Abstract
Background
Fluoxetine (FLX), a selective serotonin reuptake inhibitor, is prescribed for the treatment of major depressive disorder in young populations. Here, we explore the short- and long-term consequences of adolescent exposure to FLX on behavioral reactivity to emotion-eliciting stimuli.
Methods
Adolescent male rats received FLX (10 mg/kg) twice daily for 15 consecutive days (postnatal days 35–49). The influence of FLX on behavioral reactivity to rewarding and aversive stimuli was assessed 24 hours (short-term) or 3 weeks after FLX treatment (long-term). A separate group of adult rats was also treated with FLX (postnatal days 65–79) and responsiveness to forced swimming was assessed at identical time intervals as with the adolescents.
Results
Fluoxetine exposure during adolescence resulted in long-lasting decreases in behavioral reactivity to forced swimming stress and enhanced sensitivity to sucrose and to anxiety-eliciting situations in adulthood. The FLX-induced anxiety-like behavior was alleviated by re-exposure to FLX in adulthood. Fluoxetine treatment during adolescence also impaired sexual copulatory behaviors in adulthood. Fluoxetine-treated adult rats did not show changes in behavioral reactivity to forced swim stress as observed in those treated during adolescence and tested in adulthood.
Conclusions
Treating adolescent rats with FLX results in long-lived complex outputs regulated by the emotional valence of the stimulus, the environment in which it is experienced, and the brain circuitry likely being engaged by it. Our findings highlight the need for further research to improve our understanding of the alterations that psychotropic exposure may induce on the developing nervous system and the potential enduring effects resulting from such treatments.
Keywords: Adolescence, antidepressant, anxiety, depression, fluoxetine, rat, sexual behavior
Until relatively recently, the existence of major depressive disorder (MDD) in pediatric populations was not well recognized. Epidemiological reports now indicate that mood disorders are quite common early in life, affecting approximately 2% to 8% of children and adolescents, respectively (1,2). Pediatric MDD can lead to impairments in various psychiatric and functional domains such as antisocial personality, bipolar disorder, substance abuse, homelessness, self-harm, and up to 75% risk of recurrent depressive episodes in adulthood (3–7). These observations indicate an adverse impact of MDD on the development of neural substrates mediating cognitive, emotional, and social functioning (8–10). Thus, depression is a serious disorder necessitating timely and appropriate therapeutic intervention.
Fluoxetine (FLX) (Prozac), a selective serotonin reuptake inhibitor (SSRI), is the first drug approved for pediatric MDD (11). Although data about the effectiveness and safety of pharmacotherapy in youngsters are sparse, it is conceivable that treatment decisions for acute management of symptoms are made under the assumption that limiting dysfunction outweighs the potential for long-term side effects (1,12–14). Decisions regarding antidepressant use in early life have been largely based on data from adults (15,16). Although reliable evidence-based indications for SSRI use and its potential long-term consequences in youngsters are lacking, prescription rates are on the rise (16–21).
The acute effects of SSRI antidepressant medications are well defined: they increase the brain’s serotonin neurotransmission; however, they exert their mood-elevating effects after prolonged (i.e., weeks) administration (22,23). Serotonin is pivotal in the regulation of adolescent brain development in both rodents and humans (24,25). There is extensive serotonergic innervation of key brain regions involved in the control of emotional, cognitive, and motivated behaviors (25–28), and dysregulation of this neurotransmitter system has been correlated with deficits in behavior and emotional regulation (29–32). Because SSRI exposure in youngsters occurs at a time of ongoing neuronal adaptations (33–35) and such treatments can last for years (7,36,37), it is not difficult to conceive the notion that antidepressant treatments impact development of brain pathways dramatically influencing neurobiological functioning later in life.
Given the prevalence of prescription antidepressant use during adolescence and the scarcity of knowledge regarding long-term effects of such treatments, it is essential that the neurobiological consequences associated with FLX exposure be characterized. Thus, this study was designed to assess the short- and long-term behavioral responsivity to a range of emotion-eliciting stimuli after FLX exposure during adolescence (postnatal day [PD] 35–49) in male rats.
Methods and Materials
Subjects
Male Sprague-Dawley rats were obtained from Charles River (Raleigh, North Carolina). For the initial experiment (Figure 1), rats arrived on the same day at PD30 (adolescent) and PD60 (~250–275 g, adults). For all other experimental conditions, rats arrived on PD30 and treatment started at PD35 or PD65 as depicted in Figure S1 in Supplement 1. The age at the start and duration of the experimental manipulations in adolescent rats (PD35–PD49) was selected because it roughly approximates adolescence in humans (33,35,38). Rats were housed in pairs in clear polypropylene boxes containing wood shavings in an animal colony maintained at 23°C to 25°C on a 12-hour light-dark cycle in which lights were on between 07:00 and 19:00 hours. Rats were provided with food and water ad libitum.
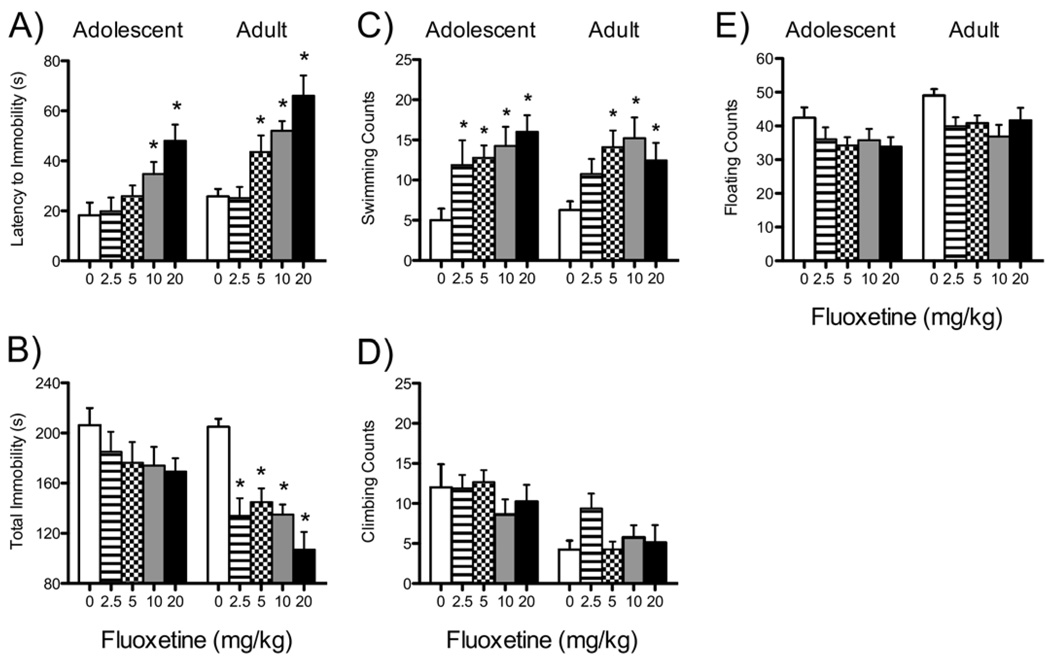
Figure 1.
Acute effects of fluoxetine on forced swimming behaviors in adolescent (PD35; n = 8–9/dose) and adult (PD70+; n = 8–10/dose) male rats (A–E). (A) Latency to become immobile, (B) total immobility, (C) swimming counts, (D) climbing counts, and (E) floating counts of rats tested on the forced swim test after three injections (same dose) of fluoxetine (0, 2.5, 5, 10, or 20 mg/kg) 1, 5, and 23 hours between swims. Data were analyzed using individual one-way analyses of variance (p < .05) between the age groups. *Significantly different from vehicle control rats within the same age group. PD, postnatal day.
Drug Treatment and Experimental Design
Fluoxetine hydrochloride was obtained from Sigma (St. Louis, Missouri), dissolved in sterile distilled water, and administered in a volume of 2 mL/kg. An initial experiment was conducted using the forced swim test (FST) to establish the FLX dose that would reliably decrease immobility as characterized in adult (250–275 g) rats (39,40). The FST consists of two swimming sessions over 2 days. The PD35 and PD65 rats were exposed to the FST on day 1 and then received intraperitoneal injections of FLX (0, 2.5, 5, 10, or 20 mg/kg) 23 hours, 5 hours, and 1 hour before re-exposure to the FST (day 2). Based on the results from this experiment (Figure 1), separate groups of PD35 rats were treated with FLX (0 or 10 mg/kg) twice daily (4 hours apart) for 15 consecutive days. Rats were randomly assigned to treatment and behavioral conditions, and the schedule of behavioral testing was counterbalanced among all groups (Table S1 in Supplement 1). Because rodents metabolize FLX about 10 times faster than humans (41), this drug schedule was selected to approximate FLX levels observed clinically. Short-term behavioral testing began 24 hours after the last injection, whereas long-term assessments started when subjects reached adulthood (Figure S1A in Supplement 1). Rats assigned to receive FLX in adulthood (treatment starting at PD65, Figure S1B in Supplement 1) were used as positive control rats (matched for drug treatment and testing time) only for the FST. Rats treated with FLX during adolescence and re-exposed to FLX as adults were tested on a single behavioral paradigm (i.e., food approach in a novel environment; Figure S5 and Table S1 in Supplement 1). Behavioral observations and analyses were performed by observers with no knowledge of the treatment conditions of each rat. All experiments were conducted in compliance with the 1996 National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by Florida State University Animal Care and Use Committee.
