Abstract
High grade invasive transitional cell carcinoma (InvTCC) kills >14,000 people yearly in the United States, and better therapy is needed. Cyclooxygenase-2 (Cox-2) is over-expressed in bladder cancer. Cox inhibitors have caused remission of InvTCC in animal studies, and cancer regression was associated with doubling of the apoptotic index in the tumor. The purpose of this study was to determine the apoptosis-inducing effects of celecoxib (a Cox-2 inhibitor) in InvTCC in humans. Patients (minimum of 10 with paired tumor samples) with InvTCC who had elected to undergo cystectomy were enrolled. The main study end point was induction of apoptosis in tumor tissues. Patients received celecoxib (400mg twice daily po for a minimum of 14 days) between the time of diagnosis (TURBT; transurethral resection) and the time of cystectomy (standard frontline treatment for InvTCC). TUNEL assay and immunohistochemistry were performed on TURBT and cystectomy samples. Of 13 cases treated with celecoxib, no residual invasive cancer was identified in 3 patients at the time of cystectomy (post celecoxib). Of the 10 patients with residual cancer, 7 had induction of apoptosis in their tumor. Induction of apoptosis was less frequent [3 of 13 cases, p < 0.04] in control patients not receiving a Cox inhibitor. Expression of VEGF in the tumor cells decreased more frequently [p < 0.026] in the treated patients as compared to non-treated control cases. The biological effects of celecoxib treatment (increased apoptosis) justify further study of the antitumor effects of Cox-2 inhibitors in InvTCC.
Introduction
Invasive urinary bladder cancer kills more than 14,000 people each year in the United States (1). Most of those deaths are due to high grade invasive transitional cell carcinoma (InvTCC) that has metastasized and is resistant to chemotherapy. Cyclooxygenase-2 (Cox-2) is differentially over-expressed in many cancers (including InvTCC) as compared to the corresponding normal tissue, thereby making it an attractive target for cancer therapy (2–10). Cox inhibitors have induced apoptosis and have caused tumor regression in athymic mice bearing human bladder cancer xenografts and in pet dogs with naturally occurring InvTCC (11–13). The antitumor activity of Cox inhibitors is thought to be due, at least in part, to inhibition of Cox and the resulting decrease in Cox products (prostaglandins and thromboxanes), although Cox independent effects have also been reported (10, 14, 15). One of the key events identified to date in the antitumor activity of Cox inhibitors in vivo in animals is induction of apoptosis (10, 11). A significant association between increase in apoptotic index and tumor regression induced by the Cox inhibitor piroxicam was noted in dogs with naturally-occurring InvTCC (12). Naturally-occurring InvTCC in dogs is very similar to human InvTCC in histopathology, molecular features, biological behavior including frequency and sites of metastasis, and response to chemotherapy (16). The compressed life span of the dog along with intact immune system and body processes make it a very attractive tool to study response to drugs and to study their mechanisms of action associated with tumor regression.
Prior to considering large, longer term trials in humans, a prospective pilot study was performed to determine the effects of short term Cox-2 inhibitor (celecoxib) treatment in inducing apoptosis in people with InvTCC. Tissue analyses were also conducted to explore some of the possible mechanisms involved in the induction of apoptosis.
Materials and Methods
Pilot study
The pilot study was performed at the Indiana University School of Medicine with the approval of and following the guidelines of the Indiana University Institutional Review Board. A prospective pilot study was performed in patients with bladder-confined InvTCC who had elected to undergo cystectomy, as part of standard care for the treatment of their cancer. A Cox-2 inhibitor, celecoxib, was scheduled to be given for a minimum of 2 weeks and a maximum of 6 weeks between the time of diagnosis (transurethral resection of bladder tumor, TURBT) and cystectomy. Tissues collected at the time of TURBT (pre celecoxib) and at the time of cystectomy (post celecoxib) were studied. The main endpoint was induction of apoptosis in the tumor with celecoxib treatment. To determine that changes in apoptotic index were most likely due to the celecoxib and not other causes, samples from similar patients undergoing cystectomy at the same institution and who were not receiving any Cox inhibitor were studied for comparison. Immunohistochemistry was used to investigate proteins of potential importance in Cox inhibitor induced apoptosis (17–25). Urine samples collected before and during celecoxib treatment were analyzed for Cox metabolites (prostaglandins E2, PGE2; thromboxane B2, TBXB2) as an indication of Cox activity.
Entry requirements included: [1] patients over 35 yrs of age with confirmed InvTCC localized to the bladder who were planning to undergo cystectomy, [2] serum creatinine concentrations ≤ 1.5 mg/dl, SGOT ≤ 45 IU/L, SGPT ≤ 35 IU/L, [3] no current use of Cox inhibitors and no recent Cox inhibitor use that had lasted for a month or longer, [4] no known hypersensitivity to celecoxib, other Cox inhibitors, or sulfonamides, [5] tissue available from the TURBT and cystectomy, and [6] informed patient consent in writing.
Patient evaluation and monitoring included: [1] CBC and serum biochemical profile prior to treatment, and serum creatinine, SGOT, and SGPT after 3 weeks of treatment, [2] interview with the study nurse prior to and after 3 and 6 weeks of treatment, [3] and a diary in which the patient was asked to record when the celecoxib was taken and any unusual symptoms noted by the patient. For patients who received less than 3 weeks of treatment, the follow-up laboratory work and interview with the study nurse were conducted at the end of the treatment period.
