Abstract
The accumulation of fat in tissues not suited for lipid storage has deleterious consequences on organ function, leading to cellular damage that underlies diabetes, heart disease, and hypertension. To combat these lipotoxic events, several therapeutics improve insulin sensitivity and/or ameliorate features of metabolic disease by limiting the inappropriate deposition of fat in peripheral tissues (i.e. thiazolidinediones, metformin, and statins). Recent advances in genomics and lipidomics have accelerated progress towards understanding the pathogenic events associated with the excessive production, underutilization, or inefficient storage of fat. Herein we review studies applying pharmacological or genetic strategies to manipulate the expression or activity of enzymes controlling lipid deposition, in order to gain a clearer understanding of the molecular mechanisms by which fatty acids contribute to metabolic disease.
Keywords: Lipotoxicity, Diabetes, Lipid, Sphingolipids, Insulin resistance, Genetic manipulation, Metabolism, Metabolic syndrome, Lipid metabolism
1. Introduction
“Gluttony is the source of all our infirmities, and the fountain of all our diseases. As a lamp is choked by a superabundance of oil, a fire extinguished by excess of fuel, so is the natural health of the body destroyed by intemperate diet.” Robert Burton, English writer, philosopher and humorist, 1621
In a 1992 paper entitled “What if Minkowski had been ageusic—an alternative angle on diabetes,” Dennis McGarry suggested that the glucose-centric view of diabetes had led researchers astray, and that the disease would be “more readily understood if viewed in the context of underlying abnormalities of lipid metabolism” [1]. This seminal paper challenged existing dogma about the aetiology of the disease, and introduced a new and controversial model suggesting that increased action of insulin, specifically its ability to drive lipid synthesis in the liver, rather than insulin resistance, was the key feature in the progression towards diabetes. Some 17 years later, we know that Dr. McGarry was not only correct in many of his predictions, but that disorders in lipid metabolism and the excess delivery of fat to peripheral tissues underlie most of the more prevalent diseases affecting mankind. In the article below, we discuss progress elucidating the molecular mechanisms by which abnormalities in lipid metabolism contribute to diabetes and other metabolic diseases.
The psychological and economic toll of diabetes is well documented, and the increasing rate of occurrence of the condition is disconcerting and unequivocal [2]. The astounding proliferation of the disease is undoubtedly related to population changes in body composition, as the prevalence of obesity in the USA has risen from percentages in the mid-teens at the time of Dr. McGarry’s article to nearly 33% today [3]. With obesity rates increasing in developed and developing countries across the globe [4], the World Health Organization estimates that a staggering number of individuals are currently dealing with complications of disrupted lipid homeostasis, 1.6 billion adults are overweight, 400 million are obese, and 180 million are diabetic [5]. Cardiovascular disease, which is the major complication of diabetes, is the cause of death of 17 million [6].
Based on our review of the literature, we surmise that the metabolic diseases associated with obesity, including diabetes and cardiovascular disease, derive from a common molecular pathogenesis characterized of two independent stages: (a) selective insulin resistance; and (b) lipotoxicity (Fig. 1).
Fig. 1.

Schematic diagram depicting the flux of nutrients during the proposed stages of metabolic disease. (A) In healthy individuals, insulin facilitates postprandial nutrient deposition by (a) inhibiting hepatic glucose production, (b) stimulating glucose uptake into muscle, and promoting triglyceride synthesis in (c) liver and (d) adipose. (B) The obese become selectively resistant to insulin effects on glucose metabolism, such that muscle glucose transport is blocked and hepatic glucose output is enhanced (denoted by red X’s). However, other effects of insulin remain intact, and the enhanced lipogenesis in the liver leads to increased circulating triglyceride levels (i.e. VLDLs). As circulating insulin levels rise, due to the residual glucose in the blood, the lipogenic effects of the hormone become dominant, and a vicious cycle ensues. Increased production of triglycerides leads to an increasing rate of their delivery to peripheral tissues, greater insulin resistance, more profound hyperinsulinemia, and exacerbation of the lipid synthesis effects of the hormone. (C) The final stages of metabolic disease involve additional defects in the adipocyte. Impairments in adipocyte plasticity or storage capacity limit its utility as a reservoir for excess fat. Moreover, impairment of insulin action in the tissue leads to enhanced lipolysis, further increasing delivery of fatty acids to the liver. Ultimately, lipid delivery to peripheral tissues exceeds their storage and oxidative capacity, and tissue damage ensues.
-
Stage One: Selective Insulin Resistance. When circulating glucose reaches a critical threshold level, pancreatic β-cells secrete insulin, the major anabolic hormone in the body. Insulin has two major actions: to lower circulating glucose levels by facilitating its uptake into skeletal muscle while inhibiting its production by the liver; and, to promote the storage of available nutrients, predominantly in the form of glycogen and fat. Obese individuals display a decreased capacity for insulin-stimulated glucose disposal, as muscle fibres adjust to utilize the calorically dense fatty acids, rather than glucose, as a primary energy source. However, insulin-stimulated lipogenesis, particularly in the liver and adipose tissue, is unaltered or enhanced. This selective insulin resistance is one of several factors which predispose individuals to cardiovascular disease and diabetes [7]
The relative importance of insulin resistance in the pathogenesis of metabolic disease is subject to debate. At best, insulin resistance is a benign predictor of metabolic disease—an independent marker of a condition where the supply of nutrients exceeds demand, and muscle is adapting to oxidize lipids, while sparing glucose. At worst, this selective insulin resistance exacerbates and accelerates the path towards metabolic disease, as it promotes hyperinsulinemia, leading to an increase in triglyceride synthesis and enhanced delivery of lipoprotein-bound fats to peripheral tissues.
Stage Two: Lipotoxicity in Peripheral Tissues. Diseases related to obesity probably result from the delivery of lipids to peripheral tissues in excess of their oxidative or storage capacities. Indeed, experimental manipulations aimed to enhance lipid delivery to peripheral tissues (e.g. pancreatic β-cells or the heart) or prevent their storage in white adipose tissue is sufficient to recapitulate many of the features of these diseases [8–19]. Moreover, pharmacological therapies for warding off the condition almost invariably reduce the accumulation of ectopic fat in non-adipose tissues (e.g. thiazolidienediones, metformin, or statins).
Ultimately, both of these stages likely result from the excess delivery of fat to peripheral tissues, and the body appears capable of sensing small changes in cellular lipids. During selective insulin resistance, elevation in intracellular lipid metabolites leads to an inhibition of GLUT4 glucose transporters, thus decreasing net rates of glucose influx into the cell. During lipotoxicity, this increased lipid deposition leads to cellular events that compromise tissue function, such as ER and oxidative stress, apoptosis, etc. However, an understanding of the mechanisms underlying these varied responses has been elusive.
In virtually all tissues, fatty acids face one of three major metabolic fates: (a) they can be converted to glycerolipids, including triglycerides, diglycerides, and major membrane phospholipids (Fig. 2); (b) they can be converted to sphingolipids, including sphingomyelin and ceramide (Fig. 3); and, (c) they can be oxidized for energy (Fig. 4). As the genes and enzymes that control these anabolic and catabolic pathways have been sequenced and characterized, accompanying experiments manipulating their expression and/or activity has revealed that modulating rates of lipid synthesis and degradation has profound effects on insulin-sensitivity, diabetes, and cardiovascular disease.
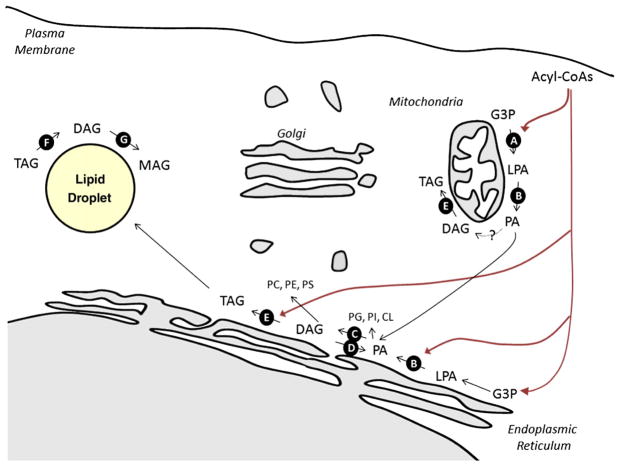
Fig. 2.
Schematic diagram illustrating key reactions in glycerolipid synthesis. Most glycerolipid synthesis occurs in the ER, though some enzymes have been found in mitochondria and/or mitochondrial associated membranes. Enzymes discussed in the text are noted with the black circles. A major substrate is glycerol-3-phosphate derived either from the reduction of the glycolytic intermediate dihydroxyacetone phosphate or from ATP-dependent phosphorylation of glycerol by glycerol kinase. Glycerol-3-phosphate undergoes successive esterifications with acyl-CoAs to produce lysophosphatidic acid (LPA) (catalyzed by GPATs, Reaction A) and phosphatidic acid (PA) (catalyzed by AGPATs, Reaction B). Phosphatidic acid is a precursor for both to membrane glycerophospholipids and triglycerides. Lipins dephosphorylate PA to produce DAG (Reaction C), and diacylglycerol kinases (Reaction D) catalyze the reverse reaction. DGATs add the final acyl moiety, to produce triglycerides. Hydrolysis of TAG includes the sequential actions of ATGL (Reaction F) and HSL (Reaction G).
Fig. 3.
Schematic diagram illustrating key reactions in sphingolipid synthesis. De novo synthesis of ceramide occurs in the ER, though some of the biosynthetic enzymes have additionally been found in ER and mitochondria. Enzymes discussed in the text are noted with the black circles. Serine palmitoyltransferase (Reaction A) catalyzes the initial step, which involves the condensation of serine and palmitoyl-CoA to form 3-ketosphinganine. 3-ketosphinganine reductase reduces 3-ketosphinganine to form the sphingoid base sphinganine. A family of (dihydro)Ceramide synthase catalyzes sphinganine acylation, producing dihydroceramide. Dyhydroceramide desaturase (Reaction B) oxidizes inactive dyhydroceramide into active ceramide. Once generated, ceramide is the common precursor of complex sphingolipids. In the golgi, glucosylceramide synthase (Reaction E) and sphingomyelin synthases convert the precursor ceramide into glucosylceramides and sphingomyelin, respectively. Glucosylceramides are the substrate for further reactions leading to the production of complex gangliosides. One of these, a GM3 synthase (reaction D), is discussed in he text. Within the lysosome (Reaction E) and plasma membrane, sphingomyelinases convert sphingomyelin back into ceramide, which can be further deacylated to produce sphingosine (Reaction F).
Fig. 4.
Schematic representation of fatty acid oxidation pathway. Fatty acyl-CoAs are transported into the mitochondrial matrix by the carnitine transport system, with CPT1 being a key and rate-limiting component (Reaction A). Once inside the mitochondrial matrix, fatty acyl-CoAs are oxidized in a series of repeated steps that each release acetyl-CoA. The basic outlines of this pathway includes the following: transfer to carnitine to acyl-CoAs by CPT-1; transport of acyl-carnitines through inner mitochondrial membranes; regeneration of acyl-CoA by carnitine acyltransferase II (CPT-2); successive rounds of β-oxidation of fatty acids. Acetyl-CoA then enters the citric acid cycle, where it is oxidized in the same fashion as the acetyl-CoA derived from glycolysis. As acetyl-CoA levels rise, acetyl-CoA carboxylases (ACCs, Reaction B) convert it into malonyl-CoA, first committed intermediated in fatty acid biosynthesis and a potent inhibitor of CPT-1. Key regulators of malonyl-CoA levels include malonyl-CoA decarboxylase (MCD, Reaction C) and fatty acid synthase (FAS, Reaction D).
2. Manipulation of glycerolipid synthesis: impact on selective insulin resistance and lipotoxicity
Energy-dense triglycerides (TAGs) have two major physiological roles: first, they are energy stores, and their accumulation, preferentially in adipocytes, allows organisms to prepare for periods when food supplies are scarce; second, they are the primary form in which lipids are transported between tissues, as they are the major constituents of lipoproteins. In most tissues, de novo TAG synthesis involves a series of enzymatic reactions localized in the endoplasmic reticulum, where fatty acyl-CoA intermediates are coupled onto a glycerol backbone (e.g. glycerolipids) (Fig. 1) [20,21]. Most of the biochemical reactions involved in this pathway were elucidated in Eugene Kennedy’s laboratory in the 1950’s [22].