Sucrose Preference
The sucrose preference test (Figure S4 in Supplement 1) consisted of a two-bottle choice paradigm, as described previously (42) (full details in Supplement 1).
Locomotor Activity
Spontaneous locomotor activity was indexed as distance traveled (cm) in an open-field (OF) apparatus for 30 minutes (see Figure S3A,B in Supplement 1 for details and results).
Novel Object Approach
This test was conducted over 2 days. Rats were introduced to the OF for 30 minutes (day 1). On day 2, rats were brought back to the OF for a 5-minute re-acclimation period, and immediately after, a novel object (a white polyvinyl chloride plastic rod [5 cm diameter, 7.5 cm height]) was placed in the center of the apparatus. Rats were allowed to explore the object for 5 minutes (light intensity: 5 lux). Latency to approach and time spent exploring the object, on initial approach, were measured. Exploration was scored only when the rat’s nose or front paws touched the object. Longer latencies were interpreted as an anxiety-like response, while exploration time was interpreted as being associated with reward (43,44).
Food Approach in a Novel Environment
This test was modified from Ansorge et al. (29) and performed under red light at the beginning of the dark phase (testing time: 5 minutes). At 17:00 hours, rats were single-housed with access to water. At the start of the dark phase (19:00 hours), rats were placed in a corner of the OF containing a single food pellet (familiar rat chow) placed on a circular white filter paper (12 cm) positioned in the center of the apparatus. Latency to approach the food and begin feeding was scored. The test ended immediately after rats started feeding or if they failed to approach food after 5 minutes, at which time they were placed back in their home cage with normal access to food and water.
Elevated Plus-Maze
The time spent and number of entries into the open arms of an elevated plus-maze (EPM) were assessed over 5 minutes, as previously described (42) (Supplement 1).
Forced Swim Test
The FST was conducted as previously described (45). Latency to immobility, total immobility, and behavioral counts (i.e., swimming, climbing, and floating) were recorded (details in Supplement 1).
Sexual Behavior
The sexual behavior experiments were carried out as previously described (46) under red light conditions between 13:00 and 18:00 hours. Male rats were given a 5-minute acclimation period to the testing arena, and testing was initiated by the introduction of a receptive female rat to the arena. Testing sessions (at PD80 and PD90, respectively) lasted 90 minutes (Supplement 1).
Statistical Analyses
Assignment of subjects to the various testing conditions was random. Behavioral data were analyzed using one-way or mixed-design (between and within variables) repeated analyses of variance (ANOVA) followed by Fisher’s least significant difference post hoc test. When appropriate, additional Student t tests were used to determine statistical significance of preplanned comparisons. Data are expressed as the mean ± SEM. Statistical significance was defined as p < .05.
Results
Establishing FST Behavioral Reactivity
Fluoxetine increased latency to immobility in adolescents [F(4,39) = 5.43, p < .001; Figure 1A, left panel; n = 8–9/group]. Rats receiving 10 or 20 mg/kg FLX displayed longer latencies to immobility when compared with control rats (p < .05). Fluoxetine had a tendency toward decreasing total immobility (p = .07; Figure 1B) and dose-dependently increased swimming counts [F(4,39) = 3.77, p < .01; Figure 1C], while having no effect on climbing or floating counts (Figure 1D,E).
Fluoxetine dose-dependently increased latency to immobility in adults [F(4,39) = 8.88, p < .001; Figure 1A, right panel; n = 8–10/group]. Rats receiving 5, 10, or 20 mg/kg FLX displayed longer latencies to immobility (p < .05) and decreased total immobility [F(4,39) = 3.12, p < .02] compared with control rats (p < .05; Figure 1B). Fluoxetine increased swimming counts [F(4,39) = 2.72, p < .04; Figure 1C], without affecting climbing or floating counts.
Effects of FLX on Body Weight
Based on the results above, 10 mg/kg FLX was selected to treat adolescent and adult rats for 15 days (twice daily). Figure S2 in Supplement 1 shows the effects of FLX on body weight gain in PD35 (n = 18/group) and PD65 (n = 7–8/group) rats. A mixed-design repeated measures ANOVA revealed that FLX significantly decreased weight gain across days [main effect: F(14,476) = 930.75, p < .0001], drug [main effect: F(1, 34) = 11.67, p < .002; Figure S2A inset in Supplement 1], and as a function of day by drug [interaction: F(14,476) = 25.31, p < .0001] in adolescent rats (Figure S2A in Supplement 1). Although body weight increased with age, the FLX-treated adolescent rats displayed lower weights than control rats (p < .05). Similarly, FLX reduced body weight in adult rats (Figure S2B in Supplement 1) as a function of injection day [F(14,182) = 14.93, p < .0001], drug [F(1,13) = 25.11, p < .0001; Figure S2B inset in Supplement 1], and day by drug [F(14,182) = 18.05, p < .0001]. Fluoxetine-treated adult rats displayed lower weights than control rats (p < .05).
Effects of Chronic FLX on Sucrose Preference
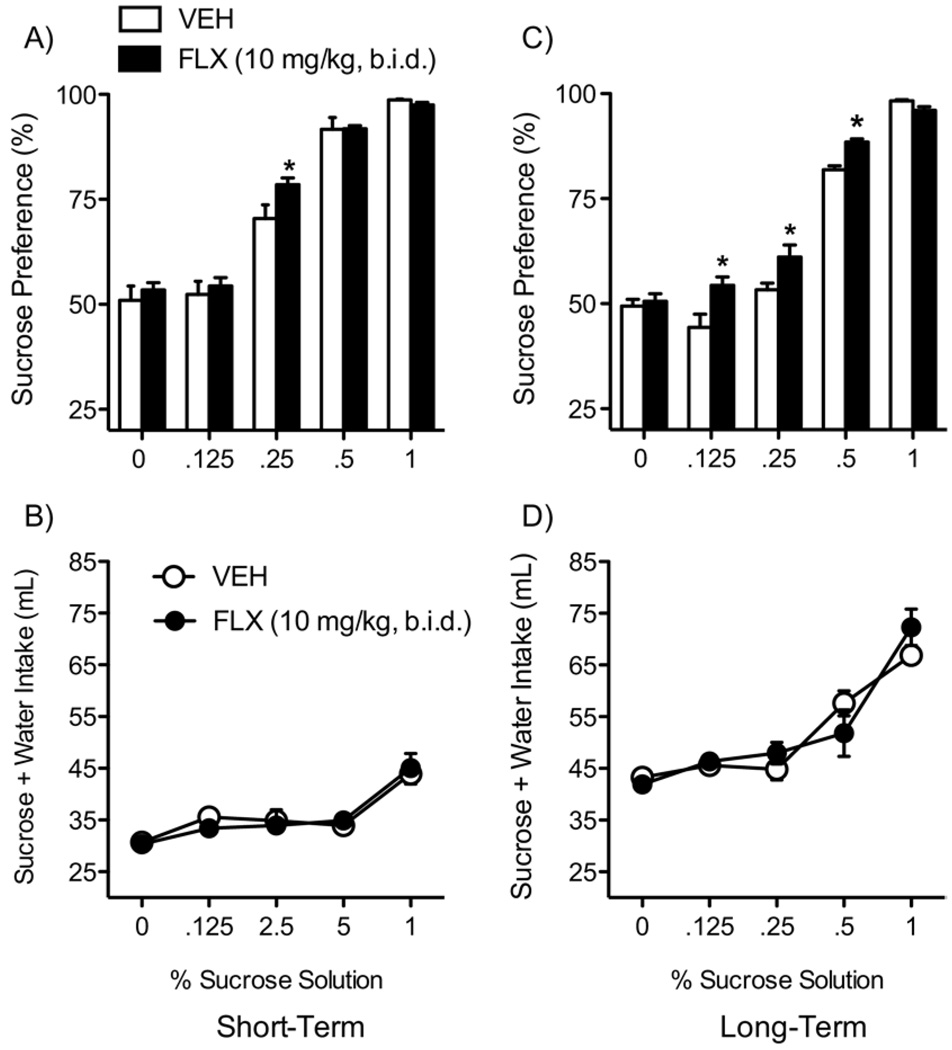
Fluoxetine did not influence total fluid intake (water + sucrose; Figure 2B) 24 hours after treatment (n = 13/group; short-term). Conversely, there was a main effect of sucrose [F(1,24) = 4.71, p < .04; Figure 2A), with FLX-treated rats preferring sucrose only at the .25% concentration (p < .05). A separate ANOVA revealed that FLX treatment during adolescence increased sucrose preference in adulthood (Figure 2C; long-term), without affecting total fluid intake (Figure 2D; n = 15/group). Sucrose preference varied by sucrose concentration [main effect: F(4,112) = 145.56, p < .05] and drug [main effect: F(1,28) = 9.08, p < .05]. Fluoxetine treatment increased sucrose preference only at the .125%, .25%, and .5% concentrations (p < .05, respectively; Figure 2C).
Figure 2.
Flouxetine (10 mg/kg, b.i.d.) exposure during adolescence regulates responses to sucrose reward (A–D). (A) Exposure to FLX significantly increased sucrose preference when compared with VEH-treated control rats (at the .25% concentration) 24 hours after treatment (p < .05; n = 13/ group). (C) Rats treated with FLX during adolescence and tested in adulthood (long-term) show a significant increase in sensitivity to the rewarding effects of sucrose (p < .05; n = 15/group). No differences in total fluid intake (sucrose + water) were detected regardless of treatment or time of testing (B and D). *Significantly different than VEH-treated control rats (p < .05). Data are presented as percent preference or total mL consumed between VEH- and FLX-exposed rats (mean ± SEM). b.i.d., twice daily; FLX,fluoxetine; VEH, vehicle.