Treatment consisted of celecoxib (Celebrex, Pfizer, New York, NY) given orally at a dose of 400 mg twice daily.
Samples
Tissues examined included formalin fixed tissues collected at the time of TURBT and cystectomy. The analyses performed on tissues included: TUNEL, and immunohistochemistry for Cox-2 (Oxford Biochemical Research, Inc., Oxford, MI), Ki67 (Zymed, Carlsbad, CA), p53 (Signet, Dedham, MA), VEGF (Zymed, Carlsbad, CA), bFGF (Upstate, Lake Placid), and survivin (Lab Vision, Fremont, CA). Urine samples from healthy control patients and urine samples from InvTCC patients before and after celecoxib were analyzed for PGE2 and TBXB2.
Sample size and analyses
The plan was to enroll a minimum of 10 patients receiving celecoxib. This number was based on the expectation that positive results (induction of apoptosis) would be noted in one or more of the patients should celecoxib have a true apoptosis-inducing activity rate of at least 20% of patients (26). Induction of apoptosis was defined as doubling or more of the apoptotic index, as this level of change in apoptotic index has been considered biologically or clinically meaningful (12, 27). If no cases had induction of apoptosis, then further study would not be indicated. SAS 9.1.3 was used for all statistical analyses. Due to the small sample size, Fisher's Exact test was used to compare differences in the frequency of the categorical responses. A p value less than 0.05 was considered significant.
Determination of apoptosis by TUNEL
Apoptosis was measured quantitatively with the use of the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end-labeling (TUNEL) assay (28). The ApopTag In Situ Apoptosis Detection kit (Chemicon, Temecula, CA) was used following the manufacturer's instructions. The percent of positive tumor cells was determined in five different high power fields, and the average recorded.
Immunohistochemistry
Immunohistochemistry was performed as described previously (12). Briefly, 5µm sections were cut from paraffin-embedded human InvTCC tissues and placed on Superfrost® slides. Sections were dewaxed in xylene and rehydrated in descending percentages of alcohol. Target Retrieval solution (Dako Corp., Carpinteria, CA) was used according to manufacturer’s instructions. The sections were then immersed in 3% hydrogen peroxide in methanol to block the endogenous peroxidase, and then blocked for avidin and biotin (Vector Laboratories, Inc., Burlingame, CA). The sections were permeabilized in TNB-BB (0.1M Tris pH 7.5/0.15M NaCl/0.5% blocking agent/0.3% Triton-X, 0.2% saponin), and incubated in primary antibody overnight at 4°C. Cox-2 immunoreactive complexes were detected using tyramide signal amplification (TSA-indirect, NEN Life Sciences), and visualized with the peroxidase substrate, AEC (Zymed Laboratories, San Francisco, CA) whereas DAB substrate (Vector Laboratories, Inc., Burlingame, CA) was used to visualize all other immunocomplexes (Ki67, VEGF, survivin and p53, bFGF). Slides were counter stained in hematoxylin-1 (Richard-Allan Scientific, Kalamazoo, MI) mounted in crystal mount, and cover-slipped in 50:50 xylene/permount (Fisher Scientific).
Two pathologists who were blinded to treatment status reviewed slides independently. The percentages of cancer cells with immunoreactivity to Cox-2, Ki67, VEGF, survivin, and p53 were determined. The intensity of immunostaining was graded on a scale of 0–3 where 0 = no staining, 1 = equivocal staining, 2 = moderate to intense staining, and 3 = highest intensity staining. A change (between TURBT and cystectomy samples) of ≥20% in the percent of cells expressing the protein of interest was considered meaningful.
PGE2 and TBXB2 concentrations
PGE2 concentrations (PGE2 ELISA Kit, Cayman Chemical Co. Ann Arbor, MI) and TBXB2 concentrations (Thromboxane B2 Enzyme Immunoassay Kit, Assay Designs, Ann Arbor, MI) in urine were measured before and after treatment with celecoxib according to manufacturer’s protocol. The data were normalized to urine creatinine concentration and reported as pg/mg creatinine.
Results
Pilot study
Subject characteristics are summarized in Table 1. Thirteen patients with high grade InvTCC received celecoxib treatment in the study. The patients receiving celecoxib [n=13] included 5 women and 8 men. The mean age was 61.4 years [range 37–83 years]. The non-treated control patients [n=13] included 9 men and 4 women with a mean age of 68 years [range 52–79 years]. Of the 13 patients receiving celecoxib, 3 did not have residual InvTCC at the time of cystectomy. In 2 of these 3 patients, no evidence of malignancy was found at the time of the cystectomy. The third patient had carcinoma in situ, but no InvTCC detected at the time of cystectomy. Analyses were performed on tumor tissues from the 10 treated patients who had InvTCC at the time of cystectomy. Samples from 13 non-treated control patients were analyzed. In treated patients, celecoxib was given for 20.2 ± 9.6 days. One of the patients received less than the planned minimum 14 day treatment (treated 9 days). Treatment was generally well tolerated. One patient reported fatigue, and one patient reported back and stomach ache while receiving celecoxib. No increases in serum creatinine, SGOT, or SGPT were observed.
Table 1.
