Abstract
Two sites of protein-DNA interaction have been identified in vivo and in vitro in the proximal promoter regions of an H4 and an H3 human histone gene. In proliferating cells, these genes are transcribed throughout the cell cycle, and both the more distal site I and the proximal site II are occupied by promoter-binding factors. In this report we demonstrate that during the shutdown of proliferation and onset of differentiation of the human promyelocytic leukemia cell line HL-60 into cells that exhibit phenotypic properties of monocytes, histone gene expression is down-regulated at the level of transcription. In vivo occupancy of site I by promoter factors persists in the differentiated HL-60 cells, but protein-DNA interactions at site II are selectively lost. Furthermore, in vitro binding activity of the site II promoter factor HiNF-D is lost in differentiated cells, and nuclear extracts from differentiated cells do not support in vitro transcription of these histone genes. Our results suggest that the interaction of HiNF-D with proximal promoter site II sequences plays a primary role in rendering cell growth-regulated histone genes transcribable in proliferating cells. It appears that while cell-cycle control of histone gene expression is mediated by both transcription and mRNA stability, with the shutdown of proliferation and onset mRNA stability, with the shutdown of proliferation and onset of differentiation, histone gene expression is regulated at the transcriptional level.
Full text
PDF



Images in this article
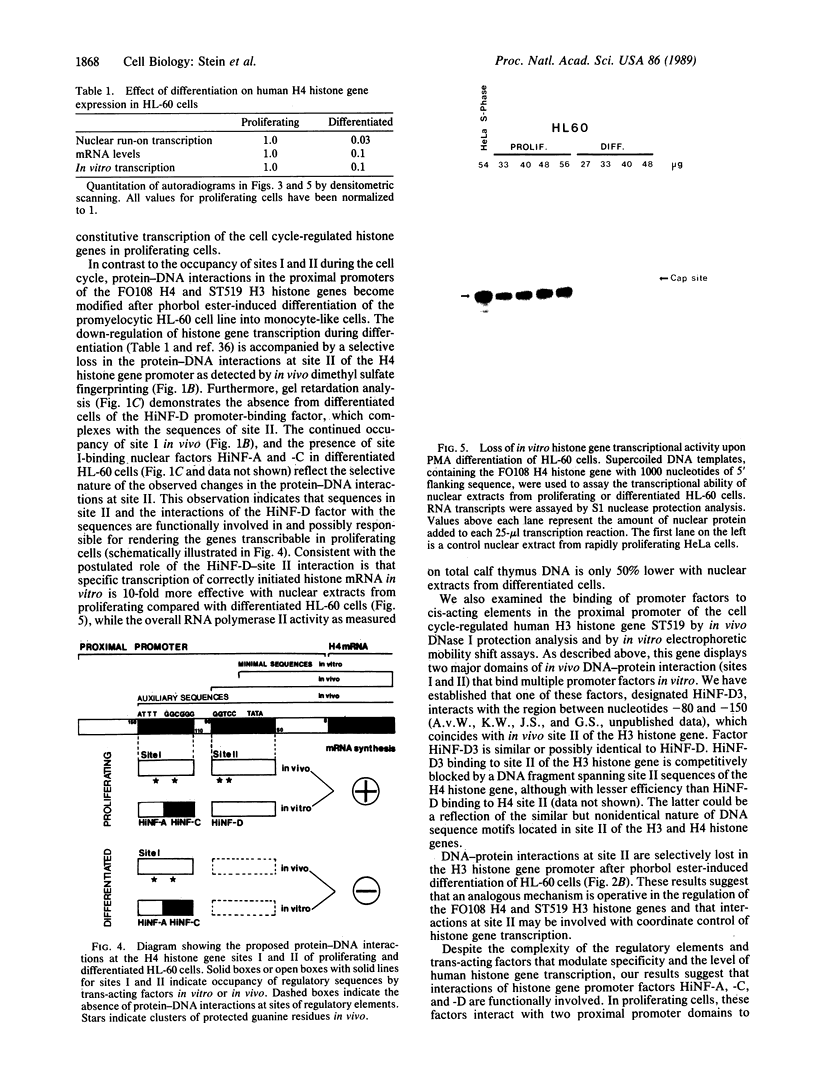
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artishevsky A., Delegeane A. M., Lee A. S. Use of a cell cycle mutant to delineate the critical period for the control of histone mRNA levels in the mammalian cell cycle. Mol Cell Biol. 1984 Nov;4(11):2364–2369. doi: 10.1128/mcb.4.11.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artishevsky A., Wooden S., Sharma A., Resendez E., Jr, Lee A. S. Cell-cycle regulatory sequences in a hamster histone promoter and their interactions with cellular factors. 1987 Aug 27-Sep 2Nature. 328(6133):823–827. doi: 10.1038/328823a0. [DOI] [PubMed] [Google Scholar]
- Baumbach L. L., Marashi F., Plumb M., Stein G., Stein J. Inhibition of DNA replication coordinately reduces cellular levels of core and H1 histone mRNAs: requirement for protein synthesis. Biochemistry. 1984 Apr 10;23(8):1618–1625. doi: 10.1021/bi00303a006. [DOI] [PubMed] [Google Scholar]
- Baumbach L. L., Stein G. S., Stein J. L. Regulation of human histone gene expression: transcriptional and posttranscriptional control in the coupling of histone messenger RNA stability with DNA replication. Biochemistry. 1987 Sep 22;26(19):6178–6187. doi: 10.1021/bi00393a034. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelvi Z. S., Studzinski G. P. Coordinate expression of c-myc, c-myb, and histone H4 genes in reversibly differentiating HL 60 cells. J Cell Physiol. 1987 Apr;131(1):43–49. doi: 10.1002/jcp.1041310108. [DOI] [PubMed] [Google Scholar]
