Abstract
Background
Phosphoinositide 3-kinase (PI 3-K) signaling plays a crucial role in neuronal growth and plasticity. Recently, we demonstrated that suicide brain is associated with decreased activation and expression of selective catalytic and regulatory subunits of PI 3-K. The present investigation examined the regulation and functional significance of compromised PI 3-K in suicide brain at the level of upstream phosphatase and tensin homolog on chromosome ten (PTEN) and downstream substrates 3-phosphoinositide-dependent kinase 1 (PDK1) and Akt.
Method
mRNA expression of Akt1, Akt3, PTEN, and PDK1 by competitive RT-PCR; protein expression of Akt1, Akt3, PTEN, PDK1, phosphorylated-Akt1 (Ser473), phosphorylated-Akt1(Thr308), phosphorylated-PDK1, and phosphorylated-PTEN by Western blot; and catalytic activities of Akt1, Akt3, and PDK1 by enzymatic assays were determined in prefrontal cortex (PFC) and hippocampus obtained from suicide subjects and nonpsychiatric controls.
Results
No significant changes in the expression of Akt1 or Akt3 were observed; however, catalytic activity of Akt1, but not of Akt3, was decreased in PFC and hippocampus of suicide subjects, which was associated with decreased phosphorylation of Akt1 at Ser473 and Thr308. The catalytic activity of PDK1 and the level of phosphorylated-PDK1 were also decreased in both brain areas without any change in expression levels of PDK1. On the other hand, mRNA and protein expression of PTEN was increased, whereas the level of phosphorylated-PTEN was decreased.
Conclusion
Our study demonstrates abnormalities in PI 3-K signaling at several levels in brain of suicide subjects and suggests the possible involvement of aberrant PI 3-K/Akt signaling in the pathogenic mechanisms of suicide.
Keywords: Akt, PDK1, PTEN, suicide, depression, prefrontal cortex, hippocampus
Introduction
Phosphoinositide 3 (PI 3)-kinase (PI 3-K)-mediated signaling is critical for many physiological functions in the brain, including cell survival, synaptic plasticity, protein synthesis, and membrane trafficking (1,2,3,4). Among various PI 3-Ks, the one that belongs to subclass IA, is activated by neurotrophins via receptors with intrinsic protein tyrosine kinase activity (5). Once activated, PI 3-K phosphorylates the 3′ position of membrane PI, phosphatidylinositol (4,5) biphosphate, to form PI(3,4,5)-tri phosphate (PIP3) (6). PIP3 is a critical signaling molecule that interacts with high affinity with several pleckstrin homology (PH) domain-containing proteins that further regulate downstream signaling cascade.
The functions of PI 3-K depend on the ability of PI 3-K to activate one of its most important downstream target Akt, which has emerged as a central node in the PI 3-K signaling (7,8,9). Mammalian genome contains three Akt genes, encoding Akt1, Akt2, and Akt3, located at chromosomes 14q32, 19q13, and 1q44, respectively (10,11,12). Akt1 and Akt3 are the predominant isoforms expressed in the brain, whereas Akt2 is predominantly expressed in skeletal muscle and embryonic brown fat (13). Activation of Akt depends on its recruitment to plasma membrane and subsequent phosphorylation at Thr308 and Ser473, located at catalytic domain and C-terminal regulatory domain, respectively (14). PI 3-K product PIP3 is crucial in recruiting Akt to the plasma membrane, where Akt is phosphorylated by 3,-phosphoinositide-dependent kinase 1 (PDK1), another substrate of PIP3. PDK1 is a Ser/Thr kinase that is targeted to the plasma membrane after interaction with PIP3 at the PH domain, where it phosphorylates Akt (15,16). PDK1 itself is regulated through transphosphorylation in the activation loop (17). The PI 3-K signaling is directly regulated by dual-function phosphatase, known as phosphatase and tensin homolog on chromosome ten (PTEN), which possesses both tyrosine phosphatase and lipid phosphatase activity (18). As lipid phosphatase, it regulates the intracellular level of PIP3 by dephosphorylating the D3 position of the inositol ring, thereby directly antagonizing signaling through the PI 3-K/Akt pathway (19). It has been shown that overexpression of PTEN is associated with decreased Akt activity (20). On the other hand, activity of PTEN is itself regulated through phosphorylation, which causes decreases in its activity (21,22).
