Abstract
The purpose of this study was to determine the 10-yr cumulative incidence of hearing impairment and associations of education, occupation and noise exposure history with the incidence of hearing impairment in a population-based cohort study of 3753 adults ages 48-92 years at the baseline examinations during 1993-1995 in Beaver Dam, WI. Hearing thresholds were measured at baseline, 2.5 yr, 5 yr, and 10-yr follow-up examinations. Hearing impairment was defined as a pure-tone average (PTA) > 25 dB HL at 500, 1000, 2000, and 4000 Hz. Demographic characteristics and occupational histories were obtained by questionnaire. The 10-yr cumulative incidence of hearing impairment was 37.2%. Age (5 yr; Hazard Ratio (HR)=1.81), sex (M v W; HR=2.29), occupation based on longest held job (Production/Operations/Farming vs others; HR=1.34), marital status (unmarried vs married; HR=1.29) and education (<16 vs 16+ yrs; HR=1.40) were associated with the 10 yr incidence. History of noisy jobs was not associated with the 10-yr incidence of hearing impairment. The risk of hearing impairment was high, with women experiencing a slightly later onset. Markers of socioeconomic status were associated with hearing impairment, suggesting that hearing impairment in older adults may be associated with modifiable lifestyle and environmental factors, and therefore, at least partially preventable.
Keywords: presbyacusis, socioeconomic status, incidence, hearing impairment, hearing loss, aging
INTRODUCTION
Although age-related hearing loss, or presbyacusis, has been regarded as a part of normal aging, growing scientific evidence suggests that hearing loss may be another preventable age-related disorder such as atherosclerosis, cardiovascular disease or osteoarthritis (Cruickshanks, et al., In Press). Human studies have demonstrated cross-sectional associations between hearing loss and modifiable factors including education, smoking, and occupation (Cruickshanks, et al., 1998a, Popelka et al., 2000, Helzner, et al., 2005, Torre et al., 2005. Agrawal Y, et al., 2008). Animal models have suggested possible interventions to reduce the occurrence of hearing loss in aging (Chen Y, et al., 2007, Tanaka C et al., 2009). There have been few longitudinal studies of hearing in aging adults (Gates et al., 1991, Brant et al., 1996, Cruickshanks et al., 2003, Lee, et al., 2005) and to our knowledge, no other population-based studies of the long-term risk of developing hearing impairment. Such studies are essential to quantify the risk of developing hearing impairment and identify factors associated with the risk of hearing loss.
Prolonged exposure to loud noise in occupational settings has long been recognized as a cause of hearing loss in working age adults but the long-term effect of occupational noise exposure, after the noise exposure has stopped, is controversial (Lee, et al., 2005, Gates et al., 2000). Subclinical damage accrued during employment may leave the cochlea with reduced residual function, placing the ear at higher risk for hearing impairment, or noise effects may only result in apparent damage during the exposure, detectable as threshold shifts. It is possible that the effect of occupational noise on hearing has been overestimated by failing to control for other factors which may affect hearing and the possible protective effects of moderate exposure to noise which may act to “toughen” or protect the ear from damage during subsequent exposure to excessive noise (Subramaniam et al., 1991, Boettcher et al., 1992). Occupations associated with noise exposure are strongly associated with having less education which may be a marker for many unhealthy lifestyle attributes (Power, et al., 2008). Socioeconomic status (SES), usually measured by education, income, or occupation, has been found to be an independent determinant of risk of cardiovascular disease, subclinical atherosclerosis, diabetes, arthritis, and early mortality, even when controlling for known established risk factors(Marmot et al., 1991, Dalstra et al., 2005, Kaplan and Keil, 1993).
In the Epidemiology of Hearing Loss Study (EHLS), low education was associated with an increased 5-yr incidence of age-related hearing impairment (Odds Ratio (OR) = 2.37, less than high school vs college or greater), when adjusting for age, sex, and occupation (Cruickshanks, et al., 2003). Although participants who had been employed in production and fabrication occupations also experienced a higher risk of hearing loss, noise exposure history was not associated with the 5-yr incidence of hearing impairment. These results suggested that SES indicators may be markers for more complex social and behavioral factors which influence the health of aging ears.
In this earlier study, we reported that about half of the participants with hearing impairments at the baseline examination experiences further declines of hearing (progression) (Cruickshanks, et al., 2003). Age, but not sex, occupation, education, or history of noise exposure was associated with the 5-yr risk of progression (Cruickshanks, et al., 2003). Identifying factors associated with the continued loss of hearing may lead to secondary prevention opportunities to slow the rate of deterioration in hearing. The long-term impact of occupational exposure to noise is of interest as some (Gates et al., 2000), but not others (Lee et al., 2005), have suggested that noise exposure may lead to accelerated rates of hearing declines for many years.
The purpose of this paper was to determine the 10-yr cumulative incidence and progression of hearing impairment and the effects of occupation, occupational noise exposure history, and education, on the 10-yr incidence and progression of hearing impairment.
METHODS
Population
During 1987-88, a private census was conducted to identify residents of the city or township of Beaver Dam who were ages 43-84 years (Linton et al., 1991). This cohort subsequently was invited to participate in the Beaver Dam Eye Study, a study of age-related ocular disorders (Klein et al., 2001). Of the 5924 eligible people, 4926 (83%) participated in the eye examination phase (1988-90). Beaver Dam Eye Study participants alive as of March 1, 1993 were eligible for the baseline examination for the Epidemiology of Hearing Loss Study (EHLS; n=4541) which occurred at the time of the five-year follow-up visit for the eye study (Cruickshanks et al., 1998b). Of those eligible for the EHLS, 3753 (82.6%) participated in the baseline hearing study, 180 (4.0%) died prior to being seen, 604 (13.3%) refused to participate, and 4 (0.1%) were lost to follow-up (Cruickshanks et al., 1998b).