2.1. Manipulation of GPAT1
Glycerol-phosphate acyltransferase (GPAT) isoforms 1–4 catalyze the first, rate-limiting step in glycerolipid synthesis, which involves the synthesis of lysophosphatidic acid from glycerol–3-phosphate and long-chain acyl-CoA (Fig. 2, Reaction A) [23,24]. In most tissues, the majority of GPAT activity (GPAT3 and 4) resides in the endoplasmic reticulum, but mitochondrial isoforms (GPATs 1 and 2) play a more prominent role in the liver. Knockout mice lacking the mitochondrial GPAT1 display a partial reduction in liver triglycerides and resistance to obesity [25,26]. In particular, the animals have a smaller fat pad, decreased body weight, a lower rate of VLDL secretion, and increased insulin sensitivity [25,26]. This profound metabolic improvement occurs despite the fact that the GPAT1−/− mice demonstrate markedly elevated acyl-CoA levels. Interestingly, the effects of global GPAT1-deficiency on glucose tolerance are highly dependent upon gender and the carbohydrate content of the diet [27], reflecting the increased role of triglyceride synthesis which accompanies carbohydrate consumption. Selective knockdown of GPAT1 in the liver produces similar findings [28], while hepatic overexpression of the enzyme induces hepatic and peripheral insulin resistance [29]. These data strongly suggest that glycerolipid intermediates modulate hepatic glucose output in addition to controlling the rate of delivery of lipids to other peripheral tissues.
2.2. Manipulation of AGPATs
Acyl-CoA:1-acylglycerol-sn-3-phosphate acyltransferases (AGPATs, also referred to as lysophosphatidate acyltransferases, or LPAATs) catalyze the second step in the Kennedy pathway, acylating lysophosphatidic acid to produce phosphatidic acid (Fig. 2, Reaction B). A number of enzymes have been identified which have sequence homology and the capacity to serve as acyltransferases, but most have only been characterized at the biochemical or cellular level [30]. The one exception is AGPAT2, which is present in high levels in adipose, liver, heart, and pancreas, and more modestly in other tissues [30]. Humans with mutations in the AGPAT2 gene are lipodystrophic, and suffer from severe insulin resistance, hepatic steatosis, hypertriglyceridemia, and diabetes [31]. These patients have virtually no adipose tissue and have extremely low serum leptin and adiponectin levels [32–35]. AGPAT2 knockout mice have a comparable phenotype, including nearly complete loss of adipose tissue, insulin resistance, hepatic steatosis, and hyperglycemia [36].
2.3. Manipulation of lipins
Phosphatidate phosphatases (i.e. lipins) catalyze the third step in the Kennedy pathway: the dephosphorylation of phosphatidic acid to form diacyglycerol (Fig. 2, Reaction C). Of the three isoforms, lipin-1 has been characterized most extensively, and the enzyme accounts for all of the phosphatidic acid phosphatase (PAP) activity in adipose tissue and skeletal muscle. In addition, lipin-1 may additionally have coactivator functions which drive the expression of pro-adipogenic genes in adipocytes [37] and lipid oxidation genes in the liver [38]. Lipin-1-deficient fld mice are lipodystrophic, displaying profound hepatic steatosis, hypertriglyceridemia, and insulin resistance [39–41].
Transgenic approaches have been used to assess the role of lipin-1 in adipose tissue and skeletal muscle, and its overexpression in these tissues induces obesity, albeit through separable mechanisms. Animals overexpressing the enzyme in adipose tissue become obese, due to the upregulation in TAG synthesis, but retain insulin sensitivity [42]. Animals overexpressing lipin1 in skeletal muscle also become obese but display marked decreases in whole-body energy expenditure and fat utilization, as well as insulin resistance [42]. In fact, replacing lipin in skeletal muscle of the fld mouse is sufficient to reduce energy metabolism, without increasing adiposity.
2.4. Manipulation of DAGK
The reversal of the step 3 reaction listed above involves the phosphorylation of DAG to produce phosphatidic acid, and at least 10-different DAG-kinases (DAGK) are capable of catalyzing this reaction (Fig. 2, Reaction D). Mice lacking the DAGKδ isoform, which localizes in the ER [43], display a particularly severe phenotype, dying within 24 h after birth [44]. Newborn DGKδ-knockout mice have open eyelids, which is characteristic of reduced epidermal growth factor signalling [44]. Moreover, studies in cell culture reveal that DGKδ deficiency leads to downregulation of EGF-receptors via a PKC-related mechanism [44]. Studies described below suggest that DGKδ may similarly modulate insulin receptor activity through a comparable mechanism.
Noting that DAGKδ was decreased in skeletal muscle from poorly controlled type 2 diabetic patients, the Zierath group investigated whether mice haploinsuifficient for the enzyme displayed alterations in their metabolic characteristics [45]. Enzyme assays revealed that such heterozygosity reduced total DAGK activity in muscle and fat, but not liver. At 9 weeks of age, the DAGK haploinsufficiency did not affect body weight, but at 36 weeks both the body weight and epididymal fat pad weight were increased. The older animals also displayed glucose intolerance and insulin resistance. Under these conditions, DAG accumulated in muscle but not liver, suggesting this tissue was a major site of action. The mice also demonstrated metabolic inflexibility as they had diminished lipid oxidation and were unable to shift between lipid and glucose oxidation during the transition between rested and active states. These changes preceded obesity. Moreover, studies in isolated issues suggested that defects in glucose transport were also evident prior to the animals showing an increase in adipose mass. Moreover, studies in cell culture revealed that the mechanism for the inhibition of insulin action likely involved PKC and the subsequent inhibition of insulin receptor substrate-1 (IRS-1).
2.5. Manipulation of DGATs
In rodents, two acylCoA:diacylglycerol acyltransferase (DGAT) isoforms catalyze incorporation of a fatty acid onto a diacylgycerol backbone, which is the final step in the biosynthetic pathway leading to triglyceride synthesis (Fig. 2E, Reaction E). DGAT2 appears to play a dominant role in most tissues, and mice lacking this isofom die within a few hours after birth due to a severe reduction in triglycerides needed for oxidative metabolism and in essential fatty acids needed for epidermal barrier function [46]. However, studies on DGAT1−/− mice provide insight into the role of glycerolipids in insulin resistance, hepatic steatosis, and lipotoxicity.
Knockout mice lacking DGAT1 are resistant to obesity and remain insulin sensitive when placed on a high fat diet [47]. The reduced adiposity is due to decreased lipid absorption in the intestine [48] and an increase in energy expenditure [47], and results in diminished accumulation of lipids in peripheral tissues, including skeletal muscle and the liver. Surprisingly, DGAT ablation is not associated with increases in other lipid intermediates, such as diacylglycerol (DAG). Many of the effects of whole body DGAT knockouts can be recapitulated by transplanting adipocytes from DGAT1-deficient mice, suggesting that the anti-obesity phenotype is due to functions within this tissue [49]. One predicts that the small adipocytes from the DGAT1 knockout mice retain a favourable endocrine profile. In particular, DGAT deficiency increases leptin sensitivity, and the anti-obesity effects of DGAT-deficiency are lost in mice lacking leptin [50]. In contrast, adiponectin appears dispensable for the improved insulin sensitivity associated with DGAT deficiency [51]. Transgenic overexpression of DGAT1 in adipose tissue of C57Bl/6 mice exacerbates obesity without impacting peripheral insulin sensitivity or glucose homeostasis [52], supporting the hypothesis that lipotoxic accumulation of fat in peripheral tissues, rather than obesity itself, is a key component of glucose intolerance associated with an elevated BMI.
Inhibition of DGAT activities within the liver has been investigated as a therapeutic strategy for limiting lipid delivery to peripheral tissues, but the studies are mixed, depending on the duration of knockdown. Short-term reduction of hepatic DGAT2 using antisense oligonucleotides reduced hepatic steatosis in obese mice and rats, improved hepatic insulin sensitivity, and promoted lipid oxidation [53,54]. Paradoxically, this experimental approach led to reductions in various glycerolipid intermediates, such as DAG and lysophosphatidic acid. Long-term knockdown of DGAT2 using antisense approaches also protected from hepatic steatosis in mice fed a methionine/choline-deficient diet, but increased levels of hepatic fatty acids, oxidative stress, fibrosis and necrosis [55]. DGAT1 knockdown did not produce comparable effects.
Overexpression of DGAT1 or 2 in the liver enhances steatosis in the absence of hepatic insulin resistance [56]. Surprisingly, these transgenic mice have increased levels of other putative lipotoxic intermediates, including DAG, ceramides, and unsaturated FFAs, without evidence of altered hepatic glucose output or peripheral insulin resistance.
Intramyocellular triglyceride levels correlate well with the severity of peripheral insulin resistance, except in endurance-trained athletes, who demonstrate markedly elevated triglyceride levels in type I (oxidative) fibers but remarkably enhanced insulin sensitivity [57–59]. These studies suggest that TAGs are inert entities in muscle, and studies with DGAT reinforce this idea. Overexpression of DGAT1 in skeletal muscle using electroporation or the creation of transgenic mice increases intramyocellular triglyceride levels and improves insulin sensitivity and glucose homeostasis [60,61]. These manipulations are associated with decreased levels of DAG and ceramide, and suggest that TG production in muscle protects from lipotoxicity caused by the accumulation of glycerolipid and sphingolipid intermediates. Of note, manipulating rates of glycerolipid synthesis here seemed to impact sphingolipid synthesis, suggesting that the rate of flux of FFAs through the two arms are interrelated. Interestingly, transgenic overexpression of DGAT2 in type II (glycolytic) fibers leads to the accumulation of TAG and reduced DAG levels, but has a paradoxical decrease in insulin sensitivity and glucose tolerance [62]. However, in this circumstance, the tissues displayed elevated FFA and ceramide content, suggesting that DGAT overexpression somehow leads to a secondary enhancement of sphingolipid synthesis.
2.6. Manipulation of SCD1
Though not technically part of the glycerolipid biosynthetic pathway, stearoyl-CoA desaturase, which catalyzes the synthesis of Δ9-monunsaturated fatty acids, markedly impacts rates of glycerolipid synthesis. As noted previously [63], SCD1-deficient animals display a phenotype remarkably similar to the DGAT1 knockout, including resistance to obesity and enhanced insulin sensitivity [64,65]. The enzyme has been shown to co-localize with DGAT2 [66], and clearly impacts rates of glycerolipid synthesis. Liver-specific depletion of SCD1 using antisense nucleotides protects from hepatic steatosis and insulin resistance [67,68].
Like DGAT, SCD1 expression in peripheral tissues appears to protect cells from lipotoxic events associated with fat exposure, while its inhibition renders them susceptible to lipotoxic insults. In human myotubes, overexpression of SCD1 enhances triglyceride synthesis and prevents inflammatory and ER-stress responses to palmitate [69], suggesting that it prevents lipotoxicity by sequestering lipids into inert triglyceride pools. In the liver, SCD1 deficiency sensitizes cells to injury, despite the concomitant decrease in triglycerides [70]. SCD1 inhibition in various other mouse models has been shown to improve insulin sensitivity and prevent steatosis but to exacerbate molecular events associated with atherosclerotic plaque formation [71] and pancreatic β-cell failure [72,73].
SCD1 clearly promotes triglyceride synthesis, though the mechanism is for this effect remains elusive. Thus, its deficiency in liver leads to diminished steatosis and enhanced lipid oxidation [74], and consequently decreasing adiposity and reducing the delivery of triglycerides to peripheral tissues. By contrast, the enzyme’s deficiency in other cell-types, including β-cells, likely leads to the formation of saturated lipid intermediates that impair tissue function. A more detailed discussion of putative metabolites and their role in lipotoxic events is included below.
2.7. Manipulation of TAG hydrolysis
Most triglycerides in the body reside in lipid droplets, which are dynamic organelles comprised of TG and cholesterol esters surrounded by a phospholipid monolayer containing regulatory proteins (e.g. perilipin, adipophilin, TIP47, etc.). The impact of these proteins on lipid dynamics is fascinating and complex, and is only starting to be fully realized. Ultimately, triglyceride hydrolysis involves the sequential action of three lipases: (a) adipose triglyceride lipase (ATGL) (Fig. 2, Reaction F), which shows higher affinity for TAG; hormone sensitive lipase (HSL) (Fig. 2, Reaction G), which is rat-limiting in the hydrolysis of DAG; and monoglyceride lipase [75,76]. HSL was cloned and characterized first, and knockout mice lacking the enzyme have a paradoxical reduction in WAT mass and resistance to obesity, owing to a downregulation of triglyceride synthesis [77,78]. In this circumstance, HSL depletion reduces β-adrenergic-stimulated lipolysis by a modest 30–40% and promotes DAG accumulation [79,80]. Other mechanisms accounting for the decrease in adiposity in the HSL knockouts include an increase in necrotic adipocyte death and an upregulation of uncoupling protein-1 [81,82].