Effects of FLX on Anxiety-Like Behaviors
Elevated Plus-Maze
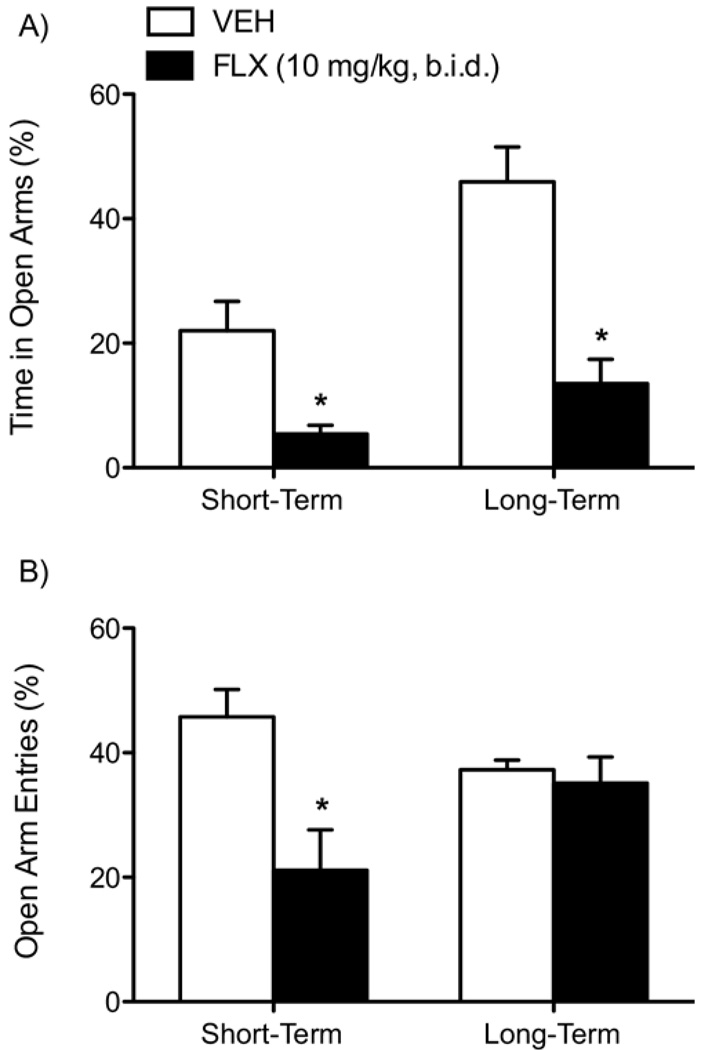
Fluoxetine induced anxiety-like behaviors 24 hours after the last injection (short-term; n = 8/group) and in adulthood (long-term; n = 8/group). Fluoxetine significantly decreased percent time spent [F(1,14) = 11.03, p < .005; Figure 3A, left panel] and percent entries [F(1,14) = 9.63, p < .008; Figure 3B, left panel] in the open arms of the EPM. Similarly, rats tested in adulthood spent significantly less percent time in the open arms [F(1,14) = 21.93, p < .0001; Figure 3A, right panel] but did not differ in percent entries into the open arms of the EPM (Figure 3B, right panel).
Figure 3.
Fluoxetine (10 mg/kg, b.i.d.) exposure during adolescence regulates anxiety-like behavior in the EPM (A and B). Short-term (n = 8/group): FLX significantly reduced time spent (A, left panel) and entries (B, left panel) into the open arms of the EPM 24 hours after the last FLX injection (p < .05). Long-term (n = 8/group): FLX also reduced time spent (A, right panel) in the open arms of the EPM, without influencing entries (B, right panel) compared with VEH-treated control rats. Data are presented as percent time spent and percent entries (mean ± SEM) into the open arms of the EPM. b.i.d., twice daily; EPM, elevated plus-maze; FLX, fluoxetine; VEH, vehicle.
Novel Object Approach in a Familiar Environment
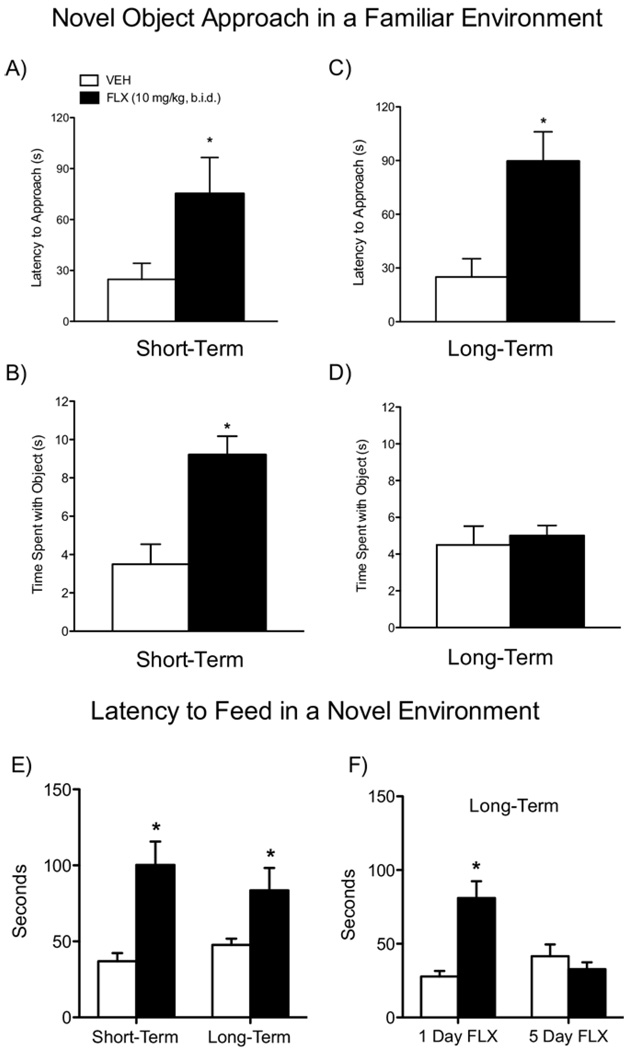
There were significant differences in the latency to approach a novel object 24 hours after treatment [t(17) = −2.16, p < .05]. Fluoxetine-treated rats took significantly longer to approach the object than control rats (Figure 4A; n = 9–10/group). Additionally, once the FLX-treated rats first approached the object, they spent significantly more time exploring it (Figure 4B) than control rats [t(17) = −3.59, p < .02]. A somewhat similar behavioral pattern was observed in rats tested in adulthood: FLX-treated rats displayed longer latencies to approach (Figure 4C; n = 14–15/group) [t(27) = −2.32, p < .03] but showed no differences in time spent exploring the object (Figure 4D).
Figure 4.
Effects of fluoxetine (10 mg/kg, b.i.d.) exposure during adolescence on the latency to approach a novel object in a familiar environment (A–D) and the latency to feed in a novel environment (E and F). Short-term (n = 9–10/group): FLX-treated rats had significantly longer latencies to approach (A) and spent significantly more time exploring (B) the novel object 24 hours after the last FLX injection. Long-term (n = 14–15/group): FLX-treated rats displayed significantly longer latencies to approach (C) but spent similar time exploring (D) the novel object compared with control rats. (E) FLX increased latency to feed in a novel environment at both short-term (n = 9/group) and long-term (n = 6/group) time points of behavioral assessment. (F) Acute exposure to FLX (10 mg/kg) did not decrease latency to feed (F, left panel; n = 6/group), while chronic exposure (5 days) to FLX (10 mg/kg) reversed this effect to control levels (F, right panel; n = 6/group) in a separate group of adult rats pretreated with FLX during adolescence. *Significantly different compared with VEH-treated control rats (p < .05). b.i.d., twice daily; FLX, fluoxetine; VEH, vehicle.
Latency to Feed in a Novel Environment
Fluoxetine-treated rats had significantly longer latencies to approach food in a novel environment 24 hours after treatment [t(16) = −4.24, p < .05; n = 9/group; Figure 4E, short-term] or in adulthood [t(10) = −2.35, p < .05; n = 6/group; Figure 4E, long-term]. We also assessed whether FLX could reverse these effects in a separate group of adult rats pretreated with FLX during adolescence. Repeated [5 days; t(10) = −3.8, p < .05], but not acute (1 day), FLX (10 mg/kg) reversed the aberrant latency to approach food in these rats (Figure 4F; n = 6/group).