Participants 75 years of age or older in 1995 were re-examined for a 2.5 yr follow-up (n=801). The entire cohort was invited to a five-year follow-up examination (1998-2000). Of the 3407 EHLS participants alive as of March of 1998, 2800 (82.2%) of the survivors participated in the five-year follow-up study, 436 (12.8%) refused, 164 (4.8%) died prior to being seen, and seven (0.2%) were lost to follow-up. During 2003-2005, 2395 (82.5%) of the survivors participated in the ten-year follow-up study, 259 (8.9%) died prior to being seen, and 248 (8.5%) refused or were lost to follow-up. (Cruickshanks et al., 2003)
Among participants seen at the 10-yr follow-up the average length of follow-up was 10.1 years. Participants in the 10-yr follow-up examination were younger than non-participants (Table 1, p<0.001). After adjusting for age and sex, non-participants were less educated, less likely to have been employed in managerial or professional occupations, and more likely to have a hearing loss at baseline. There were 1925 participants with normal hearing at the baseline examination, and therefore at risk of developing hearing impairment during the follow-up period. Of these, 1636 (89.1% of the 1837 alive in 1998) participated in five-yr follow-up examinations and 1465 (91.6% of the 1600 alive in 2003) participated in the 10-yr follow-up examinations. This study was approved by the Health Sciences Institutional Review Board of the University of Wisconsin.
Table 1.
Baseline (1993-95) Characteristics by Participation in EHLS 5 and 10-yr Follow-up Examinations
| 1993-1995 | 1998-2000 | 2003-20005 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 3753 | n = 3407 | n = 2902 | ||||||||
| Participants | Non-participants | Participants | Non-participants | |||||||
| Age group (yrs) | n | % | n | % | n | % | n | % | n | % |
| 48-59 | 1298 | 34.6 | 1108 | 39.6 | 156 | 25.7 | 1064 | 44.4 | 160 | 31.6 |
| 60-69 | 1103 | 29.4 | 871 | 31.1 | 164 | 27.0 | 778 | 32.5 | 149 | 29.4 |
| 70-79 | 942 | 25.1 | 637 | 22.8 | 176 | 29.0 | 472 | 19.7 | 138 | 27.2 |
| 80-92 | 410 | 10.9 | 184 | 6.6 | 111 | 18.3 | 81 | 3.4 | 60 | 11.8 |
| Sex | ||||||||||
| Men | 1589 | 42.3 | 1158 | 41.4 | 258 | 42.5 | 981 | 41.0 | 211 | 41.6 |
| Women | 2164 | 57.7 | 1642 | 58.6 | 349 | 57.5 | 1414 | 59.0 | 296 | 58.4 |
| Education (yrs) | ||||||||||
| < 12 | 914 | 24.4 | 552 | 19.7 | 203 | 33.4 | 394 | 16.5 | 156 | 30.8 |
| 12 | 1714 | 45.7 | 1329 | 47.5 | 260 | 42.8 | 1165 | 48.6 | 237 | 46.8 |
| 13-15 | 577 | 15.4 | 454 | 16.2 | 83 | 13.7 | 413 | 17.3 | 60 | 11.8 |
| 16+ | 546 | 14.6 | 463 | 16.6 | 61 | 10.0 | 421 | 17.6 | 54 | 10.7 |
| Marital Status | ||||||||||
| Married | 2410 | 68.2 | 1916 | 70.8 | 322 | 64.1 | 1686 | 73.0 | 291 | 66.3 |
| Single | 131 | 3.7 | 100 | 3.7 | 21 | 4.2 | 85 | 3.7 | 16 | 3.6 |
| Divorced/Widowed | 992 | 28.1 | 691 | 25.5 | 159 | 31.7 | 540 | 23.3 | 132 | 30.1 |
| Employment | ||||||||||
| Full time | 1161 | 31.0 | 992 | 35.5 | 129 | 21.3 | 951 | 39.7 | 131 | 25.8 |
| Part time | 459 | 12.2 | 367 | 13.1 | 71 | 11.7 | 330 | 13.8 | 70 | 13.8 |
| Homemaker | 399 | 10.6 | 294 | 10.5 | 70 | 11.6 | 258 | 10.8 | 56 | 11.1 |
| Retired | 1623 | 43.3 | 1063 | 38.0 | 320 | 52.8 | 793 | 33.1 | 229 | 45.2 |
| Un/Dis/SL | 107 | 2.9 | 82 | 2.9 | 16 | 2.6 | 62 | 2.6 | 21 | 4.1 |
| Occupation - Longest Held Job |
||||||||||
| Managerial or Professional |
665 | 18.7 | 553 | 20.7 | 81 | 14.1 | 496 | 21.6 | 77 | 16.0 |
| Technical sales | 850 | 23.9 | 672 | 25.2 | 121 | 21.0 | 545 | 25.9 | 108 | 22.4 |
| Service | 750 | 21.1 | 550 | 20.6 | 123 | 21.4 | 454 | 19.8 | 102 | 21.2 |
| Farming or forestry | 131 | 3.7 | 84 | 3.1 | 29 | 5.0 | 70 | 3.1 | 18 | 3.7 |
| Production | 419 | 11.8 | 296 | 11.1 | 74 | 12.9 | 247 | 10.8 | 58 | 12.0 |
| Operator or Fabricator |
745 | 20.9 | 577 | 19.4 | 148 | 25.7 | 432 | 18.8 | 119 | 24.7 |
| Hearing Status | ||||||||||
| Normal | 1925 | 54.1 | 1636 | 60.1 | 201 | 39.0 | 1465 | 62.9 | 223 | 50.8 |
| Mild Loss | 948 | 26.7 | 687 | 25.3 | 160 | 31.1 | 578 | 24.8 | 118 | 26.9 |
| Mod/Sev | 683 | 19.2 | 398 | 14.6 | 154 | 29.9 | 288 | 12.4 | 98 | 22.3 |
Examinations
Informed consent was obtained at each examination. The same standardized methods were used at each examination which included an otoscopic evaluation, screening tympanogram (GSI 37 Autotymp, Grason-Stadler, Inc., Madison, WI), and pure-tone air- and bone-conduction audiometry (Nondahl et al., 1996, Cruickshanks et al., 1998b). Audiometric testing was conducted according to the guidelines of the American Speech-Language-Hearing Association (ASHA, 1987) in sound-treated booths. Pure-tone air-conduction thresholds (measured in dB Hearing Level (HL)) were obtained for each ear at 500, 1000, 2000, 3000, 4000, 6000 and 8000 Hz. Bone-conduction thresholds were measured at two frequencies at baseline (500 and 4000 Hz) and three at each follow-up examination (500, 2000 and 4000 Hz). Masking was used as necessary.