Reports on glucose homeostasis in HSL−/− mice have shown mixed and contradictory results. In some studies, HSL knockout mice have signs of enhanced glucose uptake, increased insulin sensitivity, protection from diet-induced insulin resistance, and decreased muscle triglycerides [83,84]. Conversely, other groups have reported that HSL−/− mice have decreased insulin sensitivity [85,86]. In addition to affecting insulin-responsive tissues, HSL depletion impacts β-cells, and islets isolated from the whole-body knockouts show impaired insulin secretion [86], owing evidently to the enzyme’s ability to liberate free cholesterol needed for the exocytosis to insulin granules [87]. Indeed, mice lacking HSL selectively in β-cells display hyperglycaemia and disrupted insulin release [88].
Unlike mice lacking HSL, those devoid of ATGL exhibit a predicted phenotype including enlarged fat pads, disrupted lipolysis, and cold sensitivity [89]. All of these findings are consistent with the animals having an impaired lipolytic response, which is decreased by more than 75%. ATGL-deficiency has severe metabolic consequences associated with the accumulation of TAG in peripheral tissues including the heart, skeletal muscle, testis, kidney, and pancreas. The lipid accumulation in cardiac myocytes causes cardiac failure and premature death, evidenced at only 12 weeks of age. The reduced availability of FFAs is accompanied by increased utilization of glucose, improved glucose tolerance, and insulin sensitivity [89]. Thus, in the ATGL knockouts, insulin resistance can be dissociated from lipotoxicity. In a small number of patients, mutations in ATGL leads to abnormal lipid deposition and myopathy [76].
Less is known about the role of ATGL in non-adipose tissues. In the liver, overexpression of ATGL (or HSL) reduces hepatic lipid stores and promotes lipid oxidation [90]. In muscle, whole-animal ATGL deficiency is associated with increased lipid accumulation, glucose uptake, and glucose utilization [89,91]. Overexpression of the enzyme in muscles using electrotransfer increased the oxidation of fatty acids and elevated DAG and ceramide levels [92]. Moreover, its forced overexpression in obese, insulin resistant rats reduced TAG content but did not restore insulin sensitivity [92]. In islets, ATGL may additionally impact insulin secretion. Peyot and colleagues [93] noted that ATGL−/− mice demonstrated increased insulin sensitivity and decreased GSIS. Moreover, islets from ATGL−/− mice or cultured islets in which ATGL was knocked down with short hairpins showed impaired insulin secretion in response to a number of different secretagogues.
2.8. Summary—molecular mechanisms through which glycerolipids contribute to selective insulin resistance and lipotoxicity
Teasing out molecular mechanisms underlying the pathogenesis of metabolic disease from the complex phenotypes of knockout mice is challenging, but the data give us clues regarding the potential role of some distinct lipid metabolites. Moreover, the findings identify several enzymes involved in glycerolipid synthesis or metabolism as potential therapeutic targets. In general, partial inhibition of triglyceride synthesis in WAT (e.g. DGAT1 knockouts) or liver e.g. (GPAT1 or SCD1 knockouts) appears to have beneficial effects owing to a reversal of selective insulin resistance in the liver, an overall lack of delivery of lipids to peripheral tissues, and an improved endocrine profile of adipocytes. In each of these cases, adipose and/or liver triglyceride levels are greatly decreased. Surprisingly, the animals are able to adequately deal with the excess lipid that is not stored as triglycerides, and the condition appears to be associated with either decreased intestinal absorption or increased oxidation of the excess fat. In contrast, complete ablation of enzymatic intermediates in the biosynthetic pathway leading to triglyceride synthesis, particularly in WAT (e.g. AGPAT2 and Lipin1), promotes a lipodystrophic syndrome characterized by increased lipid deposition and severe malfunction of peripheral tissues, resembling genetically engineered “fatless” mice where transgenic manipulations lead to complete adipocyte apoptosis [94,95].
The anti-obesity and/or anti-steatosis properties of the whole-animal knockouts make it difficult to glean whether glycerolipids play direct roles in skeletal muscle or mediate other lipotoxic events associated with obesity (e.g. β-cell or cardiomyocyte apoptosis, etc.). In these whole-body knockouts, the changes in muscle insulin sensitivity are likely to be secondary to changes in the adipocyte (e.g. effects on adipokines) or the liver (e.g. synthesis of VLDLs, and thus delivery to peripheral tissues). Nonetheless, our interpretation of the studies with GPAT1−/− and DAGK−/+ mice is that glycerolipid intermediates likely contribute to the desensitization of insulin action in liver and muscle, respectively. But which glycerolipid metabolite is the culprit? As described above, overexpression of DGAT or SCD1 in peripheral tissues appears to protect cells from lipotoxic events associated with fat exposure by diverting lipids into inert TAG stores [60,61,69]. Conversely, inhibition of these enzymes renders peripheral tissues susceptible to lipotoxic insults [55,70–73]. These observations are consistent with studies in cultured cells suggesting that one can ward off the lipotoxic effects of saturated fats by supplementing with oleate, which promotes incorporation of the excess lipid into the triglyceride pool [96–98]. Thus, TAG is unlikely to be anything other than an inert bystander.
An idea which has received considerable attention is that intermediates in the de novo synthesis pathway are antagonists of glucose transport and modulators of cell function. Indeed, both DAG and PA are well established as cell signalling intermediates, though their role in signalling is normally attributed to plasma membrane functions, rather than those in the ER. For example, DAG involved in cell signalling is generally produced at the cell membrane via the hydrolysis of phosphatidylcholine. However, in the lipin1 deficient animals, Nadra et al. [99] found that the accumulation of phosphatidic acid, through its ability to activate the MEK/ERK signalling pathway, accounted for peripheral neuropathy. Thus, intermediates in the de novo glycerolipid pathway are capable of serving as signalling intermediates, as well as lipotoxic modulators of cell function.
But which glycerolipids serve as modulators of insulin sensitivity in muscle or liver? Because of the data involving the DAGKδ−/+ mice [98], DAG emerges as a strong candidate. Indeed, DAG has received considerable attention as an inducer of insulin resistance, and the prevailing hypothesis has been that that its ability to activate PKC isoforms leads to the phosphorylation of insulin receptor substrates and the inhibition of phosphatidylinositol 3-kinase [100,101], with the latter being an obligate intermediate in insulin-stimulated glucose uptake and anabolic metabolism. The aforementioned studies by Chibalin et al. [98], which revealed that PKC inhibitors restored insulin sensitivity in cultured myotubes, support this hypothesis. However, there have been two lines of evidence that introduce doubts about the importance of DAG as a modulator of glucose homeostasis. First, though DAG often correlates with insulin sensitivity, in some experimental models large increases in liver or muscle DAG are not sufficient to induce insulin resistance [56,102]. Second, the James lab has recently suggested that insulin resistance (i.e. the inhibition of glucose transport in muscle) caused by lipids occurs independently of signalling to IRS-1, the supposed target of DAG/PKC-induced insulin resistance [103]. Indeed, effects on IRS-1 are unlikely to account for the selective insulin resistance associated with obesity, as inhibition of PI3K would be predicted to prevent hepatic lipogenesis [104]. Curiously, in cultured myotubes, overexpression of another DAGK isoform, DAGKε, prevented lipid (i.e. linoleate) activation of PKCs, but exacerbated insulin resistance [105]. In this study, the authors identified phosphatidic acid as the primary antagonist of insulin action [105].
A unique feature of the glycerolipid pathway is its dependence upon mono-unsaturated fats, as evidenced by the impact of SCD expression or oleate supplementation to drive lipids into the triglyceride pool [96–98]. As described below, saturated and unsaturated fats induce insulin resistance by separable mechanisms, with the former being reliant on sphingolipid signalling [102]. An intriguing possibility is that the fatty acid composition of glycerolipid intermediates influences their signalling capabilities. Indeed, prior studies suggest that DAG comprised of unsaturated acyl-chains is a far more potent activator of PKC [106], raising the possibility that distinct, unsaturated DAG subspecies will emerge as important contributors to insulin resistance and metabolic disease.
3. Manipulation of sphingolipid synthesis: impact on selective insulin resistance and lipotoxic diseases
Many of the circulating factors associated with obesity (e.g. inflammatory cytokines, saturated fatty acids, glucocorticoids, etc.) induce sphingolipid synthesis. As in de novo glycerolipid synthesis, most of the action occurs in the ER, where the substrates palmitoyl-CoA, serine, and another fatty acyl-CoA are converted to ceramide [107]. Vesicular transport mechanisms or ceramide transport proteins move ceramide to the golgi [108], where additional biosynthetic reactions convert it to sphingomyelin, ceramide-phosphate, glucosylceramides, and other complex sphingolipids. Manipulation of enzymes controlling sphingolipid synthesis or degradation in vitro of in vivo has a potent effect on insulin resistance and ameliorates lipotoxic responses associated with obesity [109].
3.1. Manipulation of serine palmitoyltransferase
Serine palmitoyltransferase (SPT), which condenses palmitoyl-CoA and serine to produce 3-ketosphinganine, is the initial, rate-limiting enzyme in the biosynthetic pathway driving ceramide production (Fig. 3, Reaction A) [110]. In seminal studies manipulating this enzyme, the Unger laboratory found that inhibiting SPT with the broad-spectrum antibiotic cycloserine prevented lipotoxic effects of palmitate in Zucker fa/fa beta cells in vitro and in vivo [111,112], leading the authors to conclude that “the ceramide pathway seems to be the most important of the destructive routes” involved in lipoapoptosis [113]. Subsequent studies involving the manipulation of this enzyme with higher specificity inhibitor myriocin and genetic manipulations strongly support this hypothesis.
In lipid-infused, high fat fed, and ob/ob mice as well as dexamethasone-treated, Zucker fa/fa, and ZDF rats, treatment with the SPT inhibitor myriocin markedly improves insulin sensitivity and glucose tolerance [102,114]. Hyperinsulinemic–euglycemic clamp studies confirm that myriocin improves insulin sensitivity in both muscle and the liver [102]. In the ZDF rats, SPT inhibition additionally preserved pancreatic insulin (Holland and Summers, unpublished observation) and prevented the onset of frank diabetes [102], confirming the Unger hypothesis that depleting ceramide preserves pancreatic β-cells. Studies in cultured cells and/or isolated tissues strongly suggest that these effects can result from tissue-autonomous mechanisms, as SPT inhibition negates lipid-induced insulin resistance and apoptosis in isolated muscles (and cultured myotubes) or pancreatic islets, respectively [96,102,111,112,115–117].
Though these findings universally support the enzyme’s role in insulin sensitivity in both skeletal muscle and the liver, some important differences emerge that warrant elucidation. The relative effects of myriocin on body weight are unclear, due to some discrepancies in the literature. In a prior study, the insulin sensitizing effects of myriocin were independent of changes in body weight or a reduction in obesity [102]. In apolipoprotein-E deficient mice (ApoE−/−) fed a high fat diet (discussed below), myriocin also had only a minor impact on body weight [118]. However, in a subsequent study in high fat fed and ob/ob mice, myriocin reduced body weight and increased energy expenditure [114]. Moreover, in this latter study myriocin reduced hepatic steatosis [114], though it has been shown by others to have no acute effect on glycerolipid synthesis (e.g. during lipid infusion [102] or lipid-incubation of hepatocytes [119]). These are important differences that have profound implications on our understanding of the mechanism of sphingolipid action.
Studies using myriocin additionally suggest that ceramide is related to cardiovascular (e.g. atherosclerosis and cardiomyopathy) complications of lipid excess, even in the absence of obesity. Several groups have found that treating ApoE−/− mice with myriocin decreases the formation of atherosclerotic lesions [118,120–122]. Moreover, the Goldberg laboratory found that myriocin ameliorated symptoms of cardiomyopathy, including cardiac hypertrophy, enlarged and abnormal myocytes, left ventricular dilatation, and reduced fractional shortening, in animals overexpressing lipoprotein lipase in the heart [123]. In these studies, myriocin reduced sphingolipid levels without impacting glycerolipid synthesis. Importantly, the Goldberg laboratory was the first to demonstrate that haploinsufficiency for a key subunit of SPT also prevented features of metabolic disease, as the manipulation rendered mice resistant to this form of lipotoxic cardiomyopathy [124].