Characteristics of Control and Celecoxib Treatment Subjects. TNM Stage was classified according to WHO TNM Classification (6th edition, 2003–2009) and was based on cystectomy samples. No significant differences (t-test/Wilcoxon rank sum for continuous variables, Fisher’s exact test for categorical variables) were found between the patients in the control vs. the treated group in regards to gender, age, grade, LVI or stage.
| Control (n=13) | Celecoxib Treatment (n=13) | ||
|---|---|---|---|
| Gender | |||
| Male | 9 | 8 | |
| Female | 4 | 5 | |
| Age | |||
| Mean | 68 | 61 | |
| Median | 67 | 63 | |
| Range | 52–79 | 37–83 | |
| Grade | |||
| II/III | 1 | 2 | |
| III/III | 12 | 11 | |
| LVI | 4 | 4a | |
| TNM Stage Classification at cystectomy (6th edition 2003–009) | |||
| T0N0M0 | 0 | 1b | |
| T0N1M0 | 0 | 1b | |
| TisN0M0 | 1c | 1b | |
| T1N0M0 | 0 | 1 | |
| T2aN1M0 | 1 | 0 | |
| T2bN0M0 | 0 | 1 | |
| T3aN0M0 | 3 | 3 | |
| T3aN1M0 | 2 | 0 | |
| T3aN2M0 | 1 | 2 | |
| T3aN3M0 | 1 | 0 | |
| T3bN3M0 | 1 | 0 | |
| T4aN0M0 | 2 | 1 | |
| T4aN1M0 | 0 | 1 | |
| T4aN2M0 | 1 | 0 | |
| T4aN3M0 | 0 | 1 | |
Invasive cancer was not detected at cystectomy for 3 patients; n=10 samples assessed for LVI.
Invasive cancer was not detected at cystectomy. All 3 cases had T2 cancer at the time of TURBT.
Invasive cancer was not detected at cystectomy. This patient had T1 cancer at the time of URBT.
Increase in apoptosis
Of the 10 treated patients with residual cancer at the time of cystectomy, the apoptotic index in the tumor doubled in 7 patients, increased (but did not double) in 1 patient, remained unchanged in 1 patient, and decreased in 1 patient (Table 2, Figure 1). In 13 untreated control patients, the apoptotic index increased in 3 patients, and remained unchanged or decreased in 10 patients. Induction of apoptosis occurred significantly more frequently (Fisher’s Exact test, P < 0.04) in celecoxib-treated patients than in control patients. In control untreated patients, the percentage of tumor cells undergoing apoptosis was 2.0±1.5 (mean±sd) at the time of TURBT, and was 2.7±3.3 at the time of cystectomy. In patients in the celecoxib treatment group, the percentage of tumor cells undergoing apoptosis increased from 6.2±15.4 at the time of TURBT to 9.9±21.2 at the time of cystectomy.
Table 2.
Change in Apoptotic Index. Change in apoptotic index between TURBT and cystectomy in patients receiving celecoxib or in patients not receiving a Cox inhibitor (controls). Induction of apoptosis occurred significantly more frequently (Fisher’s Exact test, P < 0.04) in celecoxib treated patients than in control patients.
| Control (n= 13) |
Celecoxib Treatment (n=10) |
|
|---|---|---|
| Apoptotic index doubled (≥ 100% increase) | 3(23%) | 7 (70%) |
| Apoptotic index increased by 20 – 99% | 0 (0%) | 1 (10%) |
| Less than 20% change in apoptotic index | 4 (31%) | 1 (10%) |
| Apoptotic index decreased by ≥ 20% | 6 (46%) | 1 (10%) |
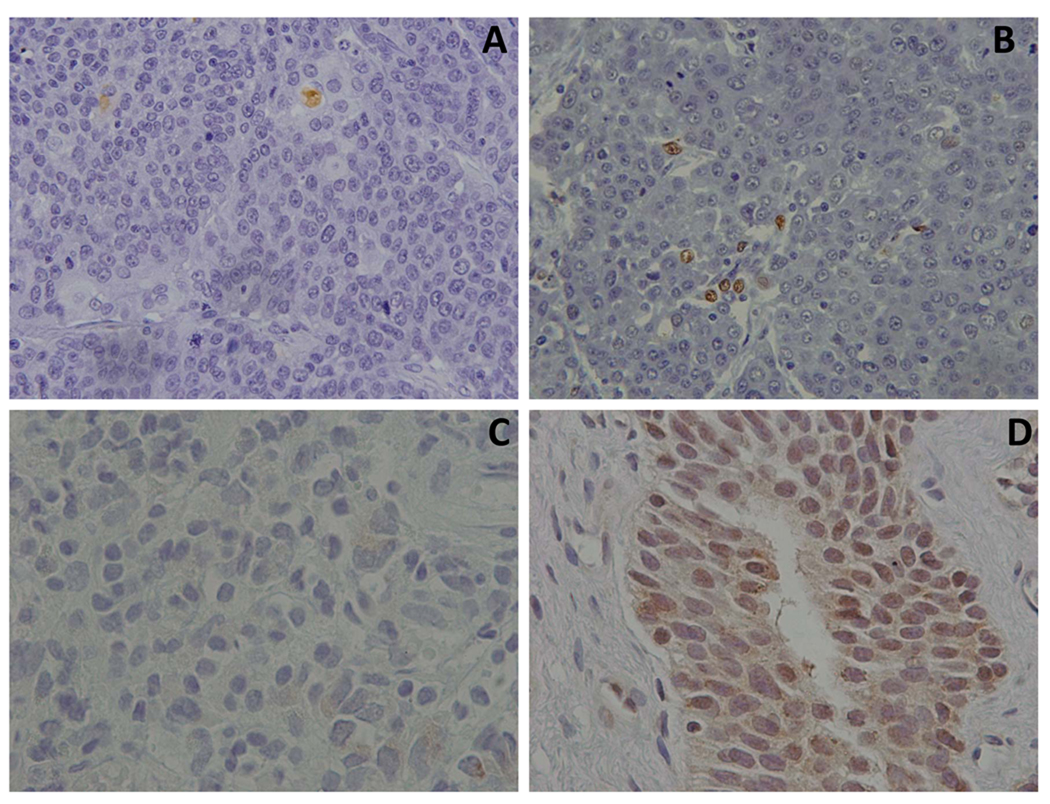
Figure 1.