At the baseline examination Virtual 320 clinical audiometers (Virtual Corporation, Seattle, WA) equipped with TDH-50 earphones and insert earphones were used. At the follow-up examinations, GSI 61clinical audiometers (Grason-Stadler, Inc., Madison, WI) equipped with TDH-50 earphones and insert earphones were used. Participants seen outside of the field examination site were tested using a Beltone 112 portable audiometer (Beltone Electronic Corp., Chicago, IL). All audiometers were calibrated and were re-calibrated every six months during the study periods (ANSI, 1996, 2004). Ambient noise levels were measured at each home or nursing home visit and were routinely monitored at the field examination site at the Beaver Dam Community Hospital to ensure that testing conditions complied with ANSI standards (ANSI, 1991,1999).
A questionnaire including occupational history was administered as an interview at the baseline and follow-up examinations. Questionnaire data on educational attainment were obtained as part of the Beaver Dam Eye Study examinations in the same cohort
Definitions
At baseline, the presence of hearing impairment was defined as a pure-tone average (PTA) of thresholds at 500, 1000, 2000 and 4000 Hz greater than 25 dB HL in either ear (Cruickshanks, et al., 1998b). Analyses of the incidence of hearing impairment were restricted to participants (n=1925) who were at risk for incident hearing impairment at the baseline examination (PTA in each ≤25dB HL at baseline). Incident hearing impairment was defined as a PTA > 25 dB HL in either ear at a follow-up examination among participants at risk for incident hearing impairment (i.e., normal hearing at baseline).
Participants with a hearing impairment at baseline (PTA > 25 db HL in either ear) were included in the analyses evaluating progression of hearing impairment if the PTA in the worse ear was less than 100 dB HL (Cruickshanks, et al., 1998b). There were 29 people with hearing impairment at baseline with a PTA ≥ 100 dB HL who were excluded from analyses because their hearing thresholds at baseline were near the limits of measurement. Progression of hearing impairment was defined as a PTA at follow-up that was more than 5 dB greater than the baseline PTA. Participants with normal hearing at the baseline examination were excluded from these analyses of progression.
Marital status at baseline was defined as married or not married, including single, widowed or divorced, although indicator variables were used to test effects of each category before combining them. Years of education was classified as less than 16 yrs vs 16 or more (less than college graduate or college graduate or more) after exploring effects of other groupings using indicator variables.
Occupations were coded according to the 1980 census classifications: managerial/professional, technical/sales/administration, service, farmer/forestry/fishing, production/crafts/repair, and operator/fabricator/laborer. Major occupation was classified on the basis of the job held the longest. After exploring the effects using indicator variables, occupation was grouped into two groups: Production, Operator or Farmer and Managerial, Technical or Service. In addition, current job classification was defined as the job held at the baseline examination, among those employed at baseline. History of a noisy job (ever) was defined as ever holding a fulltime job where one needed to speak in a raised voice or louder to be heard by someone two feet away, being a farmer and driving a tractor without a cab, or having served in the military as a pilot, airplane or tank crew member, or worked in the engine room of a ship, or used grenades, mortars, shoulder-held grenade launchers or multi-person weapons systems. Current noisy job (at baseline) was computed for those employed at baseline.
Analyses were conducted using SAS version 9.1 software (SAS Institute, Inc., Gary, NC). Univariate analyses used the chi-square test of association for categorical variables, Mantel Haenszel chi-square test of trend for ordinal data (Mantel 1963), and t-tests of mean differences for continuous data. Age-adjusted comparisons between participants and nonparticipants (Table 1) utilized the Cochran-Mantel-Haenszel statistic for general association as did age-adjusted comparisons of prevalence and incidence rates between men and women (Landis et al., 1978). Product-limit (Kaplan-Meier) survival estimates were used to calculate cumulative incidence and progression (Kaplan and Meier, 1958; Cox and Oakes, 1984). The discrete hazards of hearing loss developing or progressing were compared with the Cox proportional hazards models (Cox, 1972), using the discrete-time version because of the relatively small number of follow-up intervals. These models were used to evaluate the associations of age, gender, socioeconomic and occupational factors and the incidence and progression of hearing loss.
RESULTS
Incidence
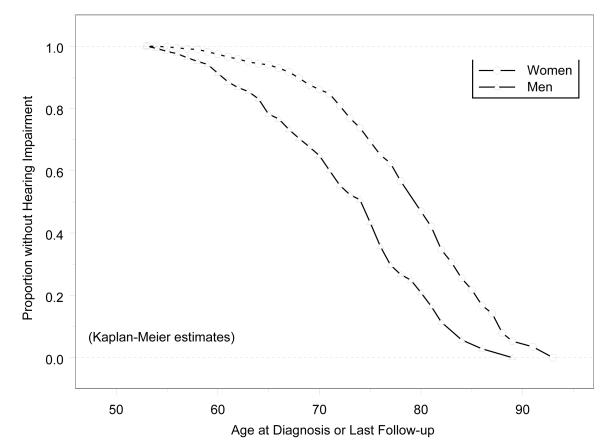
The 10-yr cumulative incidence of hearing impairment was 37.2% (Table 2). The incidence was higher in older age groups and higher among men than women. The 10-yr cumulative incidence was about twice the 5-yr incidence rate among the youngest age groups but not among those 70 yrs of age or older. Figure 1 shows the sex-specific survival curves using incidence of hearing impairment as the outcome and demonstrates that women develop hearing impairments about six years later than men. The mean age at onset was 74 yrs for women vs. 68 yrs for men (p<0.0001). The mean PTA at baseline for participants at risk of hearing impairment was 14.8 dB.
Table 2.