3.2. Manipulation of dihydroceramide desaturase
Dihydroceramide desaturase (DES) inserts a critical double bond into dihydroceramide, converting it into the bioactive molecule ceramide (Fig. 3, Reaction B). Of the two DES isoforms, DES1 is essential for ceramide synthesis in most tissues, with DES2 playing more specialized roles in making phytosphingolipids which are in the gut [102]. Mice that are haploinsufficient for DES1 are refractory to dexamethasone-induced insulin resistance. Moreover, muscles isolated from these mice remain insulin sensitive, even in a profoundly hyperlipidemic environment [102]. Interestingly, the small molecule fenretinide has recently been identified as a potent inhibitor of DES1 [125], and the compound improves insulin sensitivity in mice fed a high fat diet [126].
3.3. Manipulation of glucosylceramide synthase and gm3 synthase
Ceramides can be glucosylated by glucosylceramide synthases (Fig. 3, Reaction C), and the product can be further modified to give rise to a large family gangliosides (Fig. 3, Reaction D). Studies with two unrelated glucosylceramide synthase inhibitors suggest that these intermediates may contribute to obesity-induced insulin resistance. Specifically, the GCS inhibitors N-(5′-adamantane-1′-yl-methoxy)-pentyl-1-deoxynojirimycin (AMP-DNM) and Genz-123346 improve glucose tolerance and increase insulin sensitivity in muscle and liver of ob/ob and DIO mice, as well as ZDF rats [127,128]. When given to ob/ob mice, inhibitors of GCS prevent hepatic steatosis by modulating the expression of various lipogenic genes [129]. However, these drugs do not impact susceptibility to atherosclerosis [130].
Through the addition of various additional carbohydrate residues, glucosylceramide gets converted into distinct gangliosides. One of these, GM3 ganglioside, is widely distributed, and serves as a precursor for most of the more complex species. Mice lacking the GM3-synthase gene display lower fasting glucose levels and improved glucose tolerance [131]. When challenged with high fat diets, the GM3-synthase null mice maintained superior glucose tolerance, improved insulin stimulated glucose uptake, and enhanced suppression of hepatic glucose output.
3.4. Manipulation of lysosomal ceramide degradation (e.g. acid ceramidase and acid sphingomyelinase)
Sphingomyelinases convert sphingomyelin back into ceramide and phosphochline (Fig. 3, Reaction E). Nikolova-Karakashian and colleagues [119] crossed mice lacking the acidic, lysosomal form of the enzyme (ASMase−/−) with mice lacking the low density lipoprotein receptor (ldlr−/−), with the latter prone to metabolic disease when placed on western diets enriched in saturated fat and cholesterol. The ablation of ASMase produced a striking phenotype including a marked reduction in hepatic triglycerides, increased insulin sensitivity, and improvements in glucose tolerance. The animals demonstrated a substantial increase in hepatic ceramide. Desipramine, an inhibitor of ASMase, but not myriocin, reduced rates of triglyceride synthesis in isolated hepatocytes. These results suggest a highly interesting link between acid sphingomyelinase activity and rates of triglyceride synthesis.
Once ceramide accumulates within the lysosome, acid ceramidases further deacylate it to produce sphingosine (Fig. 3, Reaction F). Mice that are haploinsufficient for acid ceramidase (Asah1−/+) show a phenotype remarkably different than that of the ASMase mice mentioned above, including the development of a progressive lipid storage disorder, with particularly abundant fat accumulation within the liver [132].
3.5. Summary—molecular mechanisms through which sphingolipids contribute to selective insulin resistance and lipotoxicity
The studies reviewed herein strongly suggest that one or more sphingolipid entities are important contributors to selective insulin resistance and lipotoxicity, as the inhibition of early steps in the sphingolipid pathway has large beneficial effects in rodent models of obesity, diabetes, atherosclerosis, and cardiomyopathy. But which metabolite is the critical factor, and what is the mechanism by which small increases in sphingolipid intermediates affect cell function?
Of the sphingolipids, ceramide, including that generated by de novo synthesis as well as by acute sphingomyelin hydrolysis, has emerged as a particularly important player in cellular responses to stress. With regards to insulin signalling, ceramide or a ceramide-metabolite inhibits activation of Akt/PKB, a serine/threonine kinase important for glucose uptake (muscle) and repression of hepatic glucose output (liver), via a two independent pathways involving protein phosphatase 2A and protein kinase Cλ/ζ [133]. In cultured myotubes, we found that blocking ceramide glucosylation or deacylation antagonized insulin signalling, leading us to conclude that ceramide was the likely signalling intermediate [134].
Taniguchi and colleagues [104] have suggested that Akt/PKB and PKCλ play differential roles on glucose output and lipogenesis, respectively. Thus, the differential effect of ceramide on Akt/PKB and atypical PKCs is intriguing, as it provides a potential explanation for selective insulin resistance (Fig. 1). However, Akt/PKB has also been implicated in events underlying lipid accumulation in liver [135], and it is still unclear whether ceramide is capable of modulating lipid synthesis in this tissue. This issue is important to resolve.
In addition to its modulation of Akt/PKB, ceramide may contribute to insulin resistance and lipoapoptosis through a number of additional mechanisms. In skeletal muscle, ceramide or a related lipid has also been linked to distal events controlling glucose transporter 4 translocation [103,136], which is a key step in postprandial glucose disposal. Moreover, ceramide has been strongly linked to the induction of mitochondrial damage and apoptosis, which can contribute to the demise of pancreatic β-cells, cardiomyocytes, and others. Specific mechanisms include the following: (a) recruitment of pro-apoptotic Bax to mitochondrial membranes [137–140]; (b) generation of reactive oxygen species through the inhibition of the mitochondrial ubiquinone pool of complex III [141,142] or regulation of NADPH oxidase [143,144]; (c) clustering of death receptors [145–147]; and (d) directly increasing mitochondrial membrane permeability [148–154].
Though the pharmacological and knockout data listed above strongly implicate sphingolipids in metabolic disease, several pieces of data argue that ceramide is not the key bioactive intermediate. First, though ceramide levels sometimes correlate with various indices of metabolic disease, sometimes disease symptoms are manifest under conditions where no ceramide elevation is detected [155]. Second, under certain experimental conditions, large inductions in liver ceramide are not associated with hepatic insulin resistance [56,156]. Third, the increase in ceramide during obesity, when observed, is typically rather small, and it is difficult to understand how subtle differences in ceramide levels could account for such profound metabolic defects. These data suggest that a ceramide-metabolite, rather than ceramide itself, may be the key bioactive molecule. Alternatively, ceramide may not be working through tissue autonomous mechanisms, and the primary site of action has not been evaluated.
Other target which have received attention include the glucosyl-ceramides, due to the therapeutic benefit of the glucosylceramide synthase inhibitors. One mechanism that has been put forth is that glucosylceramides interfere with insulin binding to its receptor, and studies in cultured cells reinforce the hypothesis that glucosylcer-amide interfere with early steps in insulin signalling [157,158]. However, as with most signalling cascades, low levels of receptor occupancy are typically sufficient to promote a full insulin response. Moreover, the aforementioned studies by Hoehn et al. argue against such a proximal defect in insulin resistance [103]. Lastly, this mechanism would not explain how the compounds reduce hepatic lipid levels [129]. A more likely explanation is that the protective effects of this class of compound are secondary to their lowering of liver triglycerides, and the mechanism underlying this effect warrants further study.
4. Manipulation of lipid oxidation: impact on selective insulin resistance and lipotoxic diseases
In the 1960s, Randle and colleagues [159] introduced the hypothesis that mitochondria served as the primary sensors of excess lipid, and that fatty acids and glucose could serve as competitive substrates for oxidation. The mechanism put forth (i.e. the Randle Hypothesis) was that increased acetyl-CoA, produced during rounds of lipid oxidation, would trigger a chain of events leading to the accumulation of glucose 6-phosphate, which in turn inhibits hexokinase. The resulting decrease in glucose phosphorylation would reduce rates of glucose entry into the cell. While it is now clear that insulin resistance can occur in the absence of increased G6P (e.g. during lipid infusion [160]), the relative role of the Randle hypothesis, mitochondrial lipid sensing, and/or mitochondrial dysfunction in insulin resistance continues to be a hot topic of interest and debate [161,162].
4.1. Manipulation of CPT-1
Lipid entry into mitochondria involves the carnitine palmitoyl-transferase system, which exchanges carnitine for coenzyme-A to create a fatty acid-carnitine conjugate capable of being transported across the inner mitochondrial membrane (Fig. 4, Reaction A). Inhibition of CPT-1 with a pharmacological reagent (i.e. etomoxir) increases lipid deposition and exacerbates insulin resistance in rats fed a high-fat diet [163]. Moreover, modest overexpression of CPT1 in distal hindlimb muscles of rats protects against lipid-induced insulin resistance [164]. These data suggest that mitochondria do not serve as primary sensors of lipid excess.
4.2. Manipulation of ACC1 and ACC2
Malonyl-CoA is a potent inhibitor of CPT1, and a key determinant of rates of lipid oxidation. Acetyl-CoA carboxylase (ACC) generates malonyl-CoA from acetyl-CoA (Fig. 4, Reaction B), and thus is an important regulator lipid oxidation. Of the two distinct isoforms, the cytosolic ACC1 generally produces cytosolic malonyl-CoA for lipogenesis, while mitochondrial ACC2 produces the malonyl-CoA which regulates CPT1. However, there is evidence of compensation between the two isoforms (see below).
Genetic ablation of ACC1 in mice is embryonically lethal [165]. However, elimination of ACC2 in mice reduces fat mass, increases energy expenditure, improves insulin sensitivity, and prevents obesity-induced diabetes [166,167]. Moreover, soraphen, an inhibitor of the ACC system, prevents weight gain and improves peripheral insulin sensitivity in mice fed a high-fat diet [168]. As a result, ACC has emerged as an attractive therapeutic target [169].
Tissue-specific manipulations of ACC isoforms reveals some surprising findings. Liver-specific ACC knockouts demonstrate marked reductions in hepatic malonyl-CoA and decreases in hepatic triglyceride [170]. However, despite these changes, these mice were not resistant to obesity or insulin resistance [170]. Harada et al. also made liver-specific ACC1 knockout mice [171]. These animals demonstrated no changes in lipogenesis or in hepatic malonyl-CoA, suggesting that ACC2 could compensate in this experimental model. Intraperitoneal injection of antisense oligonucleotide inhibitors against ACC1 orACC2 has also been shown to effectively reduce each isoform. Knockdown of ACC1, but not ACC2, was associated with decreased lipogenesis [172]. After high fat feeding, inhibiting both isoforms simultaneously, but not either one independently, reduced hepatic malonyl-CoA, lowered hepatic lipids, and improved peripheral insulin sensitivity [172].
4.3. Manipulation of MCD
Malonyl-CoA-decarboxylase (MCD) promotes β-oxidation by converting malonyl-CoA into acetyl-CoA (Fig. 4, Reaction C), thus relieving the inhibition of CPT1. Koves and colleagues [173] revisited the hypothesis that glucose and lipids served as competitive substrates for oxidation by testing MCD−/− mice fed a high fat diet. These mice demonstrated improved glucose tolerance and insulin sensitivity, as assessed by glucose and insulin tolerance tests. These data are consistent with observations that mitochondrial fatty acid β-oxidation is sometimes enhanced in muscles from insulin resistant rodents or diabetic patients [162,174–177]. Noting that high fat feeding leads to an increase in acyl-carnitines, Muoio and colleagues hypothesized that increased flux of acyl-CoAs into mitochondria induces stress, as the substrates exceed the oxidative capacity of this organelle. They hypothesized this “incomplete β-oxidation” leads to the formation of intermediates capable of inhibit correct mitochondrial function [173,178]. This finding is consistent with studies in isolated human myocytes, where knockdown of MCD increased glucose oxidation [179,180].
Of particular note, the MCD effect appears to be specific to skeletal muscle, as MCD1 overexpression in the liver has opposing effects, preventing hepatic steatosis and reversing insulin resistance [181]. Surprisingly, the whole-animal MCD−/− mice described above did not display noticeable changes in lipid oxidation in the liver [173]. Of note, the liver isoform of CPT1 is much less sensitive to malonyl-CoA, potentially explaining the muscle-specific findings in the MCD−/− mice.
4.4. Manipulation of FAS
Fatty acid synthase (FAS) reduces malonyl-CoA by converting it to palmitoyl-CoA during de novo lipogenesis (Fig. 4, Reaction D). Deletion of the gene producing cytoplasmic FAS is embryonically lethal [182]. Manipulation of this reaction with pharmacological inhibitors has potent effects on body weight, due to hypothalamic effects on food intake [183]. Conditional ablation of FAS in the hypothalamus and pancreatic recapitulates the resistance to weight gain and decreased appetite, and reveals a role in physical activity and increased energy expenditure [184,185].