Photomicrographs of InvTCC tissues collected prior to and following celecoxib treatment. Panels A and B demonstrate results of TUNEL assay (Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling) (Chemicon, Temecula, CA) used to detect apoptotic tumor cells. Panel A: photomicrograph from the pre-treatment (TURBT) sample with minimal apoptosis detected. Panel B: photomicrograph from the post treatment sample from the same patient with multiple apoptotic cells noted (note brown nuclear staining). In panels C and D, immunohistochemistry was used to detect survivin expression before and after celecoxib treatment. Note the marked increase in survivin expression between the pre-treatment sample (Panel C) and the post treatment sample (Panel D) from the same patient treated with celecoxib.
Expression of Cox-2, VEGF, survivin, p53, Ki67 and bFGF
The percentage of cells with immunoreactivity to Cox-2 ranged from <10% to 55% in treated and <10% to 75% in non-treated patients. There were no consistent changes in Cox-2 expression with treatment. It was interesting to note that doubling of the apoptotic index following treatment with celecoxib even occurred in patients with relatively low expression of Cox-2 (those having <10% of tumor cells expressing Cox-2).
There was variability in the change in VEGF expression in the treated and non-treated patients (Table 3). Reduction in VEGF (≥20% reduction in percentage of tumor cells expressing VEGF) was significantly more frequent in patients receiving celecoxib [4 of 9] than in untreated patients [0 of 11] [p < 0.027]. VEGF, however, increased in 3 patients and remained unchanged in 2 patients receiving celecoxib. In non-treated patients, VEGF expression increased [by ≥ 20%] in 7 patients and remained the same in 4 patients. There was no association between change in VEGF expression and change in apoptotic index. Immunoreactivity to bFGF was noted in endothelial cells in blood vessels within the tumor and in a small percentage of stromal cells in some cases. No differences in bFGF were noted between treated and non-treated patients, and no changes with celecoxib treatment were detected.
Table 3.
VEGF Expression (Percentage of Tumor Cells Expressing VEGF) at TURBT and Cystectomy. In untreated control patients the mean ± standard deviation of the percentage of cells expressing VEGF was 40.5±29.9 at the time of TURBT and 56.3±21.4 at the time of cystectomy. In patients treated with celecoxib, the mean ± standard deviation of the percentage of cells expressing VEGF was 51.1±16.9 at the time of TURBT and 46.4±25.6 at the time of cystectomy. Expression of VEGF in the tumor cells decreased (by ≥20%) more frequently (p < 0.026) in the treated patients as compared to non-treated control cases.
| VEGF Expression | |||
|---|---|---|---|
| Control (n=11) | Celecoxib Treatment (n=9) | ||
| TURBT | Cystectomy | TURBT | Cystectomy |
| 0 | 10 | 20 | 20 |
| 10 | 30 | 40 | 40 |
| 10 | 55 | 50 | 25 |
| 20 | 50 | 50 | 70 |
| 25 | 50 | 50 | 85 |
| 30 | 60 | 50 | 80 |
| 57 | 70 | 50 | 20 |
| 70 | 70 | 70 | 45 |
| 70 | 82 | 80 | 33 |
| 75 | 62 | ||
| 79 | 80 | ||
Of the 23 patients in the study with InvTCC at cystectomy, p53 was detected in 14 [60.8%] tumors. No significant differences were noted between treated and control patients at baseline or at the time of cystectomy. There were no significant differences between treated and control patients in regards to the expression of Ki67 or survivin, or any changes with treatment. It was of interest to note, however, that the survivin located in the nucleus increased in 6 of 10 patients receiving celecoxib, compared to 3 of 11 non-treated patients.
Urine PGE2 and TBXB2
Paired urine samples (pre- and post- treatment) were available from 9 patients treated with celecoxib. Stable metabolites of PGE2 and TBXB2 were measured in urine from the patients treated with celecoxib and from 30 and 8 patients, respectively, without urinary tract cancer or infection (Figure 2). Interestingly, the PGE2 and TBXB2 concentrations did not decrease in all patients receiving celecoxib as expected. In fact, the PGE2 concentrations increased in 5 of 9 patients, and TBXB2 concentrations increased in 8 of 9 patients.