10 year Cumulative Incidence and Progression of Hearing Impairment by Age at Baseline
| Cumulative Incidence | |||||
|---|---|---|---|---|---|
| 5 years | 10 years | ||||
| Age group (yrs) |
At risk at Baseline |
Cases | Rate per 100 (95%CI) | Cases | Rate per 100 (95%CI) |
| 48-59 | 990 | 99 | 11.2 ( 9.10, 13.3) | 183 | 21.8 (19.0, 24.6) |
| 60-69 | 544 | 111 | 22.4 ( 18.8, 26.1) | 209 | 45.5 (40.9, 50.1) |
| 70-79 | 303 | 110 | 47.4 ( 40.9, 53.8) | 155 | 73.7 (67.4, 80.0) |
| 80-92 | 38 | 27 | 92.6 (72.5, 99.5 ) | 29 | 100 --- |
| total | 1925 | 347 | 21.1 (19.2, 23.1 ) | 576 | 37.2 (34.8, 39.6) |
| Women | |||||
| 48-59 | 605 | 37 | 6.8 (4.70, 8.90) | 80 | 15.6 (12.5, 18.8) |
| 60-69 | 407 | 61 | 17.6 (13.6, 21.6) | 131 | 40.7 (35.3, 46.1) |
| 70-79 | 242 | 83 | 44.1 (37.0, 51.2) | 120 | 70.6 (63.4, 77.9) |
| 80-92 | 34 | 23 | 91.5 (69.7, 99.4) | 25 | 100 --- |
| total | 1288 | 204 | 18.5 (16.2, 20.8) | 356 | 34.3 (31.4, 37.2) |
| Men | |||||
| 48-59 | 385 | 62 | 18.3 (14.2, 22.5) | 103 | 31.7 (26.6, 36.8) |
| 60-69 | 187 | 50 | 33.6 (26.0, 41.1) | 78 | 56.8 (48.3, 65.3) |
| 70-79 | 61 | 27 | 61.1 (46.7, 75.6) | 35 | 87.1 (75.6, 98.5) |
| 80-92* | 4 | --- | --- | --- | --- |
| total | 637 | 143 | 26.7 (23.0, 30.5) | 220 | 43.2 (38.8, 47.5) |
| Cumulative Progression | |||||
|---|---|---|---|---|---|
| 5 years | 10 years | ||||
| At risk at Baseline |
Cases | Rate per 100 (95% CI) | Cases | Rate per 100 (95% CI) | |
| 48-59 | 256 | 65 | 31.7 (25.3, 38.1) | 122 | 61.7 (54.8, 68.5) |
| 60-69 | 462 | 160 | 45.2 (40.0, 50.4) | 257 | 77.6 (73.0, 82.2) |
| 70-79 | 589 | 235 | 59.6 (54.7, 64.5) | 314 | 88.1 (84.4, 91.8) |
| 80-92 | 324 | 119 | 73.2 (65.9, 80.5) | 139 | 98.7 (84.1, 100) |
| total | 1631 | 579 | 52.0 (49.0, 54.9) | 832 | 80.4 (77.9, 83.0) |
| Women | |||||
| 40-59 | 69 | 16 | 30.2 (17.8, 42.6) | 35 | 69.2 (56.3, 82.1) |
| 60-69 | 159 | 64 | 51.2 (42.4, 60.0) | 94 | 79.4 (71.8, 86.9) |
| 70-79 | 291 | 126 | 62.3 (55.5, 69.0) | 170 | 91.4 (87.0, 95.8) |
| 80-92 | 211 | 86 | 73.5 (65.0, 82.1) | 102 | 98.4 (82.1, 100) |
| total | 730 | 292 | 58.8 (54.4, 63.2) | 401 | 86.9 (83.6, 90.2) |
| Men | |||||
| 40-59 | 187 | 49 | 32.2 (24.8,39.7) | 87 | 59.1 (51.1, 67.1) |
| 60-69 | 303 | 96 | 41.9 (35.5, 48.3) | 163 | 76.7 (70.8, 82.5) |
| 70-79 | 298 | 109 | 56.7 (49.7, 63.8) | 144 | 84.3 (78.2, 90.3) |
| 80-92 | 113 | 33 | 72.7 (58.6, 86.8) | 33 | 100-- |
| total | 901 | 287 | 46.5 (42.6, 50.4) | 427 | 75.4 (71.7, 79.0) |
Incidence rate not shown due to small cell size.
Figure 1.
Cumulative Proportion of Participants without Hearing Impairment by Age at Detection or Last Follow-up: Epidemiology of Hearing Loss Study. All participants included in this analysis had normal hearing (PTA ≤25 dB HL) at the baseline examination.
Progression
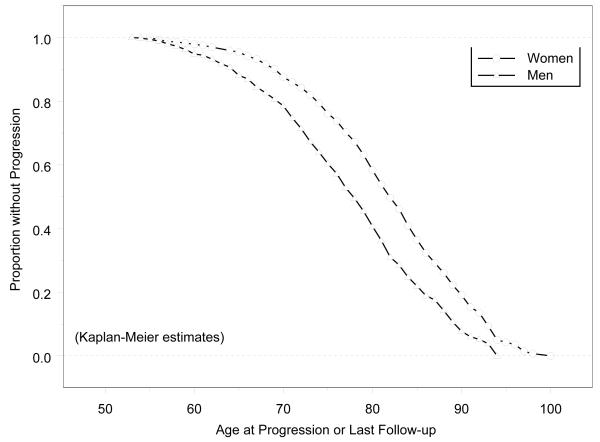
Among those with a baseline hearing impairment, most experienced a further decline in hearing, as the PTA worsened in 80.4% of these participants (Table 2). Among participants less than 60 years of age at baseline, the ten-year progression rate was about twice the 5-yr rate, but slowed for older age groups. Age-specific progression rates differed little between men and women. Figure 2 shows the sex-specific survival curves using progression of hearing impairment as the outcome and indicates that the gender gap was about five years, similar to gap in incidence. The mean age at progression was 79 years for women vs. 74 years for men (p<0.0001).
Figure 2.
Cumulative Proportion of Participants without Progression of Hearing Impairment by Age at Progression or Last Follow-up: Epidemiology of Hearing Loss Study. All participants included in this analysis had hearing impairment (PTA >25 dB HL) at the baseline examination.