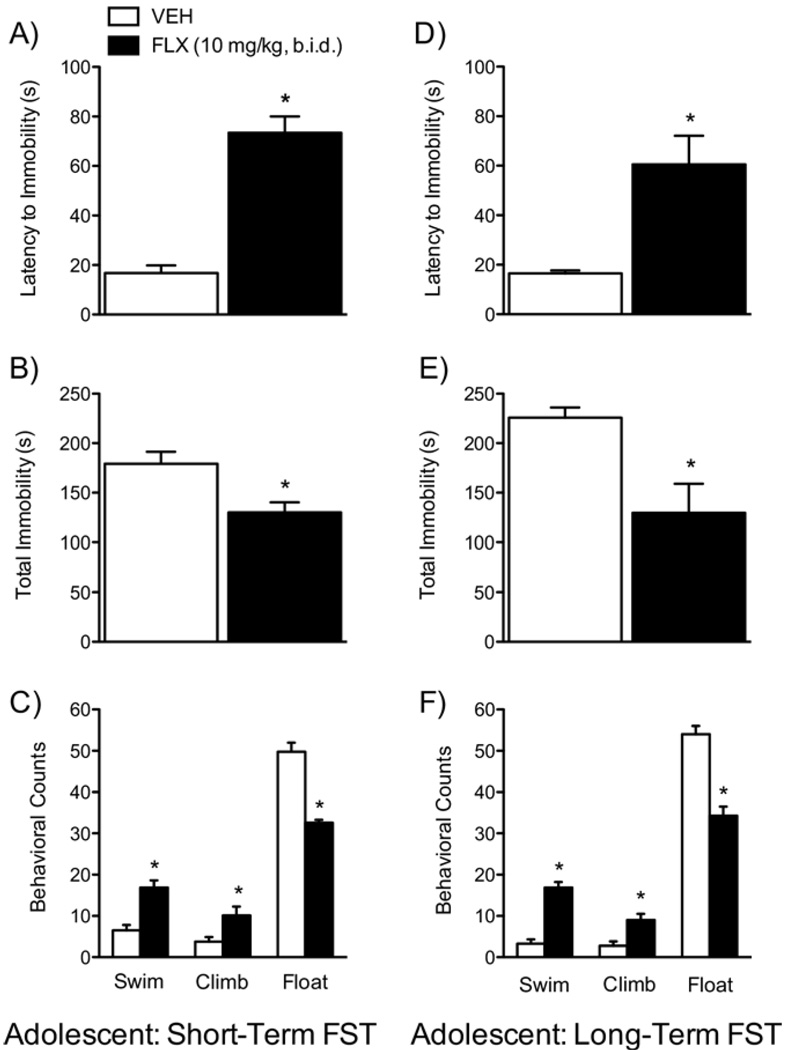
Effects of FLX on the FST
We used the FST to assess rats’ responsiveness to stress 24 hours after treatment (Figure 5A–C) or when they reached adulthood (Figure 5D–F). Fluoxetine-treated rats displayed longer latencies to immobility [t(9) = −6.1, p < .05] and decreased total immobility [t(9) = 3.01, p < .05] compared with control rats 24 hours after treatment (Figure 5A,B; n = 5–6/ group). Fluoxetine induced higher swimming [t(9) = −3.87, p < .05] and climbing counts [t(9) = −2.67, p < .05], with lower floating counts [t(9) = 9.16, p < .05; Figure 5C] than control rats. Fluoxetine-treated rats during adolescence and tested in adulthood also displayed a behavioral profile similar to the short-term group (Figure 5D–F; n = 15/group): longer latencies to immobility [t(28) = −2.39, p < .02; Figure 5D], lower total immobility [t(28) = 3.40, p < .05; Figure 5E], higher swimming counts [t(28) = −3.78, p < .001], higher climbing counts [t(28) = −3.34, p < .05], and lower floating counts [t(28) = 3.35, p < .002; Figure 5F].
Figure 5.
Effects of fluoxetine (10 mg/kg, b.i.d.) on behavioral responsivity to swim stress (A–F). Short-term (n = 5–6/group): FLX-treated rats displayed significantly longer latencies to immobility (A), lower total immobility (B), higher swimming and climbing counts and lower floating counts (C) when compared with VEH-treated control rats. Long-term (n = 15/group): FLX-treated rats displayed similar behavioral profile (D–F) as those tested in the short-term condition when compared with their VEH-treated control rats. *Significantly different from VEH-treated rats (p < .05). Data are presented as latencies to become immobile and total immobility (in seconds) and as cumulative 5-second intervals of swimming, climbing, and floating counts (mean ± SEM). b.i.d., twice daily; FLX, fluoxetine; FST, forced swim test; VEH, vehicle.
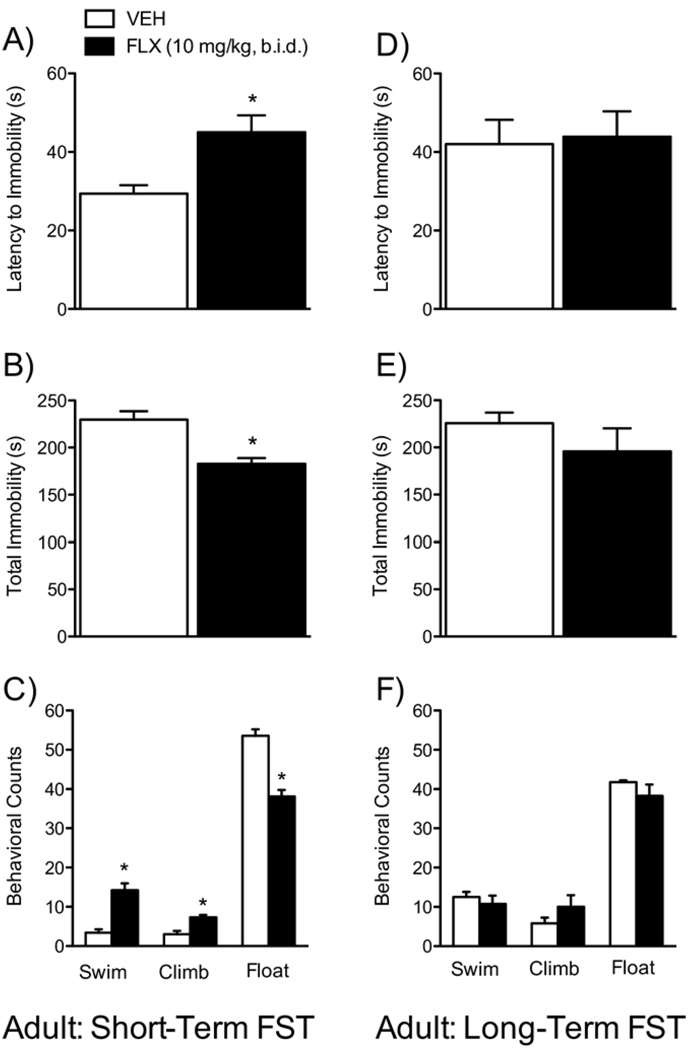
A separate group of adult rats was tested on the FST after chronic FLX (matched drug treatment and testing schedule, as with the adolescent group above; Figure 6A–F) to determine whether these FLX-induced effects on the FST are specific to adolescent treatment. These adult FLX-treated rats showed a similar behavioral profile as the FLX-treated adolescents only when tested 24 hours after the last injection (Figure 6A–C; n = 7/group): longer latencies to immobility [t(12) = −4.35, p < .001; Figure 6A], decreased total immobility [t(12) = 3.48, p < .005; Figure 6B], higher swimming counts [t(12) = −4.42, p < .001], higher climbing counts [t(12) = −4.25, p < .001; Figure 6C], and lower floating counts [t(12) = 6.06, p < .0001; Figure 6C]. Fluoxetine had no effects when the long-term adult group was tested 21 days after treatment (Figure 6D–F, n = 7/group).
Figure 6.
Effects of fluoxetine (10 mg/kg, b.i.d.) treatment in adult rats (matched control group) on behavioral responsivity to forced swim stress (A–F). Short-term (n = 7/group): FLX-treated rats displayed significantly longer latencies to immobility (A), lower total immobility (B), higher swimming and climbing counts and lower floating counts (C) when compared with VEH-treated control rats. Long-term (n = 7/group): no differences were observed in any of the measures assessed between the groups. *Significantly different from VEH-treated rats (p < .05). Data are presented as latencies to become immobile and total immobility (in seconds) and as cumulative 5-second intervals of swimming, climbing, and floating counts (mean ± SEM). b.i.d., twice daily; FLX, fluoxetine; FST, forced swim test; VEH, vehicle.
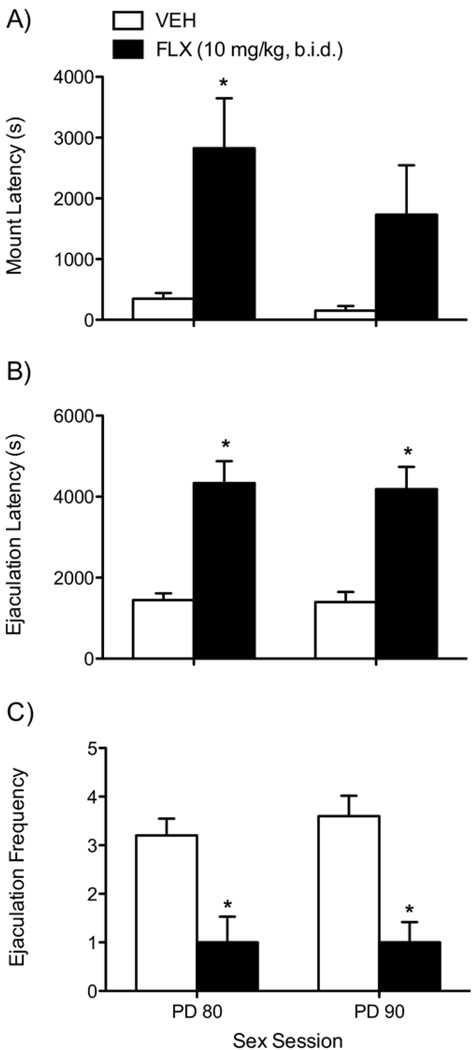
Effects of Adolescent FLX Exposure on Sexual Behavior
Fluoxetine-exposed rats exhibited deficits in sexual activity when assessed in two separate 90-minute sexual behavior sessions (PD80 and PD90, respectively; Figure 7A–C; n = 10/ group). A repeated measures (sex session) ANOVA indicated that mount latency varied only as a function of drug [F(1,18) = 7.38, p < .01; Figure 7A]. Fluoxetine-pretreated rats displayed longer mount latency than control rats at PD80 (p < .05; Figure 7A, left panel) but not at PD90 (Figure 7A, right panel). Fluoxetine also influenced ejaculation latency between the groups [F(1,18) = 28.31, p < .0001], with FLX-exposed rats displaying longer times to reach the first ejaculation at PD80 (p < .05; Figure 7B, left panel) and PD90 (p < .05; Figure 7B, right panel). Ejaculation frequency was affected by FLX [F(1,18) = 20.01, p < .0001; Figure 7C], with FLX-exposed rats showing lower ejaculation frequency than control rats at both PD80 (p < .05) and PD90 (p < .05) sessions.