In the periphery, liver-specific FAS knockouts (FASKOL) have a paradoxical predisposition to hypoglycemia and hepatic steatosis, particularly when starved or when placed on a no fat/high carbohydrate diet that promotes lipogenesis [186]. The phenotype is attributable to a reduction in β-oxidation, owing to a three-fold increase in malonyl-CoA levels [186]. Moreover, most of the effects were reversed by treating with agonists of the PPARα transcription factor [186], suggesting that “new fat” produced by the FAS reaction served as endogenous ligands for this important transcriptional regulator of genes involved in lipid metabolism.
4.5. Summary—molecular mechanisms through which mitochondrial lipid oxidation influences insulin resistance and lipotoxicity
The data herein demonstrate that increasing lipid oxidation in the liver (e.g. MCD overexpression, ACC knockdowns) prevents hepatic steatosis and insulin resistance. Thus, these enzymes emerge as putative therapeutic targets, particularly for siRNA or antisense strategies which can effect a conditional effect in the liver.
In contrast, studies in skeletal muscle gave mixed findings, and are more difficult to interpret. Whole animal CPT inhibition induces insulin resistance, and CPT overexpression in muscle improves insulin sensitivity. In contrast, ablation of MCD, which blocked lipid oxidation in muscle, had the opposite effect. How does one reconcile these paradoxical findings? It is worth noting that metformin, which is widely prescribed as insulin sensitizing therapeutic, acts essentially as a mitochondrial poison which alters intracellular ATP/AMP ratios, leading to an activation of AMP-protein kinase [187,188]. An apparently important action of AMPK is to promote lipid oxidation, which it does by reducing ACC and increasing MCD activities, respectively. Muoio and Newgard [178] propose that metformin is working primarily working in the liver, and this idea warrants further scrutiny. Alternatively, one wonders whether metformin is associated with compensatory changes which improve the capacity of the TCA cycle to metabolize incoming fat.
Considering the role of incomplete lipid oxidation in the induction of insulin resistance in muscle, one is tempted to speculate on potential mechanisms. Muoio and colleagues noted through metabolic profiling that acylcarnitines were elevated in insulin resistant rodents, but it is unclear whether acylcarnitines are the key signal, or are merely a marker of the incomplete oxidative state. Indeed, acylcarnitines are generally considered to be part of a detoxification method for removing excess acyl group from mitochondria, and carnitine supplementation improves insulin sensitivity [189]. Another possibility is that reactive oxygen species (ROS) are the culprits, and many investigators have proposed that excessive lipid supply forces the mitochondria to produce ever-increase amounts of ROS, impairing mitochondrial function and inducing a concomitant development of insulin resistance. Houstis et.al. [190] found that chronic treatment with the antioxidant agent manganese (iii) tetrakis (4-benzoic acid) porphyrin (MnTBAP) improved insulin sensitivity and glucose homeostasis in insulin-resistant ob/ob mice. More recently, Hoehn and colleagues [103] found that ROS savengers prevented insulin resistance in L6-myotubes exposed to a number of factors which induce insulin resistance, including various exogenous fatty acids. In this study, sphingolipid synthesis inhibitors also prevented the insulin resistance, suggesting that sphingolipids and ROS may function by interrelated mechanisms.
5. Future directions
Experimental manipulation of enzymes controlling lipid synthesis and metabolism has profound effects on selective insulin resistance and lipotoxicity, and many of the enzymes discussed herein have emerged as putative therapeutic drug targets. What is clear, however, is that no single lipid entity fully accounts for the lipotoxic events underlying metabolic disease, and a wide array of metabolites likely control tissue function by additive and synergistic mechanisms. One might anticipate that these different lipid species may have differing levels of importance in individual patients or patient populations.
A problem with each of these experimental approaches, particularly when it comes to trying to elucidate precise molecular mechanisms involved in lipid sensing and action, is that the inhibition of a single enzyme in a cascade of interrelated events is likely to have a large impact on the entire lipidome, as fatty acyl-CoAs get diverted from one pathway to the next. For example, inhibiting early steps in glycerolipid or sphingolipid synthesis impacts all of the downstream metabolites. Moreover, inhibition of one pathway will increase the flux of nutrients into the other. Thus, the implementation of blunt, sledgehammer approaches to tackle delicate and intricate biological questions has not yet produced a particularly strong understanding of mechanism. Advances in lipidomics, when combined in full force with conditional genetic knockouts, knockdown approaches, and more specific and efficacious small molecule inhibitors, will accelerate progress towards understanding how specific lipid metabolites perturb metabolic homeostasis.
Acknowledgments
The authors acknowledge research support from the National Institutes of Health (DK081456) and the Agency for Science, Technology and Research of Singapore (A*STAR).
References
- 1.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 2.CDC. US Center for Disease Control—National Diabetes Fact Sheet. Vol. 2009. 2009. [Google Scholar]
- 3.CDC. US Center for Disease Control—Obesity Trends. Vol. 2009. 2009. [Google Scholar]
- 4.Low S, Chin MC, Deurenberg-Yap M. Review on epidemic of obesity. Ann Acad Med Singapore. 2009;38:57–59. [PubMed] [Google Scholar]
- 5.WHO. World Health Organization - Diabetes Programme. Vol. 2009. 2009. [Google Scholar]
- 6.WHO. World Health Organization - Cardiovascular Disease Programme. Vol. 2009. 2009. [Google Scholar]
- 7.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, Lutz EP, Kako Y, Velez-Carrasco W, Goldberg IJ, Breslow JL, Shulman GI. Tissue-specific over-expression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci U S A. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira LD, Pulawa LK, Jensen DR, Eckel RH. Overexpressing human lipoprotein lipase in mouse skeletal muscle is associated with insulin resistance. Diabetes. 2001;50:1064–1068. doi: 10.2337/diabetes.50.5.1064. [DOI] [PubMed] [Google Scholar]
- 10.Listenberger LL, Schaffer JE. Mechanisms of lipoapoptosis: implications for human heart disease. Trends Cardiovasc Med. 2002;12:134–138. doi: 10.1016/s1050-1738(02)00152-4. [DOI] [PubMed] [Google Scholar]
- 11.Borradaile NM, Schaffer JE. Lipotoxicity in the heart. Curr Hypertens Rep. 2005;7:412–417. doi: 10.1007/s11906-005-0035-y. [DOI] [PubMed] [Google Scholar]
- 12.Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–822. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, Goldberg IJ. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49:2101–2112. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pappan KL, Pan Z, Kwon G, Marshall CA, Coleman T, Goldberg IJ, McDaniel ML, Semenkovich CF. Pancreatic beta-cell lipoprotein lipase independently regulates islet glucose metabolism and normal insulin secretion. J Biol Chem. 2005;280:9023–9029. doi: 10.1074/jbc.M409706200. [DOI] [PubMed] [Google Scholar]
- 16.Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319–336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 17.Unger RH. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003;144:5159–5165. doi: 10.1210/en.2003-0870. [DOI] [PubMed] [Google Scholar]
- 18.Unger RH, Orci L. Lipotoxic diseases of nonadipose tissues in obesity. Int J Obes Relat Metab Disord. 2000;24(Suppl 4):S28–32. doi: 10.1038/sj.ijo.0801498. [DOI] [PubMed] [Google Scholar]
- 19.Unger RH, Zhou YT. Lipotoxicity of beta-cells in obesity and in other causes of fatty acid spillover. Diabetes. 2001;50(Suppl 1):S118–121. doi: 10.2337/diabetes.50.2007.s118. [DOI] [PubMed] [Google Scholar]
- 20.Prentki M, Madiraju SR. Glycerolipid metabolism and signaling in health and disease. Endocr Rev. 2008;29:647–676. doi: 10.1210/er.2008-0007. [DOI] [PubMed] [Google Scholar]
- 21.Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res. 2004;43:134–176. doi: 10.1016/s0163-7827(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy EP. Biosynthesis of complex lipids. Fed Proc. 1961;20:934–940. [PubMed] [Google Scholar]
- 23.Wendel AA, Lewin TM, Coleman RA. Glycerol-3-phosphate acyltransferases: Rate limiting enzymes of triacylglycerol biosynthesis. Biochim Biophys Acta. 2008;179(6):501–506. doi: 10.1016/j.bbalip.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gimeno RE, Cao J. Thematic review series: glycerolipids. Mammalian glycerol-3-phosphate acyltransferases: new genes for an old activity. J Lipid Res. 2008;49:2079–2088. doi: 10.1194/jlr.R800013-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Hammond LE, Gallagher PA, Wang S, Hiller S, Kluckman KD, Posey-Marcos EL, Maeda N, Coleman RA. Mitochondrial glycerol-3-phosphate acyltransferase-deficient mice have reduced weight and liver triacylglycerol content and altered glycerolipid fatty acid composition. Mol Cell Biol. 2002;22:8204–8214. doi: 10.1128/MCB.22.23.8204-8214.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang XM, Choi CS, Coleman RA, Shulman GI. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA: glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab. 2005;2:55–65. doi: 10.1016/j.cmet.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Yazdi M, Ahnmark A, William-Olsson L, Snaith M, Turner N, Osla F, Wedin M, Asztely AK, Elmgren A, Bohlooly YM, Schreyer S, Linden D. The role of mitochondrial glycerol-3-phosphate acyltransferase-1 in regulating lipid and glucose homeostasis in high-fat diet fed mice. Biochem Biophys Res Commun. 2008;369:1065–1070. doi: 10.1016/j.bbrc.2008.02.156. [DOI] [PubMed] [Google Scholar]
- 28.Xu H, Wilcox D, Nguyen P, Voorbach M, Suhar T, Morgan SJ, An WF, Ge L, Green J, Wu Z, Gimeno RE, Reilly R, Jacobson PB, Collins CA, Landschulz K, Surowy T. Hepatic knockdown of mitochondrial GPAT1 in ob/ob mice improves metabolic profile. Biochem Biophys Res Commun. 2006;349:439–448. doi: 10.1016/j.bbrc.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 29.Nagle CA, An J, Shiota M, Torres TP, Cline GW, Liu ZX, Wang S, Catlin RL, Shulman GI, Newgard CB, Coleman RA. Hepatic overexpression of glycerol-sn-3-phosphate acyltransferase 1 in rats causes insulin resistance. J Biol Chem. 2007;282:14807–14815. doi: 10.1074/jbc.M611550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeuchi K, Reue K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am J Physiol Endocrinol Metab. 2009;296:E1195–E1209. doi: 10.1152/ajpendo.90958.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal AK, Arioglu E, De Almeida S, Akkoc N, Taylor SI, Bowcock AM, Barnes RI, Garg A. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet. 2002;31:21–23. doi: 10.1038/ng880. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal AK, Simha V, Oral EA, Moran SA, Gorden P, O’Rahilly S, Zaidi Z, Gurakan F, Arslanian SA, Klar A, Ricker A, White NH, Bindl L, Herbst K, Kennel K, Patel SB, Al-Gazali L, Garg A. Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J Clin Endocrinol Metab. 2003;88:4840–4847. doi: 10.1210/jc.2003-030855. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal AK, Garg A. Genetic basis of lipodystrophies and management of metabolic complications. Annu Rev Med. 2006;57:297–311. doi: 10.1146/annurev.med.57.022605.114424. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal AK, Garg A. Congenital generalized lipodystrophy: significance of triglyceride biosynthetic pathways. Trends Endocrinol Metab. 2003;14:214–221. doi: 10.1016/s1043-2760(03)00078-x. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal AK, Barnes RI, Garg A. Genetic basis of congenital generalized lipodystrophy. Int J Obes Relat Metab Disord. 2004;28:336–339. doi: 10.1038/sj.ijo.0802487. [DOI] [PubMed] [Google Scholar]
