology, pharmokinetics, clinical efficacy and adverse effects. Pharmacotherapy. 1985;5:109–126. doi: 10.1002/j.1875-9114.1985.tb03409.x. [DOI] [PubMed] [Google Scholar]
- 10.Sears MR. Clinical application of beta-agonists. Practical Allergy Immunol. 1992;7:98–100. [Google Scholar]
- 11.Gross NJ. Ipratropium bromide. N Engl J Med. 1988;8:486–494. doi: 10.1056/NEJM198808253190806. [DOI] [PubMed] [Google Scholar]
- 12.Silverman M. The role of anticholinergic antimuscarinic bronchodilator therapy in children. Lung. 1990;168:304–309. doi: 10.1007/BF02718146. [DOI] [PubMed] [Google Scholar]
- 13.Chapman K. An international perspective on anticholinergic therapy. Am J Med. 1996;100:2–4S. doi: 10.1016/s0002-9343(96)80013-1. [DOI] [PubMed] [Google Scholar]
- 14.Guill MF, Maloney MJ, DuRant RH. Comparison of inhaled metaproterenol, inhaled atropine sulfate, and their combination in treatment of children with acute asthma. Ann Allergy. 1987;59:367–371. [PubMed] [Google Scholar]
- 15.Cook JJ, Fergusson DM, Dawson KP. Ipratropium and fenoterol in the treatment of acute asthma. Pharmatherapeutica. 1985;4:383–386. [PubMed] [Google Scholar]
- 16.Reisman J, Galdes-Sebalt M, Kazim F, Canny G, Levison H. Frequent administration by inhalation of salbutamol and ipratropium bromide in the initial management of severe acute asthma in children. J Allergy Clin Immunol. 1988;81:16–20. doi: 10.1016/0091-6749(88)90214-x. [DOI] [PubMed] [Google Scholar]
- 17.Watson WTA, Becker AB, Simons FER. Comparison of ipratropium solution, fenoterol solution, and their combination administered by nebulizer and face mask to children with acute asthma. J Allergy Clin Immunol. 1988;82:1012–1018. doi: 10.1016/0091-6749(88)90138-8. [DOI] [PubMed] [Google Scholar]
- 18.Osmond MH, Klassen TP. Efficacy of ipratropium bromide in acute childhood asthma—a meta-analysis. Acad Emerg Med. 1995;2:651–656. doi: 10.1111/j.1553-2712.1995.tb03607.x. [DOI] [PubMed] [Google Scholar]
- 19.Schuh S, Johnson DW, Callahan S, Canny G, Levison H. Efficacy of frequent nebulized ipratropium bromide added to frequent high-dose albuterol therapy in severe childhood asthma. J Pediatr. 1995;126:639–645. doi: 10.1016/s0022-3476(95)70368-3. [DOI] [PubMed] [Google Scholar]
- 20.Qureshi FA, Zaritsky A, Lakkis H. Efficacy of nebulized ipratropium in severe asthmatic children. Ann Emerg Med. 1997;29:205–211. doi: 10.1016/s0196-0644(97)70269-5. [DOI] [PubMed] [Google Scholar]
- 21.Ducharme FM, Davis GM. Randomized controlled trial of ipratropium bromide and frequent low doses of salbutamol in the treatment of mild and moderate acute asthma. Pediatr Res. 1995;37:136A. doi: 10.1016/s0022-3476(98)70054-x. [DOI] [PubMed] [Google Scholar]
- 22.Peto R, Collins R, Gray R. Large-scale randomized evidence: large, simple trials and overviews of trials. J Clin Epidemiol. 1995;48:23–40. doi: 10.1016/0895-4356(94)00150-o. [DOI] [PubMed] [Google Scholar]
- 23.Sackett DL. Applying overviews and meta-analyses at the bedside. J Clin Epidemiol. 1995;48:61–66. doi: 10.1016/0895-4356(94)00085-5. [DOI] [PubMed] [Google Scholar]
- 24.Greenhalgh T. Papers that summarize other papers (systematic reviews and meta-analyses) BMJ. 1997;315:672–675. doi: 10.1136/bmj.315.7109.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized controlled trials: is blinding necessary? Control Clin Trials. 1995;134:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 26.Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow-up data. Biometrics. 1985;41:55–68. [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 28.Olkin I. Statistical and theoretical considerations in meta-analysis. J Clin Epidemiol. 1995;48:133–146. doi: 10.1016/0895-4356(94)00136-e. [DOI] [PubMed] [Google Scholar]
- 29.Hasselblad V, Hedges LV. Meta-analysis of screening and diagnostic tests. Psychol Bull. 1995;117:167–178. doi: 10.1037/0033-2909.117.1.167. [DOI] [PubMed] [Google Scholar]
- 30.Robins J, Breslow N, Greenland S. Estimators of the Mantel-Haenszel variance consistent in both sparse data and large-strata limiting models. Biometrics. 1986;42:311–323. [PubMed] [Google Scholar]
- 31.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck R, Robertson C, Galdès-Sebaldt M, Levison H. Combined salbutamol and ipratropium bromide by inhalation in the treatment of severe acute asthma. J Pediatr. 1985;107:605–608. doi: 10.1016/s0022-3476(85)80033-0. [DOI] [PubMed] [Google Scholar]
- 33.Phanichyakam P, Kraisarin C, Sasisakulporn C. Comparison of inhaled terbutaline and inhaled terbutaline plus ipratropium bromide in acute asthmatic children. Asian Pac J Allergy Immunol. 1990;8:45–48. [PubMed] [Google Scholar]
- 34.Warner JO. Asthma: a follow up statement from an international paediatric asthma consensus group. Arch Dis Child. 1992;67:240–248. doi: 10.1136/adc.67.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan KS, Daya S, Jahad AR. The importance of quality of primary studies in producing unbiased systematic reviews. Arch Intern Med. 1996;156:661–666. [PubMed] [Google Scholar]
- 36.Stern JM, Simes RJ. Publication bias: evidence of delayed publication in a cohort study of clinical research projects. BMJ. 1997;315:640–645. doi: 10.1136/bmj.315.7109.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Payne SM, Donahue C, Rappo P, McNamara JJ, Bass J, First L, et al. Variations in pediatric pneumonia and bronchitis/asthma admission rates. Is appropriateness a factor? Arch Pediatr Adolesc Med. 1995;149:162–169. doi: 10.1001/archpedi.1995.02170140044006. [DOI] [PubMed] [Google Scholar]
- 38.Homer CJ, Szilagyi P, Rodewald L, Bloom SR, Greenspan P, Yazdgerdi S, et al. Does quality of care affect rates of hospitalization for childhood asthma? Pediatrics. 1996;98:18–23. [PubMed] [Google Scholar]
- 39.Cook DJ, Guyatt GH, Ryan G, Clifton J, Buckingham L, Willan A, et al. Should unpublished data be included in meta-analyses? Current convictions and controversies. JAMA. 1993;269:2749–2753. [PubMed] [Google Scholar]
- 40.Sim I, Hlatky MA. Growing pains of meta-analysis. BMJ. 1996;313:702–703. doi: 10.1136/bmj.313.7059.702. [DOI] [PMC free article] [PubMed] [Google Scholar]