Figure 7.
Effects of fluoxetine (10 mg/kg, b.i.d.) exposure during adolescence in adult male rat sexual behavior (A–C; n = 10/group). Rats were given two 90-minute sessions (at postnatal day 80 and 90, respectively) to copulate with a receptive female. FLX treatment during adolescence increased the latency to mount an estrous receptive female (A), latency to reach the first ejaculation (B), and the total number of ejaculations (C) compared with VEH-treated control rats in the first sex session (PD80). During the second sex session (PD90), FLX treatment during adolescence increased latency to ejaculate (B, right panel) and decreased ejaculation frequency (C, right panel) without affecting latency to mount (A, right panel). *Significantly different from VEH-treated rats (p < .05). b.i.d., twice daily; FLX, fluoxetine; PD, postnatal day; VEH, vehicle.
Discussion
Antidepressants are often prescribed to pediatric populations (21); yet, there is a scarcity of knowledge regarding the short-term and/or long-lasting neurobiological consequences of such treatments during early life (11). Thus, this study was designed to assess enduring behavioral outcomes in response to rewarding and aversive situations resulting from repeated FLX exposure during adolescence in male rats. This approach was taken because serotonin and compounds that regulate its function interact with mesolimbic reward systems, part of the circuitry controlling emotional and motivated behaviors (47–52). We report that exposure to FLX during PD35 to PD49 leads to decreased responsiveness to stressful situations, increased sensitivity to natural reward, and anxiety-eliciting situations, including deficits in sexual behavior, in adulthood.
Exposure to FLX during adolescence increased rats’ normal sensitivity to sucrose (a natural reward) in adulthood, while only inducing a minimal increase in preference (at the .25% concentration) in rats tested 24 hours after treatment. Because antidepressants reduce body weight and caloric intake in animals and humans (53–55), decreases in sucrose preference were expected. However, the lack of changes in overall liquid intake (sucrose + water) between the groups indicates that increases in preference are likely due to the ability of FLX to alter rats’ responsiveness to the rewarding effects of sucrose in adulthood. Therefore, it is possible that the young rats tested short-term did not respond robustly to sucrose because of the ability of FLX to decrease caloric intake and palatability of sweet solutions (56,57). To further explore reward sensitivity after FLX administration, time spent exploring a novel object in a familiar environment was measured (43,58,59). Fluoxetine-treated adolescent rats spent significantly longer exploring the object 24 hours after treatment, indicating that interacting with the novel object was rewarding (60). However, no changes in object exploration were observed long-term and it consequently failed to complement the sucrose preference findings. Brain reward pathways, such as the nucleus accumbens (NAc) and its dopaminergic input from the ventral tegmental area, mediate responses to natural rewards (52,61,62). Ingesting sweet solutions and exploring novel objects activate this circuit (52,63,64) and disruption of this neural projection decreases interest for sucrose and novelty (61,65–67). As in the present study, research assessing the effects of antidepressant treatment on reward-related behavior reveals a complex picture. Antidepressants can decrease (68,69), increase (70,71), or have no effects (72) on responding for rewarding brain stimulation, with equivocal results when assessing responding for natural rewards (56,73). Nevertheless, antidepressants do sensitize brain reward pathways (74–76): they increase the firing activity of ventral tegmental area dopamine neurons (77), increase dopamine neurotransmission in the striatum (78–80), and enhance cocaine and morphine reward (81,82). Therefore, it is conceivable that FLX exposure during adolescence enhances reward processes that are likely discernable only in adulthood; however, more detailed studies assessing this notion are needed.
Our findings further indicate that FLX enhances reactivity to anxiogenic stimuli as measured in the EPM 24 hours after treatment in adolescent rats. This anxiety-like response was long-lived because the FLX-treated adolescent rats tested in adulthood showed similar anxiety-like responding. We also used latency to approach a novel object in a familiar environment and latency to start feeding in a novel environment as additional indexes of anxiety-like behaviors. When exposed to novel environments, rats face a conflict between their motivation to explore the environment (novelty preference) and fear of potential negative consequences (83,84). Thus, longer latencies to approach a novel object or to start feeding have been interpreted as indicative of higher levels of anxiety (29). Similar to the EPM findings, FLX-exposed rats took longer to approach a novel object in a familiar environment and to start feeding in a novel environment at both short- and long-term testing time points. Because familiarity of environment increases novelty seeking and the FLX-treated rats had longer latencies to approach the novel object in a familiar environment, it is conceivable that FLX exposure during adolescence induces “trait” and not situational anxiety (83,85); however, an alternate explanation could be that they have increased caution and less impulsivity (86). These results are supported by reports indicating that administration of SSRIs early in life results in long-lasting anxiogenic phenotypes (29,32,87). We also show that chronic, but not acute, re-exposure (i.e., 5 days) to FLX in adulthood alleviates the FLX-induced anxiety-like behavior observed in the start-to-feeding test, findings consistent with previous reports (88). Furthermore, these findings are supported by studies showing that initial exposure to antidepressants, which have been used successfully for the management of anxiety disorders, exacerbate anxiogenic-like behaviors in humans (89–91) and animals (92–94), but these alterations dissipate after prolonged exposure (95–97). Under the appropriate conditions, behavioral reactivity in the OF can also be used as an index of anxiety (98); thus, it must be noted that the overall activity observed in the OF (Figure S3A,B in Supplement 1) does not complement our findings of increased anxiety-like behaviors. Nevertheless, reports show that emotionality-related behavior from the OF and the EPM do not produce a common anxiety-related factor in adolescent rats (99), indicating that emotionality is multidimensional and that these tests do not always complement each other (100–103).
Fluoxetine-treated rats showed lower levels of behavioral despair when exposed to forced swimming. Rats tested 24 hours after treatment showed coping patterns commonly categorized as antidepressant-like behaviors (39,104,105), and this effect was also present in the long-term group (i.e., those treated during adolescence and tested in adulthood). These findings were not due to FLX-induced changes in motor activity because rats tested 24 hours after day 1 of FST showed no differences in distance traveled in the OF (Figure S3C,D in Supplement 1). An antidepressant-like phenotype after adolescent FLX counters reports showing that early-life (PD4–PD21) FLX administration renders mice vulnerable to stressful situations in adulthood (29,32,56). However, other studies using similar age and treatment regimen in mice also find equivocal results (87,88,106–108). To determine if these effects were specific to age of FLX exposure, we treated adult rats and exposed them to forced swimming 24 hours or 21 days after the last injection (i.e., matched drug regimen and testing time as the adolescents). Only those adult rats tested 24 hours after treatment displayed reduced behavioral despair in the FST, while the long-term group did not differ from control rats. Our results suggest that the FLX-induced effects in the FST may be specific to adolescent FLX treatment, and this assumption is supported by studies demonstrating that altered behavioral profiles induced by antidepressants are dependent on age of exposure (29,32,56,88). The mechanism(s) underlying these effects are unknown. In adults, antidepressants regulate complex cellular and intracellular signaling mechanisms such as brain-derived neurotrophic factor, extracellular signal-regulated kinase, and cyclic adenosine monophosphate-responsive element binding protein activity, factors associated with the regulation of mood and motivation, resulting in lasting synaptic changes influencing behavioral functioning (109–112). Fluoxetine actions in the nervous system are complex, and more detailed assessments of these phenomena accounting for length of exposure and discontinuation and developmental periods are clearly needed (35,97,113–115).