- 36.Cortes VA, Curtis DE, Sukumaran S, Shao X, Parameswara V, Rashid S, Smith AR, Ren J, Esser V, Hammer RE, Agarwal AK, Horton JD, Garg A. Molecular mechanisms of hepatic steatosis and insulin resistance in the AGPAT2-deficient mouse model of congenital generalized lipodystrophy. Cell Metab. 2009;9:165–176. doi: 10.1016/j.cmet.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reue K, Brindley DN. Thematic Review Series: glycerolipids. Multiple roles for lipins/phosphatidate phosphatase enzymes in lipid metabolism. J Lipid Res. 2008;49:2493–2503. doi: 10.1194/jlr.R800019-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finck BN, Gropler MC, Chen Z, Leone TC, Croce MA, Harris TE, Lawrence JC, Jr, Kelly DP. Lipin 1 is an inducible amplifier of the hepatic PGC-1alpha/PPARalpha regulatory pathway. Cell Metab. 2006;4:199–210. doi: 10.1016/j.cmet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Langner CA, Birkenmeier EH, Ben-Zeev O, Schotz MC, Sweet HO, Davisson MT, Gordon JI. The fatty liver dystrophy (fld) mutation. A new mutant mouse with a developmental abnormality in triglyceride metabolism and associated tissue-specific defects in lipoprotein lipase and hepatic lipase activities. J Biol Chem. 1989;264:7994–8003. [PubMed] [Google Scholar]
- 40.Langner CA, Birkenmeier EH, Roth KA, Bronson RT, Gordon JI. Characterization of the peripheral neuropathy in neonatal and adult mice that are homozygous for the fatty liver dystrophy (fld) mutation. J Biol Chem. 1991;266:11955–11964. [PubMed] [Google Scholar]
- 41.Reue K, Xu P, Wang XP, Slavin BG. Adipose tissue deficiency, glucose intolerance, and increased atherosclerosis result from mutation in the mouse fatty liver dystrophy (fld) gene. J Lipid Res. 2000;41:1067–1076. [PubMed] [Google Scholar]
- 42.Phan J, Reue K. Lipin, a lipodystrophy and obesity gene. Cell Metab. 2005;1:73–83. doi: 10.1016/j.cmet.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Nagaya H, Wada I, Jia YJ, Kanoh H. Diacylglycerol kinase delta suppresses ER-to-Golgi traffic via its SAM and PH domains. Mol Biol Cell. 2002;13:302–316. doi: 10.1091/mbc.01-05-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crotty T, Cai J, Sakane F, Taketomi A, Prescott SM, Topham MK. Diacylglycerol kinase delta regulates protein kinase C and epidermal growth factor receptor signaling. Proc Natl Acad Sci U S A. 2006;103:15485–15490. doi: 10.1073/pnas.0604104103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chibalin AV, Leng Y, Vieira E, Krook A, Bjornholm M, Long YC, Kotova O, Zhong Z, Sakane F, Steiler T, Nylen C, Wang J, Laakso M, Topham MK, Gilbert M, Wallberg-Henriksson H, Zierath JR. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell. 2008;132:375–386. doi: 10.1016/j.cell.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 46.Chen HC. Enhancing energy and glucose metabolism by disrupting triglyceride synthesis: Lessons from mice lacking DGAT1. Nutr Metab (Lond) 2006;3:10. doi: 10.1186/1743-7075-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV., Jr Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet. 2000;25:87–90. doi: 10.1038/75651. [DOI] [PubMed] [Google Scholar]
- 48.Buhman KK, Smith SJ, Stone SJ, Repa JJ, Wong JS, Knapp FF, Jr, Burri BJ, Hamilton RL, Abumrad NA, Farese RV. DGAT1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis. J Biol Chem. 2002;277:25474–25479. doi: 10.1074/jbc.M202013200. [DOI] [PubMed] [Google Scholar]
- 49.Chen HC, Jensen DR, Myers HM, Eckel RH, Farese RV., Jr Obesity resistance and enhanced glucose metabolism in mice transplanted with white adipose tissue lacking acyl CoA:diacylglycerol acyltransferase 1. J Clin Invest. 2003;111:1715–1722. doi: 10.1172/JCI15859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen HC, Smith SJ, Ladha Z, Jensen DR, Ferreira LD, Pulawa LK, McGuire JG, Pitas RE, Eckel RH, Farese RV. Increased insulin and leptin sensitivity in mice lacking acyl CoA:diacylglycerol acyltransferase 1. J Clin Invest. 2002;109:1049–1055. doi: 10.1172/JCI14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Streeper RS, Koliwad SK, Villanueva CJ, Farese RV., Jr Effects of DGAT1 deficiency on energy and glucose metabolism are independent of adiponectin. Am J Physiol Endocrinol Metab. 2006;291:E388–394. doi: 10.1152/ajpendo.00621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen HC, Stone SJ, Zhou P, Buhman KK, Farese RV. Dissociation of obesity and impaired glucose disposal in mice overexpressing acyl coenzyme a: diacylglycerol acyltransferase 1 in white adipose tissue. Diabetes. 2002;51:3189–3195. doi: 10.2337/diabetes.51.11.3189. [DOI] [PubMed] [Google Scholar]
- 53.Yu XX, Murray SF, Pandey SK, Booten SL, Bao D, Song XZ, Kelly S, Chen S, McKay R, Monia BP, Bhanot S. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology. 2005;42:362–371. doi: 10.1002/hep.20783. [DOI] [PubMed] [Google Scholar]
- 54.Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, Kim S, Distefano A, Samuel VT, Neschen S, Zhang D, Wang A, Zhang XM, Kahn M, Cline GW, Pandey SK, Geisler JG, Bhanot S, Monia BP, Shulman GI. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem. 2007;282:22678–22688. doi: 10.1074/jbc.M704213200. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, Monia BP, Li YX, Diehl AM. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 56.Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV, Sr, Hevener AL, Farese RV. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Dube JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab. 2008;294:E882–888. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 59.van Loon LJ, Goodpaster BH. Increased intramuscular lipid storage in the insulin-resistant and endurance-trained state. Pflugers Arch. 2006;451:606–616. doi: 10.1007/s00424-005-1509-0. [DOI] [PubMed] [Google Scholar]
- 60.Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest. 2007;117:1679–1689. doi: 10.1172/JCI30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roorda BD, Hesselink MK, Schaart G, Moonen-Kornips E, Martinez-Martinez P, Losen M, De Baets MH, Mensink RP, Schrauwen P. DGAT1 overexpression in muscle by in vivo DNA electroporation increases intramyocellular lipid content. J Lipid Res. 2005;46:230–236. doi: 10.1194/jlr.M400416-JLR200. [DOI] [PubMed] [Google Scholar]
- 62.Levin MC, Monetti M, Watt MJ, Sajan MP, Stevens RD, Bain JR, Newgard CB, Farese RV, Sr, Farese RV., Jr Increased lipid accumulation and insulin resistance in transgenic mice expressing DGAT2 in glycolytic (type II) muscle. Am J Physiol Endocrinol Metab. 2007;293:E1772–1781. doi: 10.1152/ajpendo.00158.2007. [DOI] [PubMed] [Google Scholar]
- 63.Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008;49:2283–2301. doi: 10.1194/jlr.R800018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci U S A. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miyazaki M, Sampath H, Liu X, Flowers MT, Chu K, Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase-1 deficiency attenuates obesity and insulin resistance in leptin-resistant obese mice. Biochem Biophys Res Commun. 2009;380:818–822. doi: 10.1016/j.bbrc.2009.01.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Man WC, Miyazaki M, Chu K, Ntambi J. Colocalization of SCD1 and DGAT2: implying preference for endogenous monounsaturated fatty acids in triglyceride synthesis. J Lipid Res. 2006;47:1928–1939. doi: 10.1194/jlr.M600172-JLR200. [DOI] [PubMed] [Google Scholar]
- 67.Gutierrez-Juarez R, Pocai A, Mulas C, Ono H, Bhanot S, Monia BP, Rossetti L. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J Clin Invest. 2006;116:1686–1695. doi: 10.1172/JCI26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flowers MT, Miyazaki M, Liu X, Ntambi JM. Probing the role of stearoyl-CoA desaturase-1 in hepatic insulin resistance. J Clin Invest. 2006;116:1478–1481. doi: 10.1172/JCI28774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peter A, Weigert C, Staiger H, Machicao F, Schick F, Machann J, Stefan N, Thamer C, Haring HU, Schleicher E. Individual stearoyl-CoA desaturase 1 (SCD1) expression modulates ER stress and inflammation in human myotubes and is associated with skeletal muscle lipid storage and insulin sensitivity in vivo. Diabetes. 2009;58(8):1757–1765. doi: 10.2337/db09-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem. 2009;284:5637–5644. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brown JM, Chung S, Sawyer JK, Degirolamo C, Alger HM, Nguyen T, Zhu X, Duong MN, Wibley AL, Shah R, Davis MA, Kelley K, Wilson MD, Kent C, Parks JS, Rudel LL. Inhibition of stearoyl-coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation. 2008;118:1467–1475. doi: 10.1161/CIRCULATIONAHA.108.793182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Attie AD, Flowers MT, Flowers JB, Groen AK, Kuipers F, Ntambi JM. Stearoyl-CoA desaturase deficiency, hypercholesterolemia, cholestasis, and diabetes. Nutr Rev. 2007;65:S35–38. doi: 10.1111/j.1753-4887.2007.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 73.Flowers JB, Rabaglia ME, Schueler KL, Flowers MT, Lan H, Keller MP, Ntambi JM, Attie AD. Loss of stearoyl-CoA desaturase-1 improves insulin sensitivity in lean mice but worsens diabetes in leptin-deficient obese mice. Diabetes. 2007;56:1228–1239. doi: 10.2337/db06-1142. [DOI] [PubMed] [Google Scholar]
- 74.Dobrzyn P, Dobrzyn A, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, Friedman JM, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc Natl Acad Sci U S A. 2004;101:6409–6414. doi: 10.1073/pnas.0401627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res. 2009;50:3–21. doi: 10.1194/jlr.R800031-JLR200. [DOI] [PubMed] [Google Scholar]
- 76.Zimmermann R, Lass A, Haemmerle G, Zechner R. Fate of fat: the role of adipose triglyceride lipase in lipolysis. Biochim Biophys Acta. 2009;1791:494–500. doi: 10.1016/j.bbalip.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 77.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 78.Harada K, Shen WJ, Patel S, Natu V, Wang J, Osuga J, Ishibashi S, Kraemer FB. Resistance to high-fat diet-induced obesity and altered expression of adipose-specific genes in HSL-deficient mice. Am J Physiol Endocrinol Metab. 2003;285:E1182–1195. doi: 10.1152/ajpendo.00259.2003. [DOI] [PubMed] [Google Scholar]
- 79.Okazaki H, Osuga J, Tamura Y, Yahagi N, Tomita S, Shionoiri F, Iizuka Y, Ohashi K, Harada K, Kimura S, Gotoda T, Shimano H, Yamada N, Ishibashi S. Lipolysis in the absence of hormone-sensitive lipase: evidence for a common mechanism regulating distinct lipases. Diabetes. 2002;51:3368–3375. doi: 10.2337/diabetes.51.12.3368. [DOI] [PubMed] [Google Scholar]
- 80.Haemmerle G, Zimmermann R, Hayn M, Theussl C, Waeg G, Wagner E, Sattler W, Magin TM, Wagner EF, Zechner R. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem. 2002;277:4806–4815. doi: 10.1074/jbc.M110355200. [DOI] [PubMed] [Google Scholar]
- 81.Strom K, Hansson O, Lucas S, Nevsten P, Fernandez C, Klint C, Moverare-Skrtic S, Sundler F, Ohlsson C, Holm C. Attainment of brown adipocyte features in white adipocytes of hormone-sensitive lipase null mice. PLoS One. 2008;3:e1793. doi: 10.1371/journal.pone.0001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 83.Park SY, Kim HJ, Wang S, Higashimori T, Dong J, Kim YJ, Cline G, Li H, Prentki M, Shulman GI, Mitchell GA, Kim JK. Hormone-sensitive lipase knockout mice have increased hepatic insulin sensitivity and are protected from short-term diet-induced insulin resistance in skeletal muscle and heart. Am J Physiol Endocrinol Metab. 2005;289:E30–39. doi: 10.1152/ajpendo.00251.2004. [DOI] [PubMed] [Google Scholar]
- 84.Voshol PJ, Haemmerle G, Ouwens DM, Zimmermann R, Zechner R, Teusink B, Maassen JA, Havekes LM, Romijn JA. Increased hepatic insulin sensitivity together with decreased hepatic triglyceride stores in hormone-sensitive lipase-deficient mice. Endocrinology. 2003;144:3456–3462. doi: 10.1210/en.2002-0036. [DOI] [PubMed] [Google Scholar]