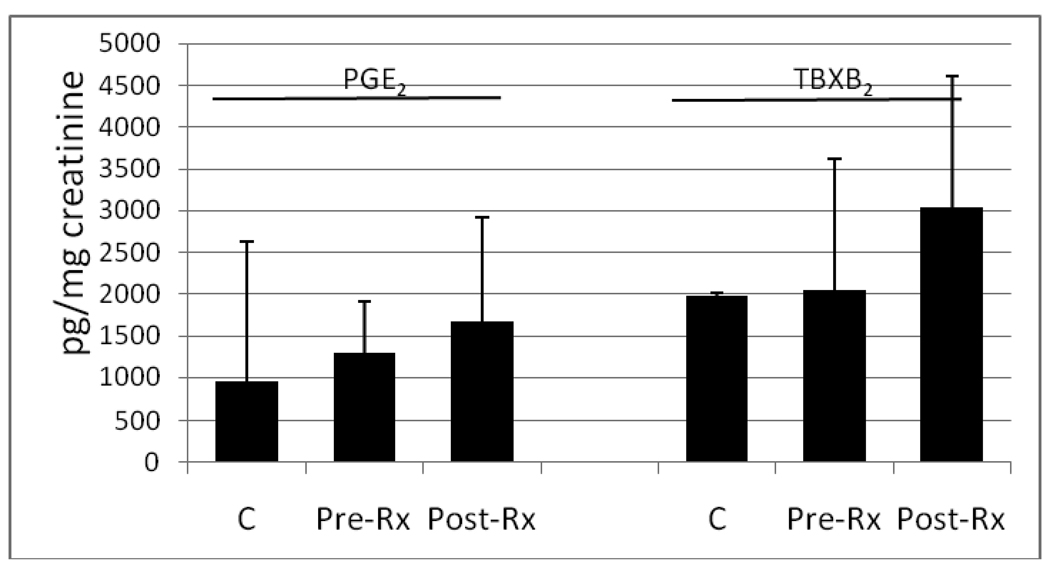
Figure 2.
PGE2 and TBXB2 concentrations (pg/mg creatinine) in the urine from normal healthy controls (C) and from patients with InvTCC prior to (Pre-Rx) and following (Post-Rx) celecoxib treatment. Data are presented as the mean ± standard deviation. For PGE2 analyses, 30 control samples, and 9 samples from patients with InvTCC before and after celecoxib were included. For TBXB2 analyses, 8 control samples, and 9 samples from patients with InvTCC before and after celecoxib were included. Although reductions in PGE2 and TBXB2 were expected with celecoxib treatment, this was not observed, possibly due to drug withdrawal the day prior to collecting the post treatment samples.
Discussion
There is continued and renewed interest in the use of Cox inhibitors in cancer treatment. Although enthusiasm for the use of Cox-2 inhibitors in primary cancer prevention has waned with the recognition of the thromboembolic risks (29), there is still substantial interest in using these drugs in cancer treatment and as agents to prevent cancer progression, especially where the outlook for the patient is guarded or worse. In InvTCC for example, the median survival of patients with metastasis treated with chemotherapy is typically ≤ 14 months (30), and new therapy approaches are needed. In studies in dogs with InvTCC, Cox inhibitors have had considerable activity as single agents (remission in 18% of animals, inhibition of growth in an additional 55% of dogs), and have greatly enhanced the remission rate with chemotherapy (12, 31). Naturally-occurring InvTCC in dogs closely mimics human InvTCC in its histopathology, molecular features (including Cox-2 expression), frequency and sites of metastases, and response to treatment (16, 32).
The purpose of the study was to determine the apoptosis-inducing effects of a Cox-2 inhibitor in humans with InvTCC. Although it would be intriguing to measure changes in tumor size in response to celecoxib, this would require longer term treatment which would not be possible within the accepted time frame for scheduling standard care (cystectomy). In addition, it is difficult to measure the size of InvTCC in the bladder in people, especially when flatter lesions in the bladder are present. Therefore, another marker of celecoxib activity was selected. Induction of apoptosis was selected because it was significantly associated with antitumor activity of Cox inhibitor in dogs with InvTCC (12). The study results were very encouraging in that celecoxib use was significantly [p<0.04] associated with induction of apoptosis. The induction of apoptosis was attributed to the celecoxib because this same level of increase was not observed in control patients who had not received celecoxib or other Cox inhibitors.
Multiple mechanisms could be involved in the induction of apoptosis by celecoxib. Broad mechanisms reported in the literature include Cox-2 dependent and independent actions leading to effects on apoptosis via mitochondrial pathway (17, 18) or activation of caspases (17, 18); through inhibition of 3-phosphoinositide-dependent protein kinase-1 (18); and through secondary effects such as those related to a decrease in VEGF (12) or VE-cadherin gene expression (19), or changes in other genes (20, 33).
In prior studies in dogs with InvTCC, Cox inhibitor antitumor effects were associated with a reduction in angiogenic factors, especially urine bFGF. VEGF-C has been shown to be an important prognostic marker in human bladder cancer (34, 35) and its expression has been correlated with Cox-2 expression (21). In the current study, celecoxib treatment was associated with a decrease in the expression of VEGF-C (but not bFGF) in tumor samples of some patients. Reduction in VEGF has been reported with celecoxib treatment in gastric cancer (22), although not in pancreatic cancer (23). Multiple mechanisms are most likely involved in the effects induced by celecoxib (24).