Lastly, we assessed whether FLX exposure during adolescence influences sexual behavior later in adulthood (see Figure S6 in Supplement 1). Fluoxetine-exposed rats showed increased latencies to mount and ejaculate and deficits in ejaculation frequency. Antidepressant treatments interfere with sexual functioning in both humans and rodents (116–118); however, these findings were unexpected, as the drug washout period for this particular group of animals was over 30 days and the behavioral deficits were observed at both PD80 and PD90 sessions. The mechanism(s) underlying these effects are also unknown. Serotonin interacts in a complex manner with several of its receptors to inhibit various aspects of sexual and ejaculatory functioning (119,120). Therefore, it is conceivable that early-life FLX induces long-lasting changes in receptors (e.g., increased sensitivity and/or density) known to inhibit sexual behavior (121). Alternatively, it is possible that sustained FLX exposure dysregulates second messenger systems, since others have shown that altered cyclic adenosine monophosphate-responsive element binding protein activity within the NAc of adult rats leads to impairments in the initiation of sexual behavior, but not the rewarding aspects of sex, in addition to increases in anxiety-like behavior (46,122,123). These findings parallel our results after adolescent FLX exposure: longer latencies to initiate sexual activity and increased sensitivity to anxiety-inducing situations in adulthood. Unfortunately, our results cannot discern whether the appetitive aspects of sexual behavior were influenced by FLX because the dependent variables assessed do not differentiate between interest and performance. Nevertheless, it is unlikely that the longer latencies to initiate sexual activity were due to a reduced reward valence, because FLX-treated rats initiated sexual behavior no differently than control rats on the PD90 session, thus indicating that this impairment is likely due to increased anxiety and not reduced reward sensitivity (46,122,123). In fact, the NAc has recently been found to exert a significant influence on anxiety-related behaviors in both rodents and humans (46,123,124).
The overall results from our study indicate that treatment with FLX during adolescence can influence responsiveness to rewarding and aversive stimuli in adulthood. These complex functional outputs are likely regulated by many factors, including the emotional valence of the stimulus, the environment in which it is experienced, and the brain circuitry likely being engaged by it. Our findings also demonstrate that FLX-induced anxiety-like behavior can be alleviated by re-exposure to FLX itself. However, it is imperative to note that the FLX-induced effects described in this study were derived from normal animals, and similar FLX treatment using established animal models for depression might yield different results. Given that our subjects were purchased, it is impossible to determine if and/or how stress of shipping may have influenced our results. Another caveat is that we did not include female subjects in our study, further limiting the inter-pretability of our results. Indeed, the results from this study should be interpreted with caution because FLX remains a safe and effective treatment for pediatric MDD.
Supplementary Material
Acknowledgments
This work was supported by Grants R03DA020089 and R21DA022351 from the National Institute on Drug Abuse, a NARSAD Young Investigator Award, and a First Year Assistant Professor Award from Florida State University to CAB-G. SDI was supported by a McKnight Fellowship from the Florida Education Fund, a Neuroscience Fellowship from the Florida State University, and a National Research Service Award (F31DA027300) from the National Institute on Drug Abuse. BLW was supported by a Neuroscience Fellowship from the Florida State University.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online
References
- 1.Kapornai K, Vetro A. Depression in children. Curr Opin Psychiatry. 2008;21:1–7. doi: 10.1097/YCO.0b013e3282f25b01. [DOI] [PubMed] [Google Scholar]
- 2.Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J, Dahl RE, et al. Childhood and adolescent depression: A review of the past 10 years. Part I. J Am Acad Child Adolesc Psychiatry. 1996;35:1427–1439. doi: 10.1097/00004583-199611000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Weissman MM, Wolk S, Goldstein RB, Moreau D, Adams P, Greenwald S, et al. Depressed adolescents grown up. JAMA. 1999;281:1707–1713. doi: 10.1001/jama.281.18.1707. [DOI] [PubMed] [Google Scholar]
- 4.Pfeffer CR. Diagnosis of childhood and adolescent suicidal behavior: Unmet needs for suicide prevention. Biol Psychiatry. 2001;49:1055–1061. doi: 10.1016/s0006-3223(01)01141-6. [DOI] [PubMed] [Google Scholar]
- 5.Rihmer Z. Suicide risk in mood disorders. Curr Opin Psychiatry. 2007;20:17–22. doi: 10.1097/YCO.0b013e3280106868. [DOI] [PubMed] [Google Scholar]
- 6.Kovacs M. Presentation and course of major depressive disorder during childhood and later years of the life span. J Am Acad Child Adolesc Psychiatry. 1996;35:705–715. doi: 10.1097/00004583-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Patten CA, Choi WS, Vickers KS, Pierce JP. Persistence of depressive symptoms in adolescents. Neuropsychopharmacology. 2001;25:S89–S91. doi: 10.1016/S0893-133X(01)00323-2. [DOI] [PubMed] [Google Scholar]
- 8.Harrison PJ. The neuropathology of primary mood disorder. Brain. 2002;125:1428–1449. doi: 10.1093/brain/awf149. [DOI] [PubMed] [Google Scholar]
- 9.Rohde P, Lewinsohn PM, Seeley JR. Are adolescents changed by an episode of major depression? J Am Acad Child Adolesc Psychiatry. 1994;33:1289–1298. doi: 10.1097/00004583-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Safer DJ. Should selective serotonin reuptake inhibitors be prescribed for children with major depressive and anxiety disorders? Pediatrics. 2006;118:1248–1251. doi: 10.1542/peds.2006-0215. [DOI] [PubMed] [Google Scholar]
- 12.Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: A comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]
- 13.Pliszka SR. The use of psychostimulants in the pediatric patient. Pediatr Clin North Am. 1998;45:1085–1098. doi: 10.1016/s0031-3955(05)70063-8. [DOI] [PubMed] [Google Scholar]
- 14.Emslie GJ, Mayes TL, Hughes CW. Depression: Recent developments and innovative treatments. Psychiatr Clin North Am. 2000;23:813–835. [Google Scholar]
- 15.Emslie GJ, Mayes TL. Mood disorders in children and adolescents: Psychopharmacological treatment. Biol Psychiatry. 2001;49:1082–1090. doi: 10.1016/s0006-3223(01)01149-0. [DOI] [PubMed] [Google Scholar]
- 16.Coyle JT, Pine DS, Charney DS, Lewis L, Nemeroff CB, Carlson GA, et al. Depression and bipolar support alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2003;42:1494–1503. doi: 10.1097/00004583-200312000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Zito JM, Safer DJ, DosReis S, Gardner JF, Soeken K, Boles M, et al. Rising prevalence of antidepressants among US youths. Pediatrics. 2002;109:721–727. doi: 10.1542/peds.109.5.721. [DOI] [PubMed] [Google Scholar]
- 18.Birmaher B. Should we use antidepressant medications for children and adolescents with depressive disorders? Psychopharmacol Bull. 1998;34:35–39. [PubMed] [Google Scholar]
- 19.Kratochvil CJ, Vitiello B, Walkup J, Emslie G, Waslick BD, Weller EB, et al. Selective serotonin reuptake inhibitors in pediatric depression: Is the balance between benefits and risks favorable? J Child Adolesc Psychopharmacol. 2006;16:11–24. doi: 10.1089/cap.2006.16.11. [DOI] [PubMed] [Google Scholar]
- 20.Coyle JT. Psychotropic drug use in very young children. JAMA. 2000;283:1059–1060. doi: 10.1001/jama.283.8.1059. [DOI] [PubMed] [Google Scholar]
- 21.Zito JM, Tobi H, de Jong-van Berg LT, Fegert JM, Safer DJ, Janhsen K, et al. Antidepressant prevalence for youths: A multi-national comparison. Pharmacoepidemiol Drug Saf. 2006;15:793–798. doi: 10.1002/pds.1254. [DOI] [PubMed] [Google Scholar]
- 22.de Montigny C, Blier P. Effects of antidepressant treatments on 5-HT neurotransmission: Electrophysiological and clinical studies. Adv Biochem Psychopharmacol. 1984;39:223–239. [PubMed] [Google Scholar]
- 23.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 24.Blakemore SJ, Choudhury S. Brain development during puberty: State of the science. Dev Sci. 2006;9:11–14. doi: 10.1111/j.1467-7687.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- 25.Azmitia EC. Serotonin and brain: Evolution, neuroplasticity, and homeostasis. Int Rev Neurobiol. 2007;77:31–56. doi: 10.1016/S0074-7742(06)77002-7. [DOI] [PubMed] [Google Scholar]
- 26.Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity during brain development: A role in autism? Int J Dev Neurosci. 2005;23:75–83. doi: 10.1016/j.ijdevneu.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Borue X, Chen J, Condron BG. Developmental effects of SSRIs: Lessons learned from animal studies. Int J Dev Neurosci. 2007;25:341–347. doi: 10.1016/j.ijdevneu.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molliver ME. Serotonergic neuronal systems: What their anatomic organization tells us about function. J Clin Psychopharmacol. 1987;7 3S–23S. [PubMed] [Google Scholar]