- 85.Mulder H, Sorhede-Winzell M, Contreras JA, Fex M, Strom K, Ploug T, Galbo H, Arner P, Lundberg C, Sundler F, Ahren B, Holm C. Hormone-sensitive lipase null mice exhibit signs of impaired insulin sensitivity whereas insulin secretion is intact. J Biol Chem. 2003;278:36380–36388. doi: 10.1074/jbc.M213032200. [DOI] [PubMed] [Google Scholar]
- 86.Roduit R, Masiello P, Wang SP, Li H, Mitchell GA, Prentki M. A role for hormone-sensitive lipase in glucose-stimulated insulin secretion: a study in hormone-sensitive lipase-deficient mice. Diabetes. 2001;50:1970–1975. doi: 10.2337/diabetes.50.9.1970. [DOI] [PubMed] [Google Scholar]
- 87.Larsson S, Wierup N, Sundler F, Eliasson L, Holm C. Lack of cholesterol mobilization in islets of hormone-sensitive lipase deficient mice impairs insulin secretion. Biochem Biophys Res Commun. 2008;376:558–562. doi: 10.1016/j.bbrc.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 88.Fex M, Haemmerle G, Wierup N, Dekker-Nitert M, Rehn M, Ristow M, Zechner R, Sundler F, Holm C, Eliasson L, Mulder H. A beta cell-specific knockout of hormone-sensitive lipase in mice results in hyperglycaemia and disruption of exocytosis. Diabetologia. 2009;52:271–280. doi: 10.1007/s00125-008-1191-9. [DOI] [PubMed] [Google Scholar]
- 89.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 90.Reid BN, Ables GP, Otlivanchik OA, Schoiswohl G, Zechner R, Blaner WS, Goldberg IJ, Schwabe RF, Chua SC, Huang LS. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J Biol Chem. 2008;283:13087–13099. doi: 10.1074/jbc.M800533200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huijsman E, van de Par C, Economou C, van der Poel C, Lynch GS, Schoiswohl G, Haemmerle G, Zechner R, Watt MJ. Adipose triacylglycerol lipase deletion alters whole body energy metabolism and impairs exercise performance in mice. Am J Physiol Endocrinol Metab. 2009;297:E505–E513. doi: 10.1152/ajpendo.00190.2009. [DOI] [PubMed] [Google Scholar]
- 92.Watt MJ, van Denderen BJ, Castelli LA, Bruce CR, Hoy AJ, Kraegen EW, Macaulay L, Kemp BE. Adipose triglyceride lipase regulation of skeletal muscle lipid metabolism and insulin responsiveness. Mol Endocrinol. 2008;22:1200–1212. doi: 10.1210/me.2007-0485. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 93.Peyot ML, Guay C, Latour MG, Lamontagne J, Lussier R, Pineda M, Ruderman NB, Haemmerle G, Zechner R, Joly E, Madiraju SR, Poitout V, Prentki M. Adipose triglyceride lipase is implicated in fuel- and non-fuel-stimulated insulin secretion. J Biol Chem. 2009;284:16848–16859. doi: 10.1074/jbc.M109.006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, Reitman ML, Vinson C. Life without white fat: a transgenic mouse. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Trujillo ME, Pajvani UB, Scherer PE. Apoptosis through targeted activation of caspase 8 (“ATTAC-mice”): novel mouse models of inducible and reversible tissue ablation. Cell Cycle. 2005;4:1141–1145. doi: 10.4161/cc.4.9.2030. [DOI] [PubMed] [Google Scholar]
- 96.Sabin MA, Stewart CE, Crowne EC, Turner SJ, Hunt LP, Welsh GI, Grohmann MJ, Holly JM, Shield JP. Fatty acid-induced defects in insulin signalling, in myotubes derived from children, are related to ceramide production from palmitate rather than the accumulation of intramyocellular lipid. J Cell Physiol. 2007;211:244–252. doi: 10.1002/jcp.20922. [DOI] [PubMed] [Google Scholar]
- 97.Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes. 2003;52:726–733. doi: 10.2337/diabetes.52.3.726. [DOI] [PubMed] [Google Scholar]
- 98.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nadra K, de Preux Charles AS, Medard JJ, Hendriks WT, Han GS, Gres S, Carman GM, Saulnier-Blache JS, Verheijen MH, Chrast R. Phosphatidic acid mediates demyelination in Lpin1 mutant mice. Genes Dev. 2008;22:1647–1661. doi: 10.1101/gad.1638008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang XM, Monia BP, Bhanot S, Shulman GI. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117:739–745. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu C, Chen Y, Zong H, Wang Y, Bergeron R, Kim JK, Cline GW, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of IRS-1 associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;2:2. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 102.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 103.Hoehn KL, Hohnen-Behrens C, Cederberg A, Wu LE, Turner N, Yuasa T, Ebina Y, James DE. IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 2008;7:421–433. doi: 10.1016/j.cmet.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taniguchi CM, Kondo T, Sajan M, Luo J, Bronson R, Asano T, Farese R, Cantley LC, Kahn CR. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 2006;3:343–353. doi: 10.1016/j.cmet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 105.Cazzolli R, Mitchell TW, Burchfield JG, Pedersen DJ, Turner N, Biden TJ, Schmitz-Peiffer C. Dilinoleoyl-phosphatidic acid mediates reduced IRS-1 tyrosine phosphorylation in rat skeletal muscle cells and mouse muscle. Diabetologia. 2007;50:1732–1742. doi: 10.1007/s00125-007-0709-x. [DOI] [PubMed] [Google Scholar]
- 106.Wakelam MJ. Diacylglycerol—when is it an intracellular messenger? Biochim Biophys Acta. 1998;1436:117–126. doi: 10.1016/s0005-2760(98)00123-4. [DOI] [PubMed] [Google Scholar]
- 107.Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50(Suppl):S91–S96. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hanada K, Kumagai K, Tomishige N, Yamaji T. CERT-mediated trafficking of ceramide. Biochim Biophys Acta. 2009;1791:684–691. doi: 10.1016/j.bbalip.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 109.Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hanada K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim Biophys Acta. 2003;1632:16–30. doi: 10.1016/s1388-1981(03)00059-3. [DOI] [PubMed] [Google Scholar]
- 111.Shimabukuro M, Higa M, Zhou YT, Wang MY, Newgard CB, Unger RH. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J Biol Chem. 1998;273:32487–32490. doi: 10.1074/jbc.273.49.32487. [DOI] [PubMed] [Google Scholar]
- 112.Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced b cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Unger RH, Orci L. Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta. 2002;1585:202–212. doi: 10.1016/s1388-1981(02)00342-6. [DOI] [PubMed] [Google Scholar]
- 114.Yang GD, Badeanlou L, Bielawski JD, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2009;297(1):E211–E224. doi: 10.1152/ajpendo.91014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cazzolli R, Carpenter L, Biden TJ, Schmitz-Peiffer C. A role for protein phosphatase 2A-like activity, but not atypical protein kinase Czeta, in the inhibition of protein kinase B/Akt and glycogen synthesis by palmitate. Diabetes. 2001;50:2210–2218. doi: 10.2337/diabetes.50.10.2210. [DOI] [PubMed] [Google Scholar]
- 116.Watson ML, Coghlan M, Hundal HS. Modulating serine palmitoyl transferase (SPT) expression and activity unveils a crucial role in lipid-induced insulin resistance in rat skeletal muscle cells. Biochem J. 2009;417:791–801. doi: 10.1042/BJ20081149. [DOI] [PubMed] [Google Scholar]
- 117.Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, Summers SA. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 2003;13:10297–10303. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- 118.Glaros EN, Kim WS, Quinn CM, Jessup W, Rye KA, Garner B. Myriocin slows the progression of established atherosclerotic lesions in apolipoprotein E gene knockout mice. J Lipid Res. 2008;49:324–331. doi: 10.1194/jlr.M700261-JLR200. [DOI] [PubMed] [Google Scholar]
- 119.Deevska GM, Rozenova KA, Giltiay NV, Chambers MA, White J, Boyanovsky BB, Wei J, Daugherty A, Smart EJ, Reid MB, Merrill AH, Nikolova-Karakashian M. Acid sphingomyelinase deficiency prevents diet-induced hepatic triacylglycerol accumulation and hyperglycemia in mice. J Biol Chem. 2009;284:8359–8368. doi: 10.1074/jbc.M807800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hojjati M, Li Z, Zhou H, Tang S, Huan C, Ooi E, Lu S, Jiang XC. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J Biol Chem. 2004;280(11):10284–10289. doi: 10.1074/jbc.M412348200. [DOI] [PubMed] [Google Scholar]
- 121.Park TS, Panek RL, Mueller SB, Hanselman JC, Rosebury WS, Robertson AW, Kindt EK, Homan R, Karathanasis SK, Rekhter MD. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2004;110:3465–3471. doi: 10.1161/01.CIR.0000148370.60535.22. [DOI] [PubMed] [Google Scholar]
- 122.Glaros EN, Kim WS, Wu BJ, Suarna C, Quinn CM, Rye KA, Stocker R, Jessup W, Garner B. Inhibition of atherosclerosis by the serine palmitoyl transferase inhibitor myriocin is associated with reduced plasma glycosphingolipid concentration. Biochem Pharmacol. 2007;73:1340–1346. doi: 10.1016/j.bcp.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 123.Park Ts, Hu Y, Okajima K, Buchanan J, Homma S, Davidson MM, Abel ED, Jiang XC, Goldberg IJ. Abstract 1386: Inhibition of ceramide biosynthesis decreases cardiomyopathy in lipoprotein lipase (LpL) lipotoxicity. 2007;116:II_284–II_285. [Google Scholar]
- 124.Hojjati MR, Li Z, Jiang XC. Serine palmitoyl-CoA transferase (SPT) deficiency and sphingolipid levels in mice. Biochim Biophys Acta. 2005;1737:44–51. doi: 10.1016/j.bbalip.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 125.Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, Kelly S, Allegood JC, Liu Y, Peng Q, Ramaraju H, Sullards MC, Cabot M, Merrill AH., Jr Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta. 2006;1758:1864–1884. doi: 10.1016/j.bbamem.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 126.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 127.Aerts JM, Ottenhoff R, Powlson AS, Grefhorst A, van Eijk M, Dubbelhuis PF, Kuipers F, Serlie MJ, Wennekes T, Overkleeft HS, Sethi JK, O’Rahilly S. Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes. 2007;56(5):1341–1349. doi: 10.2337/db06-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhao H, Przybylska M, Wu IH, Zhang J, Siegel C, Komarnitsky S, Yew NS, Cheng SH. Inhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetes. Diabetes. 2007;56:1210–1218. doi: 10.2337/db06-0719. [DOI] [PubMed] [Google Scholar]
- 129.Zhao H, Przybylska M, Wu IH, Zhang J, Maniatis P, Pacheco J, Piepenhagen P, Copeland D, Arbeeny C, Shayman JA, Aerts JM, Jiang C, Cheng SH, Yew NS. Inhibiting glycosphingolipid synthesis ameliorates hepatic steatosis in obese mice. Hepatology. 2009;50:85–93. doi: 10.1002/hep.22970. [DOI] [PubMed] [Google Scholar]
- 130.Glaros EN, Kim WS, Rye KA, Shayman JA, Garner B. Reduction of plasma glycosphingolipid levels has no impact on atherosclerosis in apolipoprotein E-null mice. J Lipid Res. 2008;49:1677–1681. doi: 10.1194/jlr.E800005-JLR200. [DOI] [PubMed] [Google Scholar]
- 131.Yamashita T, Hashiramoto A, Haluzik M, Mizukami H, Beck S, Norton A, Kono M, Tsuji S, Daniotti JL, Werth N, Sandhoff R, Sandhoff K, Proia RL. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci U S A. 2003;100:3445–3449. doi: 10.1073/pnas.0635898100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li CM, Park JH, Simonaro CM, He X, Gordon RE, Friedman AH, Ehleiter D, Paris F, Manova K, Hepbiloikler S, Fuks Z, Sandhoff K, Kolesnick R, Schuchman EH. Insertional mutagenesis of the mouse acid ceramidase gene leads to early embryonic lethality in homozygotes and progressive lipid storage disease in heterozygotes. Genomics. 2002;79:218–224. doi: 10.1006/geno.2002.6686. [DOI] [PubMed] [Google Scholar]
- 133.Stratford S, Hoehn KL, Liu F, Summers SA. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem. 2004;279:36608–36615. doi: 10.1074/jbc.M406499200. [DOI] [PubMed] [Google Scholar]