Recently, we reported that catalytic activity and expression of specific regulatory and catalytic subunits of subclass IA PI 3-K are altered in PFC and hippocampus of suicide subjects (23). The functional significance of these abnormalities further downstream at the level of substrates is not clearly understood, however. Because functional response of PI 3-K depends on the activation of Akt, the present study was undertaken to examine whether activation of Akt was altered in brain of suicide subjects and whether this alteration was associated with changes in expression of specific Akt isoform(s). Moreover, we examined whether PDK1 and/or PTEN interplay any role in altered activation of Akt in suicide brain.
Methods and Materials
Subjects
The study was performed in the same PFC and hippocampus samples from suicide (n = 28) and nonpsychiatric control subjects (referred as normal controls, n = 21) in which PI 3-K studies were performed previously (23). Hippocampii were available only for 26 suicide subjects. Postmortem brain samples were obtained from Maryland Psychiatric Research Center, University of Maryland, Baltimore. Detailed dissection of tissues is described in our earlier publication (23). Toxicology screening for alcohol, a comprehensive battery for illicit drug use, and screening for antidepressant/psychoactive drugs taken prior to death were performed in blood/urine of each subject. Psychological autopsies were performed using the Diagnostic Evaluation After Death (24) and the Structured Clinical Interview for the DSM-IV (25), as described earlier (26). This study was approved by the Institutional Review Board of the University of Illinois at Chicago, and written informed consent was obtained from next-of-kin for each subject. Detailed demography of subjects is provided in Table 1.
Table 1.
Characteristics of subjects in the suicide and control groups
| Subject No. |
Age (y) |
Race | Sex | PMI (h) |
Brain pH |
Cause of death |
Drug toxicity (at the time of death) |
Psychiatric diagnosis |
|---|---|---|---|---|---|---|---|---|
| Suicide Group | ||||||||
| 1 | 22 | Black | Female | 16 | 5.3 | Drug overdose |
Propranolol | Major depression |
| 2 | 24 | White | Male | 7 | 5.6 | GSW | None | Major depression |
| 3 | 21 | White | Male | 17 | 6.1 | GSW | None | Major depression |
| 4 | 27 | White | Male | 24 | 6.4 | GSW | None | Major depression |
| 5 | 38 | White | Male | 24 | 6.3 | Drug overdose |
Diphenhydramine | Major depression |
| 6 | 36 | White | Female | 10 | 6.5 | GSW | Butalbital, diphenhydramine, acetaminophen |
Major depression |
| 7 | 41 | White | Female | 27 | 5.9 | Drug overdose |
Amitriptyline, desipramine, diphenhydramine, nortriptyline, pseudoephedrine, salicylate |
Major depression |
| 8 | 44 | White | Female | 11 | 5.6 | Drug overdose |
Nortriptyline | Major depression |
| 9 | 46 | White | Female | 16 | 6.1 | Drug overdose |
Nortriptyline | Major depression |
| 10 | 46 | White | Female | 21 | 5.3 | Drug overdose |
Amitriptyline, desipramine | Major depression |
| 11 | 53 | White | Male | 23 | 6.1 | Jumping | None | Major depression |
| 12 | 24 | White | Male | 22 | 6.5 | Hanging | None | Schizoaffective disorder |
| 13 | 40 | White | Male | 26 | 5.6 | GSW | Ethanol | Adjustment disorder |
| 14 | 68 | White | Female | 26 | 6.1 | GSW | None | Schizoaffective disorder |
| 15 | 37 | Black | Male | NA | 5.8 | CO intoxication |