Education, Occupation, and Occupational Noise Exposure
Age and sex were associated with increased risk of developing hearing impairments during the 10-yr follow-up period (Hazard Ratio (HR) =1.81 for 5 yrs of age and 2.29 for men vs women) (Table 3, Model 1). In addition, less education (HR=1.40), being unmarried (HR=1.29), and in the Production, Operator or Farmer occupation group (POF) for the longest held job (HR=1.34) were associated with the incidence of hearing impairment. On average, the reported duration of the longest held job was 22 years. Education and occupation were strongly associated (p<0.0001); participants whose occupation was in the POF group were less likely to have attended or graduated from college than people working in other sectors (88.5% vs 53.6%)
Table 3.
Cox Proportional Hazards Model for 10-yr Incidence of Hearing Impairment
| Model 1 | Model 2 | |
|---|---|---|
| Risk Factors | Hazard Ratio (95%CI) | Hazard Ratio (95%CI) |
| Age (5 yr) | 1.81 (1.69,1.94) | 1.81 (1.70,1.94) |
| Men (vs. Women) | 2.29 (1.81, 2.90) | 2.49 (1.96,3.15) |
| Education (<16 yrs vs 16 or more yrs) |
1.40 (1.05, 1.87) | 1.56 (1.19,2.06) |
| Marital Status (Not Married vs Married) |
1.29 (1.03, 1.62) | 1.32 (1.06,1.66) |
| Occupation – Longest Held Job (Production, Operator or Farmer vs Managerial, Technical or Service) |
1.34 (1.06, 1.69) | Not included |
| Reported Noisy Job | Not included | 1.00 (0.81,1.24) |
Not all participants with jobs in the POF occupation group were exposed to loud noise at work. Among those at risk for incident hearing impairment, only 46.6% of the 414 people working in these sectors also reported having to speak in a raised voice or louder to be heard at work. In addition, people may have acquired significant noise exposure in other jobs or through military service. To assess the effects of noise at work, a similar model was constructed including history of a noisy job (Table 3, Model 2). A positive history of occupational noise exposure was not associated with the cumulative incidence of hearing impairment (Table 3, Model 2). All other covariates remained statistically significantly associated with the incidence of hearing impairment with little change in effect sizes. In another multivariable model age (HR=1.81, 95%CI=1.69,1.94), sex (HR=2.32, 95%CI=1.81,2.97), education (HR=1.40, 95%CI=1.05,1.87), marital status (HR=1.29, 95%CI=1.03, 1.62), and occupation (HR=1.35, 95%CI=1.06,1.71), but not history of a noisy job (HR=0.96, 95%CI=0.77,1.20), were associated with the incidence of hearing impairment.
Restricting analyses to those employed at the baseline examination, neither current occupation (Table 4, Model 1; HR=1.14, 95%CI=0.81, 1.61) nor having reported holding a noisy job (Table 4, Model 2; HR=1.16, 95%CI=0.81, 1.66) were associated with risk of incident hearing impairment during the 10-yr follow-up period. Age, sex, and education remained statistically significant predictors in both models (Table 4), and marital status was significant in model 2 but not model 1, although the effect size estimates were similar.
Table 4.
Cox Proportional Hazards Model for 10-yr Incidence of Hearing Impairment Among Participants Employed at the Baseline Examination
| Model 1 | Model 2 | |
|---|---|---|
| Risk Factors | Hazard Ratio (95%CI) | Hazard Ratio (95%CI) |
| Age (5 yr) | 1.54 (1.35,1.76) | 1.83 (1.70,1.96) |
| Men (vs. Women) | 2.13 (1.54, 2.95) | 2.49 (2.00,3.10) |
| Education (<16 yrs vs 16 or more yrs) |
1.95 (1.29, 2.93) | 1.55 (1.18, 2.04) |
| Marital Status (Not Married vs Married) |
1.31 (0.93, 1.84) | 1.32 (1.05, 1.65) |
| Occupation – Current Job (Production, Operator or Farmer vs Managerial, Technical or Service) |
1.14 (0.81, 1.61) | Not included |
| Reported Noisy Job (Current) | Not included | 1.16 (0.81, 1.66) |
In analyses with progression of hearing impairment as the outcome, only age (HR for 5 yrs =1.37, 95%CI=1.29, 1.46) and sex (HR men vs women =0.77, 95%CI=0.60, 0.97) were significantly associated with risk of increased hearing loss. Neither occupation (longest or current) nor reported employment in noisy jobs (longest or current) were associated with risk of progression.
DISCUSSION
Results from this population-based cohort study indicate that the ten-year cumulative incidence of hearing impairment was high, consistent with our previous report with shorter follow-up time (Cruickshanks et al., 2003). Among younger age groups, the 10-yr rate was about twice the 5-yr rate. While this pattern was less consistent at older ages, that may reflect the effects of selective survival. There are no other long-term incidence studies with which to compare these findings but they suggest that the risk of hearing impairment remains high at older ages. There does not appear to be a risk period after which one is likely to remain free of hearing impairment. In fact, age was significantly associated with the incidence, indicating that older adults are at higher risk than younger adults.
Although the U.S. Preventive Services Task Force has recommended that clinicians question their older patients about hearing impairments and provide counseling about hearing aids, these recommendations are currently being updated (USPSTF). Medicare does not usually cover associated treatment costs associated with hearing aids. Hearing impairment results in significant difficulty with communication, has been linked to depression and social isolation, and associated with lower quality of life (Weinstein and Ventry 1982, Dalton, et al., 2003). Few older adults with hearing impairment receive treatment(Popelka et al., 1998), and treatment options are limited primarily to hearing aids which may not restore optimal auditory function although they have been shown to improve speech recognition (Larson et al., 2002). Our findings suggest that age-related hearing impairment represents a significant public health problem, particularly as the number of older adults continues to grow, with improvements in life expectancy and the aging of the baby-boom cohort.