- 29.Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 30.Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Harmer CJ, Bhagwagar Z, Perrett DI, Vollm BA, Cowen PJ, Goodwin GM. Acute SSRI administration affects the processing of social cues in healthy volunteers. Neuropsychopharmacology. 2003;28:148–152. doi: 10.1038/sj.npp.1300004. [DOI] [PubMed] [Google Scholar]
- 32.Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 34.Seeman P, Bzowej NH, Guan HC, Bergeron C, Becker LE, Reynolds GP, et al. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- 35.Andersen SL, Navalta CP. Altering the course of neurodevelopment: A framework for understanding the enduring effects of psychotropic drugs. Int J Dev Neurosci. 2004;22:423–440. doi: 10.1016/j.ijdevneu.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Bhagwagar Z, Cowen PJ. “It’s not over when it’s over”: Persistent neurobiological abnormalities in recovered depressed patients. Psychol Med. 2008;38:307–313. doi: 10.1017/s0033291707001250. [DOI] [PubMed] [Google Scholar]
- 37.Duval F, Lebowitz BD, Macher JP. Treatments in depression. Dialogues Clin Neurosci. 2006;8:191–206. doi: 10.31887/DCNS.2006.8.2/fduval. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spear LP, Brake SC. Periadolescence: Age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- 39.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: Recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 40.Porsolt RD. Animal models of depression: Utility for transgenic research. Rev Neurosci. 2000;11:53–58. doi: 10.1515/revneuro.2000.11.1.53. [DOI] [PubMed] [Google Scholar]
- 41.Wegerer V, Moll GH, Bagli M, Rothenberger A, Ruther E, Huether G. Persistently increased density of serotonin transporters in the frontal cortex of rats treated with fluoxetine during early juvenile life. J Child Adolesc Psychopharmacol. 1999;9:13–24. doi: 10.1089/cap.1999.9.13. discussion 25–26. [DOI] [PubMed] [Google Scholar]
- 42.Bolaños CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol Psychiatry. 2003;54:1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- 43.Bevins RA, Besheer J. Novelty reward as a measure of anhedonia. Neurosci Biobehav Rev. 2005;29:707–714. doi: 10.1016/j.neubiorev.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Friedman A, Frankel M, Flaumenhaft Y, Merenlender A, Pinhasov A, Feder Y, et al. Programmed acute electrical stimulation of ventral tegmental area alleviates depressive-like behavior. Neuropsychopharmacology. 2009;34:1057–1066. doi: 10.1038/npp.2008.177. [DOI] [PubMed] [Google Scholar]
- 45.Iñiguez SD, Warren BL, Parise EM, Alcantara LF, Schuh B, Maffeo ML, et al. Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology. 2009;34:1609–1624. doi: 10.1038/npp.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iniguez SD, et al. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;12:200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 48.Yadid G, Friedman A. Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog Brain Res. 2008;172:265–286. doi: 10.1016/S0079-6123(08)00913-8. [DOI] [PubMed] [Google Scholar]
- 49.Naranjo CA, Tremblay LK, Busto UE. The role of the brain reward system in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:781–823. doi: 10.1016/s0278-5846(01)00156-7. [DOI] [PubMed] [Google Scholar]
- 50.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.aan het Rot M, Mathew SJ, Charney DS. Neurobiological mechanisms in major depressive disorder. CMAJ. 2009;180:305–313. doi: 10.1503/cmaj.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelley AE, Berridge KC. The neuroscience of natural rewards: Relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simansky K, Eberle-Wang K. Serotonergic mechanisms and ingestion: Pharmacological facts and physiological promises. Appetite. 1993;21:220. doi: 10.1016/0195-6663(93)90010-h. [DOI] [PubMed] [Google Scholar]
- 54.Halford JC, Harrold JA, Lawton CL, Blundell JE. Serotonin (5-HT) drugs: Effects on appetite expression and use for the treatment of obesity. Curr Drug Targets. 2005;6:201–213. doi: 10.2174/1389450053174550. [DOI] [PubMed] [Google Scholar]
- 55.Halford JC, Blundell JE. Pharmacology of appetite suppression. Prog Drug Res. 2000;54:25–58. doi: 10.1007/978-3-0348-8391-7_2. [DOI] [PubMed] [Google Scholar]
- 56.Popa D, Lena C, Alexandre C, Adrien J. Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: Evidence from sleep, stress, and behavior. J Neurosci. 2008;28:3546–3554. doi: 10.1523/JNEUROSCI.4006-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asin KE, Davis JD, Bednarz L. Differential effects of serotonergic and catecholaminergic drugs on ingestive behavior. Psychopharmacology (Berl) 1992;109:415–421. doi: 10.1007/BF02247717. [DOI] [PubMed] [Google Scholar]
- 58.Besheer J, Bevins RA. The role of environmental familiarization in novel-object preference. Behav Processes. 2000;50:19–29. doi: 10.1016/s0376-6357(00)00090-5. [DOI] [PubMed] [Google Scholar]
- 59.Hughes RN. Neotic preferences in laboratory rodents: Issues, assessment and substrates. Neurosci Biobehav Rev. 2007;31:441–464. doi: 10.1016/j.neubiorev.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 60.Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: Possible implications for age differences in substance abuse and other risk-taking behaviors [published online ahead of print September 16] Brain Cogn. 2009 doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hajnal A, Norgren R. Accumbens dopamine mechanisms in sucrose intake. Brain Res. 2001;904:76–84. doi: 10.1016/s0006-8993(01)02451-9. [DOI] [PubMed] [Google Scholar]
- 62.Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, et al. The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. J Neurosci. 2008;28:10272–10277. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berlyne DE. The arousal and satiation of perceptual curiosity in the rat. J Comp Physiol Psychol. 1955;48:238–246. doi: 10.1037/h0042968. [DOI] [PubMed] [Google Scholar]
- 64.Klebaur JE, Bardo MT. The effects of anxiolytic drugs on novelty-induced place preference. Behav Brain Res. 1999;101:51–57. doi: 10.1016/s0166-4328(98)00145-4. [DOI] [PubMed] [Google Scholar]
- 65.Shimura T, Kamada Y, Yamamoto T. Ventral tegmental lesions reduce overconsumption of normally preferred taste fluid in rats. Behav Brain Res. 2002;134:123–130. doi: 10.1016/s0166-4328(01)00461-2. [DOI] [PubMed] [Google Scholar]
- 66.Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361:1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wise RA. Dopamine and food reward: Back to the elements. Am J Physiol Regul Integr Comp Physiol. 2004;286:R13. doi: 10.1152/ajpregu.00590.2003. [DOI] [PubMed] [Google Scholar]
- 68.Lee K, Kornetsky C. Acute and chronic fluoxetine treatment decreases the sensitivity of rats to rewarding brain stimulation. Pharmacol Biochem Behav. 1998;60:539–544. doi: 10.1016/s0091-3057(98)00020-3. [DOI] [PubMed] [Google Scholar]
- 69.Katz RJ, Carroll BJ. Intracranial reward after Lilly 110140 (fluoxetine HCl): Evidence for an inhibitory role for serotonin. Psychopharmacology (Berl) 1977;51:189–193. doi: 10.1007/BF00431739. [DOI] [PubMed] [Google Scholar]
- 70.Konkle AT, Bielajew C. Feeding and reward interactions from chronic paroxetine treatment. Pharmacol Biochem Behav. 1999;63:435–440. doi: 10.1016/s0091-3057(99)00044-1. [DOI] [PubMed] [Google Scholar]
- 71.Redgrave P, Horrell RI. Potentiation of central reward by localised perfusion of acetylcholine and 5-hydroxytryptamine. Nature. 1976;262:305–307. doi: 10.1038/262305a0. [DOI] [PubMed] [Google Scholar]
- 72.Matthews K, Baldo BA, Markou A, Lown O, Overstreet DH, Koob GF. Rewarding electrical brain stimulation: Similar thresholds for Flinders Sensitive Line Hypercholinergic and Flinders Resistant Line Hypocholinergic rats. Physiol Behav. 1996;59:1155–1162. doi: 10.1016/0031-9384(95)02212-0. [DOI] [PubMed] [Google Scholar]
- 73.Qi X, Lin W, Li J, Li H, Wang W, Wang D, Sun M. Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress. Neurobiol Dis. 2008;31:278–285. doi: 10.1016/j.nbd.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 74.Collu M, Poggiu AS, Serra G. Antidepressants sensitize the dopamine mesolimbic system mediating reward. Behav Pharamcol. 1996;7 suppl 1:18. [Google Scholar]
- 75.Collu M, Poggiu AS, Pani L, Serra G. Fluoxetine-induced conditioned place preference: A preliminary study. Synapse. 1997;25:309–311. doi: 10.1002/(SICI)1098-2396(199703)25:3<309::AID-SYN11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 76.Subhan F, Deslandes PN, Pache DM, Sewell RD. Do antidepressants affect motivation in conditioned place preference? Eur J Pharmacol. 2000;408:257–263. doi: 10.1016/s0014-2999(00)00771-8. [DOI] [PubMed] [Google Scholar]
- 77.Sekine Y, Suzuki K, Ramachandran PV, Blackburn TP, Ashby CR., Jr Acute and repeated administration of fluoxetine, citalopram, and paroxetine significantly alters the activity of midbrain dopamine neurons in rats: An in vivo electrophysiological study. Synapse. 2007;61:72–77. doi: 10.1002/syn.20349. [DOI] [PubMed] [Google Scholar]
- 78.Serra G, Collu M, D’Aquila PS, Gessa GL. Role of the mesolimbic dopamine system in the mechanism of action of antidepressants. Pharmacol Toxicol. 1992;71 suppl 1:72–85. doi: 10.1111/j.1600-0773.1992.tb01631.x. [DOI] [PubMed] [Google Scholar]
- 79.Bolaños CA, Trksak GH, Cohen OS, Jackson D. Differential serotonergic inhibition of in vitro striatal [3H]acetylcholine release in pre-natally cocaine-exposed male and female rats. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1339–1348. doi: 10.1016/s0278-5846(02)00299-3. [DOI] [PubMed] [Google Scholar]