CO | NA |
| 16 | 26 | Black | Male | NA | 6.5 | Hanging | Cocaine | NA |
| 17 | 50 | White | Male | 7 | 6.1 | GSW | None | No psychiatric illness |
| 18 | 24 | Black | Male | 22 | 6.6 | GSW | None | No psychiatric illness |
| 19 | 75 | White | Male | 18 | 6.7 | GSW | None | Adjustment disorder/conduct disorder |
| 20 | 36 | White | Female | 18 | 6.9 | GSW | None | Schizophrenia |
| 21 | 21 | White | Male | 22 | 6.5 | Hanging | None | Adjustment disorder |
| 22 | 41 | Black | Male | 12 | 6.3 | Multiple injuries |
None | No psychiatric illness |
| 23 | 87 | White | Male | 16 | 6.2 | GSW | None | Adjustment disorder |
| 24 | 43 | White | Male | 12 | 6.5 | Drug overdose |
Acetaminophen | Polysubstance abuse |
| 25 | 34 | White | Male | 16 | 6.2 | GSW | Ethanol | Alcohol abuse |
| 26 | 39 | White | Male | 30 | 6.5 | Asphyxia | Cocaine | Drug abuse |
| 27 | 30 | White | Male | 32 | 6.4 | Hanging | Cocaine, ethanol | Drug/alcohol abuse |
| 28 | 51 | White | Female | 28 | 6.7 | Drug overdose |
Amitriptyline | Bipolar disorder |
| Mean | 42.9 | 5 Black | 9 Female | 18.7 | 6.1 | |||
| SD | 13.9 | 23 White | 19 Male | 7.6 | 0.4 | |||
| Control Group | ||||||||
| 29 | 45 | White | Male | 22 | 6.5 | ASCVD | None | - |
| 30 | 22 | Black | Male | 19 | 6.2 | GSW | None | - |
| 31 | 83 | White | Male | 20 | 5.6 | ASCVD | None | - |
| 32 | 63 | White | Female | 30 | 5.7 | Ovarian cancer |
None | - |
| 33 | 31 | Black | Male | 8 | 5.6 | GSW | None | - |
| 34 | 35 | White | Male | 24 | 5.6 | Crash injury | None | - |
| 35 | 33 | White | Male | 15 | 6.0 | GSW | None | - |
| 36 | 37 | Black | Male | 5 | 6.6 | ASCVD | None | - |
| 37 | 37 | White | Male | 24 | 6.3 | ASCVD | None | - |
| 38 | 65 | Black | Female | 23 | 5.6 | ASCVD | None | - |
| 39 | 38 | Black | Male | 16 | 5.8 | Lung sarcoidosis |
None | - |
| 40 | 40 | White | Female | 7 | 6.5 | ASCVD | None | - |
| 41 | 23 | Black | Male | 15 | 6.7 | GSW | None | - |
| 42 | 42 | White | Female | 23 | 6.2 | Pneumonia | None | - |
| 43 | 46 | Black | Male | 9 | 6.2 | Auto accident | None | - |
| 44 | 48 | White | Male | 26 | 6.1 | ASCVD | None | - |
| 45 | 52 | White | Male | 30 | 6.3 | ASCVD | None | - |
| 46 | 37 | Black | Male | 9.5 | 6.1 | ASCVD | None | - |
| 47 | 43 | White | Male | 17.5 | 5.7 | NA | None | - |
| 48 | 41 | White | Male | 24 | 6.2 | NA | None | - |
| 49 | 41 | Black | Male | 27 | 5.8 | Liver cirrhosis | None | - |
| Mean | 40.1 | 17 White | 4 Female | 19.3 | 6.2 | |||
| SD | 16.2 | 9 Black | 17 Male | 6.9 | 0.4 | |||
Age: t47 = 0.63, P = .53; PMI: t45 = 0.29, P = .77; brain Ph: t47 = 1.00, P = .32
ASCVD, atherosclerotic cardiovascular disease; CO, carbon monoxide; GSW, gunshot wound; NA, not available
mRNA Quantitation
The quality of RNA was rigorously characterized as described earlier (26) and only samples with an RNA integrity number >7 were used. mRNA levels of Akt1, Akt3, PTEN, and PDK1 were determined by competitive RT-PCR (26,27). Cyclophilin was used as a housekeeping gene. The sequences of external and internal primers are given in Table S1 (see Supplement 1). Decreasing concentrations of internal standard cRNAs (Ak1 and PDK1: 12-.75; Akt3: 50-3.12; PTEN: 75-4.7 pg) were added to 1 µg of total RNA. The PCR mixture was amplified for 25 cycles and the aliquots were run on 1.5% agarose gel. The results are expressed as attomoles of mRNA/ µg of total RNA.