In this study men were more likely to develop a hearing impairment than women, after adjusting for age, and were affected about 6 years sooner than women. This pattern is well-known from earlier cross-sectional studies, and in our cohort, is not explained by differences in age, occupation or exposure to noise suggesting that other behaviors, or hormonal factors, may contribute to the relative protection experienced by women (Cruickshanks et al., 2003, Cruickshanks et al., In Press). Nonetheless, hearing impairment is an important health problem for women as well as men, as the incidence was high in both groups (34% for women and 43% for men). Women need to be aware of the importance of monitoring hearing function as they age, and physicians need to ensure that female, as well as male patients are referred for appropriate treatment. Other studies, including animal models, suggest that some of the female advantage may be due to hormonal effects lost at menopause (Guimaraes et al., 2004, Hederstierna et al., 2009).
Most people with hearing impairment at the baseline examination experienced a decline in hearing during this 10-yr period, consistent with earlier reports of declining thresholds over time (Gates et al., 1991, Brant et al., 1996, Cruickshanks et al., 2003, Lee, et al., 2005, Wiley et al., 2008). Men were slightly less likely to experience a decline which may reflect their worse hearing at baseline (closer to the limits of audiometers to measure hearing limiting the change that could be measured) or true gender differences in the rate of decline. We have previously reported frequency-specific analyses which demonstrated that 10-yr thresholds were worse for men than women at most frequencies, adjusting for baseline threshold level (Wiley et al., 2008). Women may experience a delay in onset but then worsen at a slightly faster rate at least at some frequencies; men may have an earlier onset and then more gradual decline (Lee et al., 2005, Wiley et al., 2008). Changing hormonal levels around menopause may be important in explaining these gender-specific patterns. Similar patterns have been observed in mice where older female mice had worse thresholds than male mice, although there was little gender difference at young ages (Guimaraes et al., 2004), consistent with accelerated rates of decline in older female mice. Recently, one small human study demonstrated that the rate of hearing decline may differ by time since menopause (Hederstierna et al., 2009). However, other studies have suggested that hormone replacement therapy may have detrimental effects on hearing (Guimaraes et al., 2006, Price et al., 2009), highlighting the need for additional investigation of the complex effects of hormones on auditory function. For both men and women the extremely high rates of continued decline point to the importance of identifying new treatments to stop the decline in hearing function over time. In addition, better methods to restore good hearing function are needed to minimize the negative impact of hearing impairment on older adults’ quality of life and ability to communicate.
Education, occupation group, and marital status, indicators of socioeconomic status were associated with the ten-year cumulative incidence of hearing impairment. These results are consistent with most earlier cross-sectional studies and with our earlier 5-yr findings (Cruickshanks et al., 2003, Cruickshanks et al., In Press). Socioeconomic status has been linked to mortality, cardiovascular disease, and other chronic diseases (Marmot et al., 1991, Kaplan and Keil, 1993, Dalstra et al., 2005). Possible mechanisms include clustering of poor health behaviors (e.g., increased exposure to smoking, higher alcohol consumption, more atherogenic diets, increased obesity, etc.) or through biological effects of increased stress (dysregulation of hormonal function and/or increased inflammation) (Power et al., 2008, Miller et al., 2009). These strong associations with socioeconomic status suggest that presbyacusis is, at least in part, a preventable disorder.
Noise exposure was not associated with the ten-year incidence or progression of hearing impairment. In this largely retired population, occupational noise exposure may have contributed to hearing impairments present at the baseline examination (Cruickshanks et al., 1998b), but there was no evidence of any residual effect on long-term risk of declining hearing sensitivity among people with normal hearing at the baseline examination. Even among those exposed to occupational noise at the baseline examination, there was no evidence of an effect. These results are consistent with the study by Lee et al., (2005) which measured hearing repeatedly, and reported no difference in the rate of change between people with and without positive noise histories. In the Framingham Study, people classified as having notched audiograms experienced greater declines at some frequencies than those without notches (Gates et al., 2000). Although the authors suggested that the notches represented noise damage, we have recently shown that some methods for defining notched audiograms do not show good agreement, and they are not consistent indicators of noise exposure history (Nondahl, et al., In Press).
Although occupational noise exposure may be an important source of auditory damage in industrial workers, it is possible the effects have been over-estimated as few studies included multivariate models, considering other factors. Some studies have suggested that noise exposure may act to “toughen” or protect the ear from damage during subsequent exposure to excessive noise (Subramaniam et al., 1991, Boettcher et al., 1992). Therefore, our failure to find an association may reflect the impact of the opposite effects of potentially protective effects of variable noise exposure typical in most occupations and damaging effects of loud occupational noise exposures. Our data suggest that, on a population-basis, there is little evidence that prior occupational noise exposure plays an important role in the onset or progression of hearing impairment in older adults followed for ten years. However, longitudinal, population-based studies of younger adults may be needed to determine the association between noise history and early onset of hearing impairment.
This study adds important information about the long-term risk of hearing impairment in older adults. Among its strengths are the large, population-based cohort design, high follow-up rates throughout the 10-yr period particularly among those at risk for incident hearing loss, use of established, standardized testing methods for measuring hearing thresholds, multiple measures of socioeconomic status, and extensive occupational histories, including noise exposure questions. However, there are limitations that are important to consider. Our definition of incident hearing impairment is clinically oriented, using standardized pure tone air and bone conduction audiometry. It is possible that other measures of hearing such as complex speech understanding tasks may be more sensitive for detecting noise effects. Our noise questions provide an imprecise estimate of noise exposure, may not accurately capture exposure to noise levels thought to damage hearing and do not provide information on cumulative dose or duration of exposure. Thus, we may have failed to find an association because of measurement error. However, our questionnaire is a modification of one used in previous studies of occupational noise exposure (Talbott et al., 1985, Talbott et al., 1990) and we did detect an association with the prevalence of hearing impairment (Cruickshanks et al., 1998b), as would be expected since prevalent cases at the baseline examination would include people who acquired hearing impairments during the time they were employed in noisy jobs. Thus, our negative finding suggests that noise exposure has at most only a small effect on the development of hearing loss at older ages, after the exposure has stopped.