- 134.Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, Summers SA. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 2003;278:10297–10303. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- 135.Li X, Monks B, Ge Q, Birnbaum MJ. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature. 2007;447:1012–1016. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- 136.JeBailey L, Wanono O, Niu W, Roessler J, Rudich A, Klip A. Ceramide- and oxidant-induced insulin resistance involve loss of insulin-dependent Rac-activation and actin remodeling in muscle cells. Diabetes. 2007;56:394–403. doi: 10.2337/db06-0823. [DOI] [PubMed] [Google Scholar]
- 137.Kashkar H, Wiegmann K, Yazdanpanah B, Haubert D, Kronke M. Acid sphingomyelinase is indispensable for UV light-induced Bax conformational change at the mitochondrial membrane. J Biol Chem. 2005;280:20804–20813. doi: 10.1074/jbc.M410869200. [DOI] [PubMed] [Google Scholar]
- 138.Birbes H, Bawab SE, Obeid LM, Hannun YA. Mitochondria and ceramide: intertwined roles in regulation of apoptosis. Adv Enzyme Regul. 2002;42:113–129. doi: 10.1016/s0065-2571(01)00026-7. [DOI] [PubMed] [Google Scholar]
- 139.Birbes H, El Bawab S, Hannun YA, Obeid LM. Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. Faseb J. 2001;15:2669–2679. doi: 10.1096/fj.01-0539com. [DOI] [PubMed] [Google Scholar]
- 140.Birbes H, Luberto C, Hsu YT, El Bawab S, Hannun YA, Obeid LM. A mitochondrial pool of sphingomyelin is involved in TNFalpha-induced Bax translocation to mitochondria. Biochem J. 2005;386:445–451. doi: 10.1042/BJ20041627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 142.Gudz TI, Tserng KY, Hoppel CL. Directinhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J Biol Chem. 1997;272:24154–24158. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- 143.Cacicedo JM, Benjachareowong S, Chou E, Ruderman NB, Ido Y. Palmitate-induced apoptosis in cultured bovine retinal pericytes: roles of NAD(P)H oxidase, oxidant stress, and ceramide. Diabetes. 2005;54:1838–1845. doi: 10.2337/diabetes.54.6.1838. [DOI] [PubMed] [Google Scholar]
- 144.Li H, Junk P, Huwiler A, Burkhardt C, Wallerath T, Pfeilschifter J, Forstermann U. Dual effect of ceramide on human endothelial cells: induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation. 2002;106:2250–2256. doi: 10.1161/01.cir.0000035650.05921.50. [DOI] [PubMed] [Google Scholar]
- 145.Gulbins E. Regulation of death receptor signaling and apoptosis by ceramide. Pharmacol Res. 2003;47:393–399. doi: 10.1016/s1043-6618(03)00052-5. [DOI] [PubMed] [Google Scholar]
- 146.Gulbins E, Grassme H. Ceramide and cell death receptor clustering. Biochim Biophys Acta. 2002;1585:139–145. doi: 10.1016/s1388-1981(02)00334-7. [DOI] [PubMed] [Google Scholar]
- 147.Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- 148.Siskind LJ, Feinstein L, Yu T, Davis JS, Jones D, Choi J, Zuckerman JE, Tan W, Hill RB, Hardwick JM, Colombini M. Anti-apoptotic Bcl-2 family proteins disassemble ceramide channels. J Biol Chem. 2008;283(11):6622–6630. doi: 10.1074/jbc.M706115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Siskind LJ, Kolesnick RN, Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. 2006;6:118–125. doi: 10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Siskind LJ, Fluss S, Bui M, Colombini M. Sphingosine forms channels in membranes that differ greatly from those formed by ceramide. J Bioenerg Biomembr. 2005;37:227–236. doi: 10.1007/s10863-005-6632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Siskind LJ. Mitochondrial ceramide and the induction of apoptosis. J Bioenerg Biomembr. 2005;37:143–153. doi: 10.1007/s10863-005-6567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Siskind LJ, Davoody A, Lewin N, Marshall S, Colombini M. Enlargement and contracture of C2-ceramide channels. Biophys J. 2003;85:1560–1575. doi: 10.1016/S0006-3495(03)74588-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Siskind LJ, Kolesnick RN, Colombini M. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J Biol Chem. 2002;277:26796–26803. doi: 10.1074/jbc.M200754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Siskind LJ, Colombini M. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J Biol Chem. 2000;275:38640–38644. doi: 10.1074/jbc.C000587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Boden G. Ceramide: a contributor to insulin resistance or an innocent bystander? Diabetologia. 2008;51:1095–1096. doi: 10.1007/s00125-008-1015-y. [DOI] [PubMed] [Google Scholar]
- 156.Minehira K, Young SG, Villanueva CJ, Yetukuri L, Oresic M, Hellerstein MK, Farese RV, Jr, Horton JD, Preitner F, Thorens B, Tappy L. Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. J Lipid Res. 2008;49:2038–2044. doi: 10.1194/jlr.M800248-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aerts JM, Ottenhoff R, Powlson AS, Grefhorst A, van Eijk M, Dubbelhuis PF, Aten J, Kuipers F, Serlie MJ, Wennekes T, Sethi JK, O’Rahilly S, Overkleeft HS. Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes. 2007;56:1341–1349. doi: 10.2337/db06-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tagami S, Inokuchi Ji J, Kabayama K, Yoshimura H, Kitamura F, Uemura S, Ogawa C, Ishii A, Saito M, Ohtsuka Y, Sakaue S, Igarashi Y. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J Biol Chem. 2002;277:3085–3092. doi: 10.1074/jbc.M103705200. [DOI] [PubMed] [Google Scholar]
- 159.Randle PJ, Garland PB, Hales LN, Newsholme EA. The glucose fatty acid cycle, its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;i:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 160.Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dumas JF, Simard G, Flamment M, Ducluzeau PH, Ritz P. Is skeletal muscle mitochondrial dysfunction a cause or an indirect consequence of insulin resistance in humans? Diabetes Metab. 2009;35:159–167. doi: 10.1016/j.diabet.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 162.Turner N, Heilbronn LK. Is mitochondrial dysfunction a cause of insulin resistance? Trends Endocrinol Metab. 2008;19:324–330. doi: 10.1016/j.tem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 163.Dobbins RL, Szczepaniak LS, Bentley B, Esser V, Myhill J, McGarry JD. Prolonged inhibition of muscle carnitine palmitoyltransferase-1 promotes intramyocellular lipid accumulation and insulin resistance in rats. Diabetes. 2001;50:123–130. doi: 10.2337/diabetes.50.1.123. [DOI] [PubMed] [Google Scholar]
- 164.Bruce CR, Hoy AJ, Turner N, Watt MJ, Allen TL, Carpenter K, Cooney GJ, Febbraio MA, Kraegen EW. Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes. 2009;58:550–558. doi: 10.2337/db08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Abu-Elheiga L, Matzuk MM, Kordari P, Oh W, Shaikenov T, Gu Z, Wakil SJ. Mutant mice lacking acetyl-CoA carboxylase 1 are embryonically lethal. Proc Natl Acad Sci U S A. 2005;102:12011–12016. doi: 10.1073/pnas.0505714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- 167.Choi CS, Savage DB, Abu-Elheiga L, Liu ZX, Kim S, Kulkarni A, Distefano A, Hwang YJ, Reznick RM, Codella R, Zhang D, Cline GW, Wakil SJ, Shulman GI. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci U S A. 2007;104:16480–16485. doi: 10.1073/pnas.0706794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schreurs M, van Dijk TH, Gerding A, Havinga R, Reijngoud DJ, Kuipers F. Soraphen, an inhibitor of the acetyl-CoA carboxylase system, improves peripheral insulin sensitivity in mice fed a high-fat diet. Diabetes Obes Metab. 2009 doi: 10.1111/j.1463-1326.2009.01078.x. [DOI] [PubMed] [Google Scholar]
- 169.Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res. 2009;50(Suppl):S138–S143. doi: 10.1194/jlr.R800079-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mao J, DeMayo FJ, Li H, Abu-Elheiga L, Gu Z, Shaikenov TE, Kordari P, Chirala SS, Heird WC, Wakil SJ. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc Natl Acad Sci U S A. 2006;103:8552–8557. doi: 10.1073/pnas.0603115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Harada N, Oda Z, Hara Y, Fujinami K, Okawa M, Ohbuchi K, Yonemoto M, Ikeda Y, Ohwaki K, Aragane K, Tamai Y, Kusunoki J. Hepatic de novo lipogenesis is present in liver-specific ACC1-deficient mice. Mol Cell Biol. 2007;27:1881–1888. doi: 10.1128/MCB.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang D, Wang A, Zhang XM, Cline GW, Yu XX, Geisler JG, Bhanot S, Monia BP, Shulman GI. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006;116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 174.Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS, Cooney GJ. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes. 2007;56:2085–2092. doi: 10.2337/db07-0093. [DOI] [PubMed] [Google Scholar]
- 175.Holloway GP, Benton CR, Mullen KL, Yoshida Y, Snook LA, Han XX, Glatz JF, Luiken JJ, Lally J, Dyck DJ, Bonen A. In obese rat muscle transport of palmitate is increased and is channeled to triacylglycerol storage despite an increase in mitochondrial palmitate oxidation. Am J Physiol Endocrinol Metab. 2009;296:E738–747. doi: 10.1152/ajpendo.90896.2008. [DOI] [PubMed] [Google Scholar]
- 176.Holloway GP, Thrush AB, Heigenhauser GJ, Tandon NN, Dyck DJ, Bonen A, Spriet LL. Skeletal muscle mitochondrial FAT/CD36 content and palmitate oxidation are not decreased in obese women. Am J Physiol Endocrinol Metab. 2007;292:E1782–1789. doi: 10.1152/ajpendo.00639.2006. [DOI] [PubMed] [Google Scholar]
- 177.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 2007;50:790–796. doi: 10.1007/s00125-007-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 179.Muoio DM, Newgard CB. Fatty acid oxidation and insulin action: when less is more. Diabetes. 2008;57:1455–1456. doi: 10.2337/db08-0281. [DOI] [PubMed] [Google Scholar]
- 180.Bouzakri K, Austin R, Rune A, Lassman ME, Garcia-Roves PM, Berger JP, Krook A, Chibalin AV, Zhang BB, Zierath JR. Malonyl coenzymeA decarboxylase regulates lipid and glucose metabolism in human skeletal muscle. Diabetes. 2008;57:1508–1516. doi: 10.2337/db07-0583. [DOI] [PubMed] [Google Scholar]
- 181.An J, Muoio DM, Shiota M, Fujimoto Y, Cline GW, Shulman GI, Koves TR, Stevens R, Millington D, Newgard CB. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med. 2004;10:268–274. doi: 10.1038/nm995. [DOI] [PubMed] [Google Scholar]
- 182.Chirala SS, Chang H, Matzuk M, Abu-Elheiga L, Mao J, Mahon K, Finegold M, Wakil SJ. Fatty acid synthesis is essential in embryonic development: fatty acid synthase null mutants and most of the heterozygotes die in utero. Proc Natl Acad Sci U S A. 2003;100:6358–6363. doi: 10.1073/pnas.0931394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lopez M, Vidal-Puig A. Brain lipogenesis and regulation of energy metabolism. Curr Opin Clin Nutr Metab Care. 2008;11:483–490. doi: 10.1097/MCO.0b013e328302f3d8. [DOI] [PubMed] [Google Scholar]
- 184.Chakravarthy MV, Zhu Y, Lopez M, Yin L, Wozniak DF, Coleman T, Hu Z, Wolfgang M, Vidal-Puig A, Lane MD, Semenkovich CF. Brain fatty acid synthase activates PPARalpha to maintain energy homeostasis. J Clin Invest. 2007;117:2539–2552. doi: 10.1172/JCI31183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chakravarthy MV, Zhu Y, Yin L, Coleman T, Pappan KL, Marshall CA, McDaniel ML, Semenkovich CF. Inactivation of hypothalamic FAS protects mice from diet-induced obesity and inflammation. J Lipid Res. 2009;50:630–640. doi: 10.1194/jlr.M800379-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, Turk J, Semenkovich CF. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 187.Kraegen EW, Bruce C, Hegarty BD, Ye JM, Turner N, Cooney G. AMP-activated protein kinase and muscle insulin resistance. Front Biosci. 2009;14:4658–4672. doi: 10.2741/3558. [DOI] [PubMed] [Google Scholar]
- 188.Hegarty BD, Turner N, Cooney GJ, Kraegen EW. Insulin resistance and fuel homeostasis: the role of AMP-activated protein kinase. Acta Physiol (Oxf) 2009;196:129–145. doi: 10.1111/j.1748-1716.2009.01968.x. [DOI] [PubMed] [Google Scholar]
- 189.Power RA, Hulver MW, Zhang JY, Dubois J, Marchand RM, Ilkayeva O, Muoio DM, Mynatt RL. Carnitine revisited: potential use as adjunctive treatment in diabetes. Diabetologia. 2007;50:824–832. doi: 10.1007/s00125-007-0605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]