Competitive RT-PCR for Akt1, Akt3, PDK1, and PTEN mRNA levels revealed amplification product arising from the mRNA templates at 321, 362, 306, and 325 bp, respectively, and the corresponding digestion products from the complementary RNA at 211, 249, 199, and 210 bp, respectively (see Figure S1 A, B C, D in Supplement 1). The points of equivalence represent the absolute amounts of Ak1, Akt3, PDK1, and PTEN mRNA present (see Figure S1 E, F, G, H in Supplement 1).
Initially, we determined mRNA levels of all Akt isoforms (Akt1, Akt2, Akt3) in PFC and hippocampus. We found that Akt1 and Akt3 were highly expressed in both PFC and hippocampus, however, the expression of Akt2 was very negligible in these brain areas. Therefore, in subsequent studies, we examined only the expression of Akt1 and Akt3.
Akt1 and Akt3 Assay
Tissue lysate preparation and immunoprecipitaions were performed as described earlier (26). Tissue lysates (500 µg protein) were immunoprecipitated with antibodies directed against Akt-1 or Akt3 (Upstate Biotechnology), pre-coupled to protein G-agarose. The immunoprecipitates were washed and subjected to in vitro kinase assays. The incorporation of 32P into Akt-specific substrate peptide RPRAATF (Upstate Biotechnology) were measured during a 10-minute incubation at 30°C in the presence of 10 µM protein kinase A inhibitor peptide. The phosphorylated substrate was separated from the residual [γ-32P]ATP with P81 phosphocellulose and the incorporated radioactivity was quantified. Endogenous phosphorylation and nonspecific immunoprecipitation were determined by substituting buffer for substrate cocktail and nonspecific sheep polyclonal IgG for Akt antibody, respectively, and were subtracted. The mean (SD) values of the 100% control were as follows: Akt1, 105,200 (9,230); and Akt3, 85,900 (11,400) dpm.
PDK1 Assay
PDK1 activity was determined using an assay kit (Upstate Biotechnology). Tissue lysates (500 µg), prepared for Akt assay, were used for immunoprecipitation using PDK1 antibody. An in-vitro kinase assay was performed in assay dilution buffer (500 mM Tris-HCl [pH 7.5], 1 mM EGTA, 1 mM EDTA, 1% 2-mercaptoethanol, 25 µM PKA inhibitor peptide, 10 µM microcystin, 100 mM magnesium acetate, and 1 mM ATP) in the presence of unactive SGK1, AKT/SGK substrate peptide, and [γ-32P]ATP (1 µCi/µl) during a 10-minute incubation at 30°C. The phosphorylated substrate was separated as described above for Akt assay. Endogenous phosphorylation was determined by substituting assay dilution buffer for the unactivated SGK substrate and was subtracted. The mean (SD) value of the 100% control was 121,500 (14,470) dpm.
Western Blot
Western blots were performed in tissue lysates prepared for Akt and PDK1 activity assays. Equal amount of protein (25 µg) was resolved onto 10% (wt/vol) SDS-polyacrylamide gel and blotted on an enhanced chemiluminescence membrane. Membranes were incubated with monoclonal antibodies for Akt1, Akt3, PTEN (Cell Signaling), PDK1 (Santa Cruz), or polyclonal phosphorylated-Akt1 (Thr308), phosphorylated-PTEN (Ser380) (Cell Signaling), phosphorylated-PDK1 (Ser241) (Santa Cruz Biotechnology), or phosphorylated-Akt1 (Ser473) (AbCam) overnight at 4°C, followed by appropriate horseradish-peroxidase-linked secondary antibody for 5 to7 hours at room temperature. Membranes were stripped and re-probed with β-actin antibody (Sigma). The optical density (OD) of the bands we quantified as described earlier (26), and the OD of each band was corrected by the OD of the corresponding β-actin band. The specificity of each antibody was determined using blocking peptide and parallel examination of positive cells (NIH/3T3, HeLa, U-937, MDA-MB-435) or recombinant proteins. In addition, to test the specificity of p-Akt1 antibodies, we expressed the tagged human Akt1 isoform in recombinant cells. All the antibodies were found to be specific.