- Briggs M. R., Kadonaga J. T., Bell S. P., Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986 Oct 3;234(4772):47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey L., Hanly S. M., Roeder R. G., Heintz N. Distinct transcription factors bind specifically to two regions of the human histone H4 promoter. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7241–7245. doi: 10.1073/pnas.83.19.7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton S., Wells J. R. A gene-specific promoter element is required for optimal expression of the histone H1 gene in S-phase. EMBO J. 1988 Jan;7(1):49–56. doi: 10.1002/j.1460-2075.1988.tb02782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman G., Iserentant D., Gheysen D., Fiers W. Characterization of the major altered leader sequence of late mRNA induced by SV40 deletion mutant d1-1811. Nucleic Acids Res. 1979 Dec 11;7(7):1799–1814. doi: 10.1093/nar/7.7.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms S. R., van Wijnen A. J., Kroeger P., Shiels A., Stewart C., Hirshman J., Stein J. L., Stein G. S. Identification of an enhancer-like element upstream from a cell cycle dependent human H4 histone gene. J Cell Physiol. 1987 Sep;132(3):552–558. doi: 10.1002/jcp.1041320319. [DOI] [PubMed] [Google Scholar]
- Huberman E., Callaham M. F. Induction of terminal differentiation in human promyelocytic leukemia cells by tumor-promoting agents. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1293–1297. doi: 10.1073/pnas.76.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger P., Stewart C., Schaap T., van Wijnen A., Hirshman J., Helms S., Stein G., Stein J. Proximal and distal regulatory elements that influence in vivo expression of a cell cycle-dependent human H4 histone gene. Proc Natl Acad Sci U S A. 1987 Jun;84(12):3982–3986. doi: 10.1073/pnas.84.12.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marashi F., Baumbach L., Rickles R., Sierra F., Stein J. L., Stein G. S. Histone proteins in HeLa S3 cells are synthesized in a cell cycle stage specific manner. Science. 1982 Feb 5;215(4533):683–685. doi: 10.1126/science.7058333. [DOI] [PubMed] [Google Scholar]
- Marashi F., Helms S., Shiels A., Silverstein S., Greenspan D. S., Stein G., Stein J. Enhancer-facilitated expression of prokaryotic and eukaryotic genes using human histone gene 5' regulatory sequences. Biochem Cell Biol. 1986 Apr;64(4):277–289. doi: 10.1139/o86-039. [DOI] [PubMed] [Google Scholar]
- Marzluff W. F., Pandey N. B. Multiple regulatory steps control histone mRNA concentrations. Trends Biochem Sci. 1988 Feb;13(2):49–52. doi: 10.1016/0968-0004(88)90027-8. [DOI] [PubMed] [Google Scholar]
- Pauli U., Chrysogelos S., Stein G., Stein J., Nick H. Protein-DNA interactions in vivo upstream of a cell cycle-regulated human H4 histone gene. Science. 1987 Jun 5;236(4806):1308–1311. doi: 10.1126/science.3035717. [DOI] [PubMed] [Google Scholar]