It was interesting that nuclear survivin expression in the tumor increased in 6 of 10 patients receiving celecoxib. Survivin, a member of the inhibitor of apoptosis proteins (IAPs), has been reported to block induction of apoptosis, interfere with the antitumor effects of chemotherapy and radiation therapy, to promote angiogenesis, and to be associated with a poor prognosis (25, 36). Survivin has cytoplasmic – nuclear shuttling activity, and its subcellular localization affects its regulatory function (25, 37–43). Although cytoplasmic survivin has consistently been linked to less favorable prognosis and poor response to therapy, the effects of nuclear survivin are less well defined (25, 37–43). In cervical cancer cells, nuclear survivin has actually been found to facilitate induction of apoptosis (39). Survivin in the nucleus appears to degrade more rapidly than that in the cytoplasm, and this could suggest that nuclear survivin expression would be less deleterious in the cancer progression. Making note of survivin expression and its subcellular localization in larger studies could help define the possible involvement between this protein and Cox inhibitor effects in InvTCC.
It was of interest that in this celecoxib pilot study, urine PGE2 and TBXB2 concentrations did not consistently decrease with celecoxib. In fact, PGE2 concentration increased in 5 of 9 patients, and TBXB2 increased in 8 of 9 patients, with celecoxib treatment. A possible explanation for these unexpected findings relates to the timing of the celecoxib dosing and the time of urine collection. Urine samples post celecoxib treatment were collected the day of cystectomy. Many patients, however, discontinued the celecoxib the day before surgery. Thus samples collected the day of surgery would not necessarily reflect celecoxib effects. It is likely that in some patients the celecoxib concentrations were not sufficient to block Cox-2 activity at the time the post treatment urine samples were collected. In fact, these samples could reflect the “rebound” effect of higher PGE2 and TBXB2 concentrations that can be produced when Cox inhibitors are withdrawn (44).
Celecoxib was generally well tolerated with only 2 patients reporting mild symptoms (fatigue, stomach ache, back ache) that may or may not have been related to treatment. The celecoxib dose selected for the study (400 mg BID) was selected because this dose has resulted in antitumor activity in patients with other cancers, and this dose is FDA approved for use in patients with familial polyposis coli (45–47).
Of the 13 cases treated with celecoxib, no residual InvTCC was identified in 3 patients at the time of cystectomy (post celecoxib). One of the 3 patients had carcinoma in situ, and the other 2 patients did not have cancer identified in the cystectomy sections. In previous studies in the Urology Clinics at Indiana University where the pilot project was performed, a small percentage [<7%] of patients not receiving any treatment have been noted to have no residual cancer at the time of cystectomy. In these patients, it is usually thought that the cancer was removed at the time of TURBT. It is not possible to know if the finding of 3 of 13 patients with no residual InvTCC after celecoxib treatment was due to celecoxib treatment or not, although this finding is intriguing.
The biological effects of celecoxib in inducing apoptosis in InvTCC provide justification for continued investigation of Cox-2 inhibitor treatment of this cancer. Further studies could include those to document cancer regression with Cox-2 inhibitors, studies of the chemotherapy enhancing effects of Cox-2 inhibitors, and work to further define mechanisms involved in the antitumor activity.
Acknowledgments
This work was supported by PHS grant R21 CA93011
References
- 1.American Cancer Society. Cancer Facts & Figures. 2009 Available from: http://www.cancer.org/downloads/STT/500809web.pdf.
- 2.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 3.Chen CN, Hsieh FJ, Cheng YM, Chang KJ, Lee PH. Expression of inducible nitric oxide synthase and cyclooxygenase-2 in angiogenesis and clinical outcome of human gastric cancer. J Surg Oncol. 2006;94:226–233. doi: 10.1002/jso.20372. [DOI] [PubMed] [Google Scholar]
- 4.Legan M, Luzar B, Marolt VF, Cor A. Expression of cyclooxygenase-2 is associated with p53 accumulation in premalignant and malignant gallbladder lesions. World J Gastroenterol. 2006;12:3425–3429. doi: 10.3748/wjg.v12.i21.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siironen P, Ristimaki A, Narko K, et al. VEGF-C and COX-2 expression in papillary thyroid cancer. Endocr Relat Cancer. 2006;13:465–473. doi: 10.1677/erc.1.01114. [DOI] [PubMed] [Google Scholar]
- 6.Iwamoto A, Ikeguchi M, Matsumoto S, et al. Tumor cyclooxygenase-2 gene suppresses local immune responses in patients with hepatocellular carcinoma. Tumori. 2006;92:130–133. doi: 10.1177/030089160609200208. [DOI] [PubMed] [Google Scholar]