Statistical Analysis
Data analyses were performed using SPSS (SPSS Inc, Chicago, IL). The differences in various measures, demography variables, and the effects of gender, race, and antidepressant toxicology were analyzed using the independent-sample t test. P values were 2-tailed. In measures where SD was significantly different between normal control and suicide groups, we also applied nonparametric Mann-Whitney test. We found that the differences in these measures were still significant between the two groups in which we had found significant differences using independent-sample t test. The differences between normal controls, depressed suicide subjects, and non-depressed suicide subjects were evaluated by one-way ANOVA followed by post-hoc (Tukey’s) comparisons. Statistical significance was assumed at p <.05. Pearson product-moment analysis was applied for all correlational analyses.
Results
AKT Studies
Akt1 and Akt3 catalytic activities
The catalytic activity of Ak1 was significantly decreased in PFC (t47 = 9.49, p <.001) and hippocampus (t45 = 6.23, p <.001) of suicide subjects, without any change in the catalytic activity of Akt3 (PFC: t47 = 1.66, p = .10; hippocampus: t45 = .42, p = .68) (Fig. 1).
Figure 1.
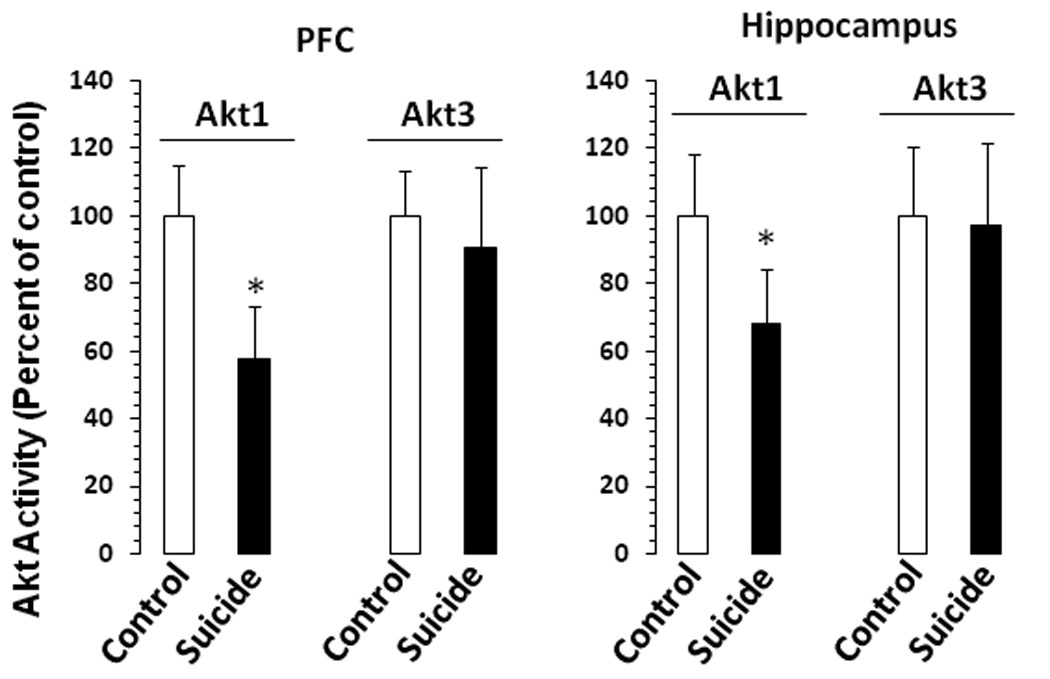
The catalytic activities of Akt1 and Akt3 in PFC and hippocampus of suicide subjects and normal controls. Akt1 and Akt3 catalytic activities were determined after immunoprecipitating Akt1 and Akt3 using specific antibodies In vitro kinase assays were performed in assay dilution buffer by measuring the incorporation of 32P into Akt-specific substrate peptide RPRAATF (Upstate Biotechnology) during a 10-minute incubation at 30°C in the presence of 10 µM protein kinase A inhibitor peptide. The phosphorylated substrate was separated from the residual [γ-32P]ATP with P81 phosphocellulose and the incorporated radioactivity was quantified. Results are presented as percent of control. PFC samples were from 21 normal controls and 28 suicide subjects, and hippocampus samples were from 21 normal controls and 21 suicide subjects. The suicide group was compared with the control group (*P<.001).