- Pauli U., Chrysogelos S., Stein J., Stein G. Native genomic blotting: high-resolution mapping of DNase I-hypersensitive sites and protein-DNA interactions. Proc Natl Acad Sci U S A. 1988 Jan;85(1):16–20. doi: 10.1073/pnas.85.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz S. W., Ross J. Autogenous regulation of histone mRNA decay by histone proteins in a cell-free system. Mol Cell Biol. 1987 Dec;7(12):4345–4356. doi: 10.1128/mcb.7.12.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumb M., Stein J., Stein G. Coordinate regulation of multiple histone mRNAs during the cell cycle in HeLa cells. Nucleic Acids Res. 1983 Apr 25;11(8):2391–2410. doi: 10.1093/nar/11.8.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovera G., Santoli D., Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümperli D. Cell-cycle regulation of histone gene expression. Cell. 1986 May 23;45(4):471–472. doi: 10.1016/0092-8674(86)90277-1. [DOI] [PubMed] [Google Scholar]
- Sierra F., Stein G., Stein J. Structure and in vitro transcription of a human H4 histone gene. Nucleic Acids Res. 1983 Oct 25;11(20):7069–7086. doi: 10.1093/nar/11.20.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittman D. B., Graves R. A., Marzluff W. F. Histone mRNA concentrations are regulated at the level of transcription and mRNA degradation. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1849–1853. doi: 10.1073/pnas.80.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding J., Kajiwara K., Mueller G. C. The metabolism of basic proteins in HeLa cell nuclei. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1535–1542. doi: 10.1073/pnas.56.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. S., Borun T. W. The synthesis of acidic chromosomal proteins during the cell cycle of HeLa S-3 cells. I. The accelerated accumulation of acidic residual nuclear protein before the initiation of DNA replication. J Cell Biol. 1972 Feb;52(2):292–307. doi: 10.1083/jcb.52.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. S., Stein J. L. Is human histone gene expression autogenously regulated? Mol Cell Biochem. 1984 Sep;64(2):105–110. doi: 10.1007/BF00224767. [DOI] [PubMed] [Google Scholar]
- Wells D. E. Compilation analysis of histones and histone genes. Nucleic Acids Res. 1986;14 (Suppl):r119–r149. doi: 10.1093/nar/14.suppl.r119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijnen A. J., Massung R. F., Stein J. L., Stein G. S. Human H1 histone gene promoter CCAAT box binding protein HiNF-B is a mosaic factor. Biochemistry. 1988 Aug 23;27(17):6534–6541. doi: 10.1021/bi00417a051. [DOI] [PubMed] [Google Scholar]
- van Wijnen A. J., Stein J. L., Stein G. S. A nuclear protein with affinity for the 5' flanking region of a cell cycle dependent human H4 histone gene in vitro. Nucleic Acids Res. 1987 Feb 25;15(4):1679–1698. doi: 10.1093/nar/15.4.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijnen A. J., Wright K. L., Massung R. F., Gerretsen M., Stein J. L., Stein G. S. Two target sites for protein binding in the promoter region of a cell cycle regulated human H1 histone gene. Nucleic Acids Res. 1988 Jan 25;16(2):571–592. doi: 10.1093/nar/16.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]