Abstract
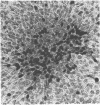
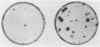
The NIH 3T3 mouse cell line is widely used as a recipient of DNA from tumors to demonstrate the presence of transforming oncogenes. We show that these cells produce transformed foci spontaneously if kept in the confluent state for more than 10 days. The formation of foci depends on the type and concentration of bovine serum used in the medium and passage history of the cells. Cells maintained in continuous exponential multiplication in the subconfluent state by transfer every 2-3 days in medium with 10% calf serum failed to develop the capacity to produce foci in 2% calf serum, but those transferred the same way in 2% calf serum or in 10% fetal bovine serum, which is a less potent growth stimulant, did develop that capacity to an increasing degree over time. The number of transformed cells increased sharply with the time that a culture remained in the confluent state. There are several morphological types and degrees of transformation, which indicates that the underlying changes are varied and the process is progressive. The results also suggest that transformation occurs in a fraction of an entire cell population that is undergoing a physiological adaptation to moderate constraints on its growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertram J. S. Effects of serum concentration on the expression of carcinogen-induced transformation in the C3H/10T1/2 CL8 cell line. Cancer Res. 1977 Feb;37(2):514–523. [PubMed] [Google Scholar]
- Farber E., Sarma D. S. Hepatocarcinogenesis: a dynamic cellular perspective. Lab Invest. 1987 Jan;56(1):4–22. [PubMed] [Google Scholar]
- Gurney T., Jr Local stimulation of growth in primary cultures of chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1969 Mar;62(3):906–911. doi: 10.1073/pnas.62.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber D. A., Fox D. A., Dynan W. S., Thilly W. G. Cell density dependence of focus formation in the C3H/10T1/2 transformation assay. Cancer Res. 1977 Jun;37(6):1644–1648. [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A. R., Cairns J., Little J. B. Timing of the steps in transformation of C3H 10T 1/2 cells by X-irradiation. Nature. 1984 Jan 5;307(5946):85–86. doi: 10.1038/307085a0. [DOI] [PubMed] [Google Scholar]
- Kennedy A. R., Fox M., Murphy G., Little J. B. Relationship between x-ray exposure and malignant transformation in C3H 10T1/2 cells. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7262–7266. doi: 10.1073/pnas.77.12.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordan L. J., Martner J. E., Bertram J. S. Quantitative neoplastic transformation of C3H/10T1/2 fibroblasts: dependence upon the size of the initiated cell colony at confluence. Cancer Res. 1983 Sep;43(9):4062–4067. [PubMed] [Google Scholar]
- Rubin H. Early origin and pervasiveness of cellular heterogeneity in some malignant transformations. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5121–5125. doi: 10.1073/pnas.81.16.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. Growth regulation, reverse transformation, and adaptability of 3T3 cells in decreased Mg2+ concentration. Proc Natl Acad Sci U S A. 1981 Jan;78(1):328–332. doi: 10.1073/pnas.78.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. Uniqueness of each spontaneous transformant from a clone of BALB/c 3T3 cells. Cancer Res. 1988 May 1;48(9):2512–2518. [PubMed] [Google Scholar]
- SANFORD K. K., EARLE W. R., SHELTON E., SCHILLING E. L., DUCHESNE E. M., LIKELY G. D., BECKER M. M. Production of malignancy in vitro. XII. Further transformations of mouse fibroblasts to sarcomatous cells. J Natl Cancer Inst. 1950 Oct;11(2):351–375. [PubMed] [Google Scholar]
- Sanford K. K., Evans V. J. A quest for the mechanism of "spontaneous" malignant transformation in culture with associated advances in culture technology. J Natl Cancer Inst. 1982 Jun;68(6):895–913. [PubMed] [Google Scholar]
- Sanford K. K., Handleman S. L., Jones G. M. Morphology and serum dependence of cloned cell lines undergoing spontaneous malignant transformation in culture. Cancer Res. 1977 Mar;37(3):821–830. [PubMed] [Google Scholar]
- Shipley G. D., Ham R. G. Improved medium and culture conditions for clonal growth with minimal serum protein and for enhanced serum-free survival of Swiss 3T3 cells. In Vitro. 1981 Aug;17(8):656–670. doi: 10.1007/BF02628401. [DOI] [PubMed] [Google Scholar]