- 7.Bhandari P, Bateman AC, Mehta RL, et al. Prognostic significance of cyclooxygenase-2 (COX-2) expression in patients with surgically resectable adenocarcinoma of the oesophagus. BMC Cancer. 2006;6:134–142. doi: 10.1186/1471-2407-6-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohammed SI, Knapp DW, Bostwick DG, et al. Expression of cyclooxygenase-2 (COX-2) in human invasive transitional cell carcinoma (TCC) of the urinary bladder. Cancer Res. 1999;59:5647–5650. [PubMed] [Google Scholar]
- 9.Byun JH, Lee MA, Roh SY, et al. Association between cyclooxygenase-2 and matrix metalloproteinase-2 expression in non-small cell lung cancer. Jpn J Clin Oncol. 2006;36:263–268. doi: 10.1093/jjco/hyl024. [DOI] [PubMed] [Google Scholar]
- 10.Dhawan D, Jeffreys AB, Zheng R, Stewart JC, Knapp DW. Cyclooxygenase-2 dependent and independent antitumor effects induced by celecoxib in urinary bladder cancer cells. Mol Cancer Ther. 2008;7:897–904. doi: 10.1158/1535-7163.MCT-07-0313. [DOI] [PubMed] [Google Scholar]
- 11.Mohammed SI, Dhawan D, Abraham S, et al. Cyclooxygenase inhibitors in urinary bladder cancer: in vitro and in vivo effects. Mol Cancer Ther. 2006;5:329–336. doi: 10.1158/1535-7163.MCT-05-0117. [DOI] [PubMed] [Google Scholar]
- 12.Mohammed SI, Bennett PF, Craig BA, et al. Effects of the cyclooxygenase inhibitor, piroxicam, on tumor response, apoptosis, and angiogenesis in a canine model of human invasive urinary bladder cancer. Cancer Res. 2002;62:356–358. [PubMed] [Google Scholar]
- 13.de Groot DJ, de Vries EG, Groen HJ, de Jong S. Non-steroidal anti-inflammatory drugs to potentiate chemotherapy effects: from lab to clinic. Crit Rev Oncol Hematol. 2007;61:52–69. doi: 10.1016/j.critrevonc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Mann JR, Backlund MG, Buchanan FG, et al. Repression of prostaglandin dehydrogenase by epidermal growth factor and snail increases prostaglandin E2 and promotes cancer progression. Cancer Res. 2006;66:6649–6656. doi: 10.1158/0008-5472.CAN-06-1787. [DOI] [PubMed] [Google Scholar]
- 15.Funk C. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 16.Knapp DW. Animal models: Naturally-occurring Canine Urinary Bladder Cancer. In: Lerner SP, Schoenberg MP, Sternberg CN, editors. Textbook of Bladder Cancer. Oxon, UK: Taylor and Francis; 2006. pp. 171–175. [Google Scholar]
- 17.Ding H, Han C, Zhu J, Chen CS, D'Ambrosio SM. Celecoxib derivatives induce apoptosis via the disruption of mitochondrial membrane potential and activation of caspase 9. Int J Cancer. 2005;113:803–810. doi: 10.1002/ijc.20639. [DOI] [PubMed] [Google Scholar]
- 18.Arico S, Pattingre S, Bauvy C, et al. Celecoxib induces apoptosis by inhibiting 3-phosphoinositide-dependent protein kinase-1 activity in the human colon cancer HT-29 cell line. J Biol Chem. 2002;277:27613–27621. doi: 10.1074/jbc.M201119200. [DOI] [PubMed] [Google Scholar]
- 19.Fontana A, Galli L, Fioravanti A, et al. Clinical and pharmacodynamic evaluation of metronomic cyclophosphamide, celecoxib, and dexamethasone in advanced hormone-refractory prostate cancer. Clin Cancer Res. 2009;15:4954–4962. doi: 10.1158/1078-0432.CCR-08-3317. [DOI] [PubMed] [Google Scholar]
- 20.Sooriakumaran P, Macanas-Pirard P, Bucca G, et al. A gene expression profiling approach assessing celecoxib in a randomized controlled trial in prostate cancer. Cancer Genomics Proteomics. 2009;6:93–99. [PubMed] [Google Scholar]
- 21.Kyzas PA, Stefanou D, Agnantis NJ. COX-2 expression correlates with VEGF-C and lymph node metastases in patients with head and neck squamous cell carcinoma. Mod Pathol. 2005;18:153–160. doi: 10.1038/modpathol.3800244. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Ran J, Tang C, et al. Effect of celecoxib on E-cadherin, VEGF, Microvessel density and apoptosis in gastric cancer. Cancer Biol Ther. 2007;6:269–275. doi: 10.4161/cbt.6.2.3629. [DOI] [PubMed] [Google Scholar]
- 23.Wei D, Wang L, He Y, Xiong HQ, Abbruzzese JL, Xie K. Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer Res. 2004;64:2030–2038. doi: 10.1158/0008-5472.can-03-1945. [DOI] [PubMed] [Google Scholar]
- 24.Dubois RN. New, long-term insights from the Adenoma Prevention with Celecoxib Trial on a promising but troubled class of drugs. Cancer Prev Res (Phila Pa) 2009;2:285–287. doi: 10.1158/1940-6207.CAPR-09-0038. [DOI] [PubMed] [Google Scholar]
- 25.Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- 26.Simon R. Optimal two stage designs for phase II clinical trials. Control Clinical Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 27.Landstrom M, Damber JE, Bergh A. Prostatic tumor regrowth after initially successful castration therapy may be related to a decreased apoptotic cell death rate. Cancer Res. 1994;54:4281–4284. [PubMed] [Google Scholar]