Our study has shown that age, sex, education, marital status, and occupation, but not occupational noise exposure, are independently associated with the 10-yr cumulative incidence of hearing impairment. Reasons for the female advantage are not known, but this pattern, along with the socioeconomic gradient, support the notion that presbyacusis is a preventable disorder affecting older adults. Women and men, and older people of any age, are at high risk of developing hearing impairments. Identifying other factors important in the etiology of hearing impairment may lead to new methods to prevent the onset of this disorder. In addition, with the aging of the population, and the high risk, hearing impairment represents an important public health problem. Additional research on barriers to hearing aid utilization, effective screening programs and treatments to restore auditory function and reduce the impact on quality of life in older adults is warranted.
Acknowledgments
This research is supported by National Institutes of Health grant AG11099 (KJ Cruickshanks) and EY06592 (R Klein).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults. Arch Intern Med. 2008;168:1522–1530. doi: 10.1001/archinte.168.14.1522. [DOI] [PubMed] [Google Scholar]
- American National Standards Institute (ANSI) Maximum Permissible Ambient Noise Levels for Audiometric Test Rooms (ANSI S3.1-1991) ANSI; New York: 1991. [Google Scholar]
- American National Standards Institute (ANSI) Specifications for Audiometers (ANSI S3.6-1996) ANSI; New York: 1996. [Google Scholar]
- ANSI . American National Standard Maximum Permissible Ambient Noise Levels for Audiometric Test Rooms. (ANSI S3.1-1999) ANSI; New York: 1999. [Google Scholar]
- American National Standards Institute . American National Standard Specification for Audiometers. (ANSI S3.6-2004) New York: 2004. [Google Scholar]
- American Speech-Language-Hearing Association (AHSA) Guidelines for Manual Pure-Tone Threshold Audiometry. ASHA. 1987;20:297–301. [PubMed] [Google Scholar]
- Boettcher FA, Sponger VP, Salvi RJ. Physiological and histological changes associated with the reduction of the threshold shift during interrupted noise exposure. Hearing Research. 1992;62:217–236. doi: 10.1016/0378-5955(92)90189-t. [DOI] [PubMed] [Google Scholar]
- Brant LJ, Gordon-Salant S, Pearson JD, Klein LL, Morrell CH, Metter EJ, Fozard JL. Risk Factors Related to Age-Associated Hearing Loss in the Speech Frequencies. J Am Acad Audiol. 1996;7:152–160. [PubMed] [Google Scholar]
- Chen Y, Huang WG, Zha DJ, Qiu JH, Wang JL, Sha SH, Schacht J. Aspirin attenuates gentamicin ototoxicity: from the laboratory to the clinic. Hear Res. 2007;226(1-2):178–82. doi: 10.1016/j.heares.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables. J Royal Stat Soc. 1972;B34:187–220. [Google Scholar]
- Cox DR, Oakes D. Analysis of Survival Data. Chapman and Hall; London: 1984. [Google Scholar]
- Cruickshanks KJ, Klein R, Klein BEK, Wiley TL, Nondahl DM, Tweed TS. Cigarette smoking and hearing loss: The Epidemiology of Hearing Loss Study. JAMA. 1998a;279(21):1715–1719. doi: 10.1001/jama.279.21.1715. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Wiley TL, Tweed TS, Klein BEK, Klein R, Mares-Perlman JA, Nondahl DM. Prevalence of hearing loss in older adults in Beaver Dam, WI: The Epidemiology of Hearing Loss Study. Am J Epidemiol. 1998b;148(9):879–86. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Tweed TS, Wiley TL, Klein BEK, Klein R, Chappell RJ, Nondahl DM, Dalton DS. The five-year incidence and progression of hearing loss: The Epidemiology of Hearing Loss Study, 2003. Arch Otolaryngol Head Neck Surg. 2003;129:1041–1046. doi: 10.1001/archotol.129.10.1041. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Zhan W, Zhong W. Epidemiology of Age-Related Hearing Impairment. In: Gordon-Salant S, Frisina R, editors. The Aging Auditory System: Perceptual Characterization and Neural Bases of Presbycusis. Springer; New York: In Press. [Google Scholar]
- Dalstra JAA, Kunst AE, Borrell C, Breeze E, Cambois E, Costa G, Geurts JJM, Lahelma E, Van Oyen H, Rasmussen NK, Regidore E, Spadea T, Mackenbach JP. Socioeconomic differences in the prevalence of common chronic diseases: an overview of eight European countries. Internat J Epidemiol. 2005;34:316–326. doi: 10.1093/ije/dyh386. [DOI] [PubMed] [Google Scholar]
- Dalton DS, Cruickshanks KJ, Klein BEK, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults. The Gerontologist. 2003;45(5):661–668. doi: 10.1093/geront/43.5.661. [DOI] [PubMed] [Google Scholar]
- Gates GA, Cooper JC. Incidence of hearing decline in the elderly. Acta Otolaryngol Suppl. 1991;111:240–248. doi: 10.3109/00016489109137382. [DOI] [PubMed] [Google Scholar]
- Gates GA, Schmid P, Kujawa SG, Nam B, D’Agostino R. Longitudinal threshold changes in older males with audiometric notches. Hearing Research. 2000;141:220–228. doi: 10.1016/s0378-5955(99)00223-3. [DOI] [PubMed] [Google Scholar]
- Guimaraes P, Zhu X, Cannon T, Kim AH, Frisina RD. Sex differences in distortion product otoacoustic emissions as a function of age in CBA mice. Hearing Research. 2004;192:83–89. doi: 10.1016/j.heares.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Guimaraes P, Frisina ST, Mapes F, Tadros SF, Frisina DR, Frisina RD. Progestin negatively affects hearing in aged women. Proc Natl Acad Sci. 2006;103:14246–14249. doi: 10.1073/pnas.0606891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstierna C, Hultcrantz M, Collins A, Rosenhall U. The menopause triggers hearing decline in healthy women. Hearing Research. 2009 Sep 23; doi: 10.1016/j.heares.2009.09.009. In Press. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Helzner EP, Cauley JA, Pratt SR, Wisniewski SR, Zmuda JM, Talbott EO, de Rekeneire N, Harris TB, et al. Race and sex differences in age-related hearing loss; The Health, Aging, and Body Composition Study. J Am Geriatr Soc. 2005;53:2119–2127. doi: 10.1111/j.1532-5415.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457–81. [Google Scholar]
- Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88:1973–1998. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BEK, Lee KE, Cruickshanks KJ. The changes in visual acuity in a population over a 10-year period. The Beaver Dam Eye Study. Ophthalmology. 2001;108(10):1757–1766. doi: 10.1016/s0161-6420(01)00769-2. [DOI] [PubMed] [Google Scholar]
- Landis RJ, Heyman ER, Koch GG. Average partial association in three-way contingency tables: a review and discussion of alternative tests. Internat Statist Review. 1978;46:237–254. [Google Scholar]
- Larson VD, Williams DW, Henderson WG, Luethke LE, Beck LB, Noffsinger D, Bratt GW, Dobie RA, Fausti SA, Haskell GB, Rappaport BZ, Shanks JE, Wilson RH. A multi-center, double blind clinical trial comparing benefit from three commonly used hearing aid circuits. Ear Hear. 2002;23:269–76. doi: 10.1097/00003446-200208000-00001. [DOI] [PubMed] [Google Scholar]
- Lee F-S, Matthews LJ, Dubno JR, Mills JH. Longitudinal study of pure-tone thresholds in older persons. Ear & Hearing. 2005;26:1–11. doi: 10.1097/00003446-200502000-00001. [DOI] [PubMed] [Google Scholar]
- Linton KLP, Klein BEK, Klein R. The validity of self-reported and surrogate-reported cataract and age-related macular degeneration in the Beaver Dam Eye Study. Am J Epidemiol. 1991;134:1438–1446. doi: 10.1093/oxfordjournals.aje.a116049. [DOI] [PubMed] [Google Scholar]
- Mantel N. Chi-square tests with one degree of freedom; extensions of the Mantel-Haenszel procedure. J Am Statist Assn. 1963;58:690–700. [Google Scholar]
- Marmot MG, Smith G. Davey, Stansfield S, Patel C, North F, Head J, White I, Brunner E, Feeney A. Health inequalities among British civil servants: the Whitehall II study. Lancet. 1991;337:1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci. 2009 Jul 14; doi: 10.1073/pnas.0902971106. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nondahl DM, Cruickshanks KJ, Wiley TL, Tweed TS, Klein BEK, Klein R. Interexaminer reliability of otoscopic signs and tympanometric measures for older adults. J Am Acad Audiol. 1996;7(4):251–259. [PubMed] [Google Scholar]
- Nondahl DM, Shi X, Cruickshanks KJ, Dalton DS, Tweed TS, Wiley TL, Carmichael LL. Notched Audiograms and Noise Exposure History in Older Adults. Ear and Hearing. doi: 10.1097/AUD.0b013e3181b1d418. In Press. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popelka MM, Cruickshanks KJ, Wiley TL, Tweed TS, Klein BEK, Klein R. Low prevalence of hearing aid use among older adults with hearing loss: The Epidemiology of Hearing Loss Study. J Am Geriatr Soc. 1998;46:1075–1078. doi: 10.1111/j.1532-5415.1998.tb06643.x. [DOI] [PubMed] [Google Scholar]
- Popelka MM, Cruickshanks KJ, Wiley TL, Tweed TS, Klein BEK, Klein R. Moderate alcohol consumption and hearing loss; A protective effect. J Am Geriatr Soc. 2000;48(10):1273–1278. doi: 10.1111/j.1532-5415.2000.tb02601.x. [DOI] [PubMed] [Google Scholar]
- Power C, Atherton K, Manor O. Co-occurrence of risk factors for cardiovascular disease by social class: 1958 British birth cohort. J Epidemiol Community Health. 2008;62(12):1030–5. doi: 10.1136/jech.2007.068817. [DOI] [PubMed] [Google Scholar]
- Price K, Zhu X, Guimares PF, Vasilyeva ON, Frisina RD. Hormone replacement therapy diminishes hearing in peri-menopausal mice. Hearing Research. 2009;252:29–36. doi: 10.1016/j.heares.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam M, Campo P, Henderson D. The effect of exposure level on the development of progressive resistance to noise. Hearing Research. 1991;52:181–188. doi: 10.1016/0378-5955(91)90197-h. [DOI] [PubMed] [Google Scholar]
- Talbott E, Helmkamp J, Matthews K, Kuller L, Cottington E, Redmond G. Occupational Noise Exposure, Noise-Induced Hearing Loss and theEpidemiology of High Blood Pressure. Am J Epidemiol. 1985;121:501–504. doi: 10.1093/oxfordjournals.aje.a114028. [DOI] [PubMed] [Google Scholar]
- Talbott EO, Findlay RC, Kuller LH, Lenknew LA, Matthews KA, Day RD, Ishii EK. Noise-Induced Hearing Loss: A Possible Marker for High Blood Pressure in Older Noise-Exposed Populations. J Occup Med. 1990;32:690–697. [PubMed] [Google Scholar]
- Tanaka C, Chen GD, Hu BH, Chi LH, Li M, Zheng G, Bielefeld EC, Jamesdaniel S, Coling D, Henderson D. The effects of acoustic environment after traumatic noise exposure on hearing and outer hair cells. Hear Res. 2009 Apr;:10–8. doi: 10.1016/j.heares.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Torre P, Cruickshanks KJ, Klein BEK, Klein R, Nondahl DM. The association between cardiovascular disease and cochlear function in older adults. J Speech Lang Hear Res. 2005;48(2):473–81. doi: 10.1044/1092-4388(2005/032). [DOI] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force (USPSTF) Agency for Healthcare Research and Quality. Rockville, MD: [Accessed 12 Aug 2009]. Screening for Hearing Impairment in Older Adults, Topic Page. http://www.ahrq.gov/clinic/uspstf/uspshear.htm. [Google Scholar]
- Weinstein BE, Ventry IM. Hearing impairment and social isolation in the elderly. J Speech Hear Res. 1982;25:593–599. doi: 10.1044/jshr.2504.593. [DOI] [PubMed] [Google Scholar]
- Wiley TL, Chappell R, Carmichael L, Nondahl DM, Cruickshanks KJ. Changes in hearing thresholds over 10 years in older adults. J Am Acad Audiol. 2008;19:281–292. doi: 10.3766/jaaa.19.4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]