mRNA and protein levels of Akt1 and Akt3
Because we observed a decrease in the catalytic activity of Akt1, we examined whether this decrease was due to a decrease in its expression. Comparison analysis showed that mRNA levels of neither Akt1 nor Akt3 were different in PFC (Akt1: t47 = 1.58, p = .12; Akt3: t47 = .31, p = .78) (Fig. 2A) or hippocampus (Akt1: t45 = .81, p = .42; Akt3: t45 = 1.53, p = .13) (Fig. 2B) between normal controls and suicide subjects. When calculated as ratios to housekeeping gene cyclophilin, similar results were noted.
Figure 2.

mRNA levels of Akt1 and Akt3 1 in the PFC (A) and hippocampus (B) of suicide subjects and normal controls. Western blots showing the immunolabeling of total Akt1, total Akt3, phospho Akt1 (Thr308), and phosphor-Akt3 (Ser473) in the PFC of 3 normal controls and 3 suicide subjects (C). Protein samples from tissue lysates were subjected to 10% polyacrylamide gel electrophoresis and transferred to enhanced chemiluminescence–nitrocellulose membranes, which were then incubated with primary antibody specific for total Akt1, total Akt3, phospho Akt1 (Thr308), phosphor-Akt3 (Ser473), or β-actin and corresponding secondary antibody. The bands were quantified as described in the “Materials and Methods” section. Ratios of the optical densities of Akts to that of β-actin were calculated. The mean ± SD of immunolabeling of total Akt1, total Akt3, phospho Akt1 (Thr308), and phosphor-Akt3 (Ser473) immunolabeling in PFC and hippocampus from normal controls and suicide subjects are given in D and E, respectively. The PFC samples were from 21 normal controls and 28 suicide subjects, and the hippocampus samples were from 21 normal controls and 21 suicide subjects. The suicide group was compared with the control group (*P<.001).
Protein levels of p-Akt1 (Ser473) and p-Akt3 (Thr308)
Representative Western blots showing immunolabeling of Akt1 and Akt3 in the PFC of normal controls and suicide subjects are given in Fig. 2C. Similar to mRNA results, no significant changes were noted in protein levels of Akt1 or Akt3 in the PFC (Akt1: t47 = .92, p = .36; Akt3: t47 = .55, p = .58, Fig. 2D) or hippocampus (Akt1: t45 = .59, p = .56; Akt3: t45 = .83, p = .41, Fig. 2E) of suicide subjects.
Because we observed a decrease in the catalytic activity of selective Akt1 and phosphorylation of Akt at Ser473 and Thr308 is essential for Akt1 catalytic activation, we examined the level of p-Akt1 using antibodies that recognize phosphorylation of Akt1 at Ser473 and Thr308. Representative Western blots showing Akt1 phosphorylation are given in Fig. 2C, and comparison results are provided in Figs. 2D and E, respectively. We observed that the levels of both p-Ser473Akt1 and p-Thr308Akt1 were significantly decreased in PFC (p-Akt1 Ser473: t47 = 9.62, p <.001; p-Akt1Thr308: t47 = 8.46, p <.001) and hippocampus (p-Akt1 Ser473: t45 = 6.88, p <.001; p-Akt1Thr308: t45 = 6.48, p <.001) of suicide subjects. Moreover, we observed significant correlations between Akt1 activity and levels of p-Ser473Akt1 (suicide: PFC, r = .60, p = .004; hippocampus, r = .47, p = .03; controls: PFC, r = .59, p <.001; hippocampus, r = .38, p = .009) and p-Thr308Akt1 (suicide: PFC, r = .47, p = .03; hippocampus, r = .56, p = .009; controls: PFC, r = .70, p<.001; hippocampus, r = .43, p = .002) in suicide and control groups.