- 80.Bolaños CA, Trksak GH, Glatt SJ, Jackson D. Prenatal cocaine exposure increases serotonergic inhibition of electrically evoked acetylcholine release from rat striatal slices at adulthood. Synapse. 2000;36:1–11. doi: 10.1002/(SICI)1098-2396(200004)36:1<1::AID-SYN1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 81.Deslandes PN, Pache DM, Buckland P, Sewell RD. Morphine, cocaine and antidepressant induced motivational activity and midbrain dopaminergic neurotransmission. Eur J Pharmacol. 2002;453:223–229. doi: 10.1016/s0014-2999(02)02451-2. [DOI] [PubMed] [Google Scholar]
- 82.Nomikos GG, Damsma G, Wenkstern D, Fibiger HC. Chronic desipramine enhances amphetamine-induced increases in interstitial concentrations of dopamine in the nucleus accumbens. Eur J Pharmacol. 1991;195:63–73. doi: 10.1016/0014-2999(91)90382-z. [DOI] [PubMed] [Google Scholar]
- 83.Ohl F. Testing for anxiety. Clin Neurosci Res. 2003;3:233–238. [Google Scholar]
- 84.Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: Differential expression of stress-related molecules. J Neurosci. 2000;20:6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Belzung C, El Hage W, Moindrot N, Griebel G. Behavioral and neurochemical changes following predatory stress in mice. Neuropharmacology. 2001;41:400–408. doi: 10.1016/s0028-3908(01)00072-7. [DOI] [PubMed] [Google Scholar]
- 86.Hyman SE. Methylphenidate-induced plasticity: What should we be looking for? Biol Psychiatry. 2003;54:1310–1311. doi: 10.1016/j.biopsych.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 87.Norcross M, Mathur P, Enoch AJ, Karlsson RM, Brigman JL, Cameron HA, et al. Effects of adolescent fluoxetine treatment on fear-, anxiety- or stress-related behaviors in C57BL/6J or BALB/cJ mice. Psychopharmacology (Berl) 2008;200:413–424. doi: 10.1007/s00213-008-1215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karpova NN, Lindholm J, Pruunsild P, Timmusk T, Castren E. Long-lasting behavioural and molecular alterations induced by early postnatal fluoxetine exposure are restored by chronic fluoxetine treatment in adult mice. Eur Neuropsychopharmacol. 2009;19:97–108. doi: 10.1016/j.euroneuro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 89.den Boer JA, Westenberg HG, Kamerbeek WD, Verhoeven WM, Kahn RS. Effect of serotonin uptake inhibitors in anxiety disorders; a double-blind comparison of clomipramine and fluvoxamine. Int Clin Psychopharmacol. 1987;2:21–32. doi: 10.1097/00004850-198701000-00002. [DOI] [PubMed] [Google Scholar]
- 90.Papp LA, Sinha SS, Martinez JM, Coplan JD, Amchin J, Gorman JM. Low-dose venlafaxine treatment in panic disorder. Psychopharmacol Bull. 1998;34:207–209. [PubMed] [Google Scholar]
- 91.Nutt DJ, Glue P, Lawson C. The neurochemistry of anxiety: An update. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14:737–752. doi: 10.1016/0278-5846(90)90044-h. [DOI] [PubMed] [Google Scholar]
- 92.Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. Int J Neuropsychopharmacol. 2001;4:399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- 93.Belzung C, Le Guisquet AM, Barreau S, Calatayud F. An investigation of the mechanisms responsible for acute fluoxetine-induced anxiogenic-like effects in mice. Behav Pharmacol. 2001;12:151–162. doi: 10.1097/00008877-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 94.Drapier D, Bentue-Ferrer D, Laviolle B, Millet B, Allain H, Bourin M, et al. Effects of acute fluoxetine, paroxetine and desipramine on rats tested on the elevated plus-maze. Behav Brain Res. 2007;176:202–209. doi: 10.1016/j.bbr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 95.Silva RC, Brandao ML. Acute and chronic effects of gepirone and fluoxetine in rats tested in the elevated plus-maze: An ethological analysis. Pharmacol Biochem Behav. 2000;65:209–216. doi: 10.1016/s0091-3057(99)00193-8. [DOI] [PubMed] [Google Scholar]
- 96.To CT, Anheuer ZE, Bagdy G. Effects of acute and chronic fluoxetine treatment of CRH-induced anxiety. Neuroreport. 1999;10:553–555. doi: 10.1097/00001756-199902250-00020. [DOI] [PubMed] [Google Scholar]
- 97.Bolaños CA, Willey MD, Maffeo ML, Powers KD, Kinka DW, Grausam KB, et al. Antidepressant treatment can normalize adult behavioral deficits induced by early-life exposure to methylphenidate. Biol Psychiatry. 2008;63:309–316. doi: 10.1016/j.biopsych.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 98.Britton DR, Britton KT. A sensitive open field measure of anxiolytic drug activity. Pharmacol Biochem Behav. 1981;15:577–582. doi: 10.1016/0091-3057(81)90212-4. [DOI] [PubMed] [Google Scholar]
- 99.McCormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models [published online ahead of print July 17] Brain Cogn. 2009 doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 100.Ramos A, Mellerin Y, Mormede P, Chaouloff F. A genetic and multifactorial analysis of anxiety-related behaviours in Lewis and SHR intercrosses. Behav Brain Res. 1998;96:195–205. doi: 10.1016/s0166-4328(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 101.Ramos A, Mormede P. Stress and emotionality: A multidimensional and genetic approach. Neurosci Biobehav Rev. 1998;22:33–57. doi: 10.1016/s0149-7634(97)00001-8. [DOI] [PubMed] [Google Scholar]
- 102.Archer J. Tests for emotionality in rats and mice: A review. Anim Behav. 1973;21:205–235. doi: 10.1016/s0003-3472(73)80065-x. [DOI] [PubMed] [Google Scholar]
- 103.Ramos A. Animal models of anxiety: Do I need multiple tests? Trends Pharmacol Sci. 2008;29:493–498. doi: 10.1016/j.tips.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 104.Porsolt RD, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 105.Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 106.Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res. 2007;176:210–215. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oh JE, Zupan B, Gross S, Toth M. Paradoxical anxiogenic response of juvenile mice to fluoxetine. Neuropsychopharmacology. 2009;34:2197–2207. doi: 10.1038/npp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Jong TR, Snaphaan LJ, Pattij T, Veening JG, Waldinger MD, Cools AR, et al. Effects of chronic treatment with fluvoxamine and paroxetine during adolescence on serotonin-related behavior in adult male rats. Eur Neuropsychopharmacol. 2006;16:39–48. doi: 10.1016/j.euroneuro.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 109.Bolaños CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. Neuromolecular Med. 2004;5:69–83. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- 110.Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 111.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 112.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carlezon WA, Jr, Konradi C. Understanding the neurobiological consequences of early exposure to psychotropic drugs: Linking behavior with molecules. Neuropharmacology. 2004;47 suppl 1:47–60. doi: 10.1016/j.neuropharm.2004.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol. 2004;15:341–352. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 115.Wiley MD, Poveromo LB, Antapasis J, Herrera CM, Bolanos Guzman CA. Kappa-opioid system regulates the long-lasting behavioral adaptations induced by early-life exposure to methylphenidate. Neuropsychopharmacology. 2009;34:1339–1350. doi: 10.1038/npp.2008.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sukoff Rizzo SJ, Schechter LE, Rosenzweig-Lipson S. A novel approach for predicting antidepressant-induced sexual dysfunction in rats. Psychopharmacology (Berl) 2008;195:459–467. doi: 10.1007/s00213-007-0924-7. [DOI] [PubMed] [Google Scholar]
- 117.Cantor JM, Binik YM, Pfaus JG. Chronic fluoxetine inhibits sexual behavior in the male rat: Reversal with oxytocin. Psychopharmacology (Berl) 1999;144:355–362. doi: 10.1007/s002130051018. [DOI] [PubMed] [Google Scholar]
- 118.Ferguson JM. The effects of antidepressants on sexual functioning in depressed patients: A review. J Clin Psychiatry. 2001;62 suppl 3:22–34. [PubMed] [Google Scholar]
- 119.Hull EM, Muschamp JW, Sato S. Dopamine and serotonin: Influences on male sexual behavior. Physiol Behav. 2004;83:291–307. doi: 10.1016/j.physbeh.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 120.Sukoff Rizzo SJ, Pulicicchio C, Malberg JE, Andree TH, Stack GP, Hughes ZA, et al. 5-HT1A receptor antagonism reverses and prevents fluoxetine-induced sexual dysfunction in rats. Int J Neuropsychopharmacol. 2009;12:1045–1053. doi: 10.1017/S1461145709000406. [DOI] [PubMed] [Google Scholar]
- 121.Li Q, Muma NA, van de Kar LD. Chronic fluoxetine induces a gradual desensitization of 5-HT1A receptors: Reductions in hypothalamic and midbrain Gi and G(o) proteins and in neuroendocrine responses to a 5-HT1A agonist. J Pharmacol Exp Ther. 1996;279:1035–1042. [PubMed] [Google Scholar]
- 122.Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barrot M, Wallace DL, Bolanos CA, Graham DL, Perrotti LI, Neve RL, et al. Regulation of anxiety and initiation of sexual behavior by CREB in the nucleus accumbens. Proc Natl Acad Sci U S A. 2005;102:8357–8362. doi: 10.1073/pnas.0500587102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sturm V, Lenartz D, Koulousakis A, Treuer H, Herholz K, Klein JC, et al. The nucleus accumbens: A target for deep brain stimulation in obsessive-compulsive- and anxiety-disorders. J Chem Neuroanat. 2003;26:293–299. doi: 10.1016/j.jchemneu.2003.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.