- 28.Pyrko P, Soriano N, Kardosh A, et al. Downregulation of survivin expression and concomitant induction of apoptosis by celecoxib and its non-cyclooxygenase-2-inhibitory analog, dimethyl-celecoxib (DMC), in tumor cells in vitro and in vivo. Mol Cancer. 2006;5:19. doi: 10.1186/1476-4598-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solomon SD, McMurray JJ, Pfeffer MA, et al. Adenoma Prevention with Celecoxib (APC) Study Investigators. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 30.Kassouf W, Swanson D, Kamat AM, et al. Partial cystectomy for muscle invasive urothelial carcinoma of the bladder: a contemporary review of the M. D. Anderson Cancer Center experience. J Urol. 2006;175:2058–2062. doi: 10.1016/S0022-5347(06)00322-3. [DOI] [PubMed] [Google Scholar]
- 31.Knapp DW, Glickman NW, Widmer WR, et al. Cisplatin versus cisplatin combined with piroxicam in a canine model of human invasive urinary bladder cancer. Cancer Chemother Pharmacol. 2000;46:221–226. doi: 10.1007/s002800000147. [DOI] [PubMed] [Google Scholar]
- 32.Knapp DW, Glickman NW, DeNicola DB, et al. Naturally-occurring canine transitional cell carcinoma of the urinary bladder, a relevant model of human invasive bladder cancer. Urol Oncol. 2000;5:47–59. doi: 10.1016/s1078-1439(99)00006-x. [DOI] [PubMed] [Google Scholar]
- 33.Jendrossek V, Handrick R, Belka C. Celecoxib activates a novel mitochondrial apoptosis signaling pathway. FASEB J. 2003;17:1547–1549. doi: 10.1096/fj.02-0947fje. [DOI] [PubMed] [Google Scholar]
- 34.Miyata Y, Kanda S, Ohba K, et al. Lymphangiogenesis and angiogenesis in bladder cancer: prognostic implications and regulation by vascular endothelial growth factors-A, -C, and -D. Clin Cancer Res. 2006;12(3 Pt 1):800–806. doi: 10.1158/1078-0432.CCR-05-1284. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki K, Morita T, Tokue A. Vascular endothelial growth factor-C (VEGF-C) expression predicts lymph node metastasis of transitional cell carcinoma of the bladder. Int J Urol. 2005;12:152–158. doi: 10.1111/j.1442-2042.2005.01010.x. [DOI] [PubMed] [Google Scholar]
- 36.Altieri DC. New wirings in the survivin networks. Oncogene. 2008;27:6276–6284. doi: 10.1038/onc.2008.303. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stauber RH, Mann W, Knauer SK. Nuclear and cytoplasmic survivin: molecular mechanism, prognostic, and therapeutic potential. Cancer Res. 2007;67:5999–6002. doi: 10.1158/0008-5472.CAN-07-0494. [DOI] [PubMed] [Google Scholar]
- 38.Connell CM, Colnaghi R, Wheatley SP. Nuclear survivin has reduced stability and is not cytoprotective. J Biol Chem. 2008;283:3289–3296. doi: 10.1074/jbc.M704461200. [DOI] [PubMed] [Google Scholar]
- 39.Temme A, Rodriguez JA, Hendruschk S, et al. Nuclear localization of Survivin renders HeLa tumor cells more sensitive to apoptosis by induction of p53 and Bax. Cancer Lett. 2007;250:177–193. doi: 10.1016/j.canlet.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 40.Marioni G, Ottaviano G, Marchese-Ragona R, et al. High nuclear expression of the apoptosis inhibitor protein survivin is associated with disease recurrence and poor prognosis in laryngeal basaloid squamous cell carcinoma. Acta Otolaryngol. 2006;126:197–203. doi: 10.1080/00016480500266685. [DOI] [PubMed] [Google Scholar]
- 41.Shinohara ET, Gonzalez A, Massion PP, et al. Nuclear survivin predicts recurrence and poor survival in patients with resected nonsmall cell lung carcinoma. Cancer. 2005;103:1685–1692. doi: 10.1002/cncr.20951. [DOI] [PubMed] [Google Scholar]
- 42.Mohamed S, Yasufuku K, Nakajima T, et al. Nuclear survivin in pN2 nonsmall cell lung cancer: prognostic and clinical implications. Eur Respir J. 2009;33:127–133. doi: 10.1183/09031936.00068708. [DOI] [PubMed] [Google Scholar]
- 43.Kim J, McNiff JM. Nuclear expression of survivin portends a poor prognosis in Merkel cell carcinoma. Mod Pathol. 2008;21:764–769. doi: 10.1038/modpathol.2008.61. [DOI] [PubMed] [Google Scholar]
- 44.Hsueh SF, Lu CY, Chao CS, et al. Nonsteroidal anti-inflammatory drugs increase expression of inducible COX-2 isoform of cyclooxygenase in spinal cord of rats with adjuvant induced inflammation. Brain Res Mol Brain Res. 2004;125:113–119. doi: 10.1016/j.molbrainres.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 45.Fort J. Celecoxib, a COX-2-specific inhibitor: the clinical data. Am J Orthop. 1999;28(3 Suppl):13–18. [PubMed] [Google Scholar]
- 46.Kaplan-Machlis B, Klostermeyer BS. The cyclooxygenase-2 inhibitors: safety and effectiveness. Ann Pharmacother. 1999;33:979–988. doi: 10.1345/aph.18415. [DOI] [PubMed] [Google Scholar]
- 47.Leese PT, Hubbard RC, Karim A, Isakson PC, Yu SS, Geis GS. Effects of celecoxib, a novel cyclooxygenase-2 inhibitor, on platelet function in healthy adults: a randomized, controlled trial. J Clin Pharmacol. 2000;40:124–132. doi: 10.1177/00912700022008766. [DOI] [PubMed] [Google Scholar]