PDK1 Studies
Catalytic activity of PDK1
Catalytic activity of PDK1 was determined in the same lysate in which Akt1 activity was assayed. The catalytic activity of PDK1 was significantly decreased in PFC (t47 = 7.96, p <.001) and hippocampus (t45 = 5.44, p <.001) of suicide subjects (Fig 3A). Further analysis revealed that catalytic activity of PDK1 was significantly correlated with Akt1 activity (suicide: PFC, r = 0.77, p = .005; hippocampus, r = 0.98, p <.001; controls: PFC, r = .33, p = .05; hippocampus, r = 0.39, p = .006).
Figure 3.
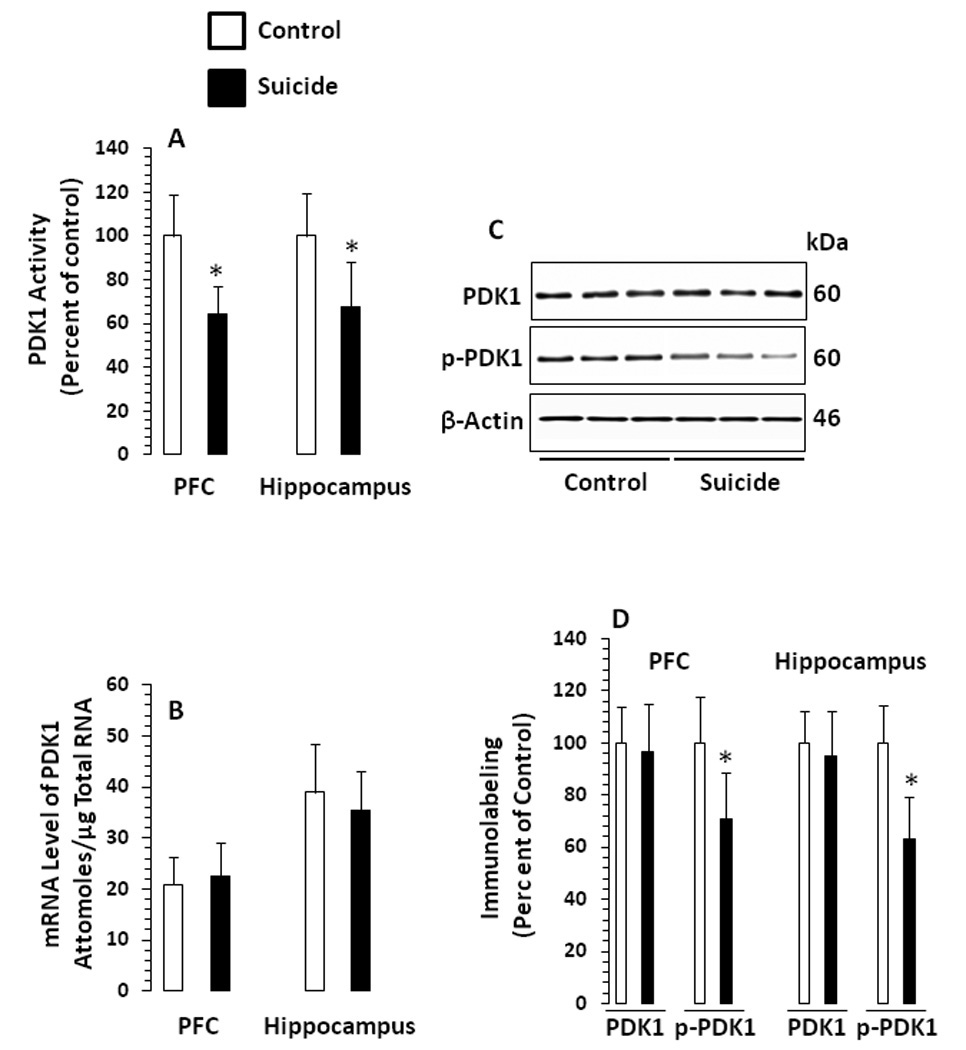
A. Catalytic activity of PDK1 in PFC and hippocampus of suicide subjects and normal controls An in vitro kinase assay was performed in the presence of unactive SGK1, AKT/SGK substrate peptide (Upstate Biotechnology) and [γ-32P]ATP (1µCi/µl) during a 10-minute incubation at 30°C. The phosphorylated substrate was separated from the residual [γ-32P]ATP with P81 phosphocellulose, and the incorporated radioactivity was quantified. B. mRNA levels of PDK1 in PFC and hippocampus of suicide subjects and normal controls. C. Western blots showing the immunolabeling of total PDK1 and phospho PDK1 in the PFC of 3 normal controls and 3 suicide subjects. Protein samples from tissue lysates were subjected to 10% polyacrylamide gel electrophoresis and transferred to enhanced chemiluminescence–nitrocellulose membranes, which were then incubated with primary antibody specific for total PDK1, phospho PDK1or β-actin and corresponding secondary antibody. The bands were quantified as described in the “Materials and Methods” section. Ratios of the optical densities of total PDK1 and phospho PDK1 to that of β-actin were calculated. D. The mean ± SD of immunolabeling of total PDK1 and phospho PDK1 immunolabeling in PFC and hippocampus from normal controls and suicide subjects. PFC samples were from 21 normal controls and 28 suicide subjects, and hippocampus samples were from 21 normal controls and 21 suicide subjects. The suicide group was compared with the control group (*P<.001).
mRNA level of PDK1
No significant changes in mRNA levels of PDK1 were observed in PFC (t47 = .91, p = .37) or hippocampus (t45 = 1.38, p = .18) of suicide subjects (Fig. 3B). Similar results were noted when the PDK1 mRNA level was calculated as a ratio to cyclophilin.
Protein levels of total and phosphorylated PDK1
Representative Western blots showing immunolabeling of PDK1 in PFC of normal controls and suicide subjects are given in Fig. 3C. As with mRNA level, the protein level of total PDK1 was not altered either in PFC (t47 = .74, p = .46) or hippocampus (t45 = 1.16, p = .25), however, phosphorylation of PDK1 at Ser241 was significantly decreased in these brain areas of suicide subjects (PFC: t47 = 5.71, p <.001; hippocampus: t47 = 8.17, p <.001 (Fig. 3D).
PTEN Studies
mRNA level of PTEN
Because overexpression of PTEN is associated with decreased Akt activity, we examined the expression of PTEN. A significantly increased mRNA level of PTEN was noted in PFC and hippocmpus of suicide subjects (PFC: t47 = 11.90, p <.001; hippocampus: t45 = 8.98, p <.001) (Fig. 4A). Data calculated as a ratio to cyclophilin reveled similar results.
Figure 4.

A. mRNA levels of PTEN in the PFC and hippocampus of suicide subjects and normal controls. B. Western blots showing the immunolabeling of PTEN in the PFC of 3 normal controls and 3 suicide subjects. Protein samples from tissue lysates were subjected to 10% polyacrylamide gel electrophoresis and transferred to enhanced chemiluminescence–nitrocellulose membranes, which were then incubated with primary antibody specific for PTEN or β-actin and corresponding secondary antibody. C. The mean ± SD of immunolabeling of total PTEN in the PFC and hippocampus from normal controls and suicide subjects is given. D. The mean ± SD of ratio of p-PTEN to total PTEN. PFC samples were from 21 normal controls and 28 suicide subjects, and hippocampus samples were from 21 normal controls and 21 suicide subjects. *P<.001.
Protein Levels of total and phosphorylated PTEN
Representative Western blots showing immunolabeling of PDK1 are given in Fig. 4B. As shown in Fig. 4C, the protein level of total PTEN was significantly increased in PFC (t47 = 5.57, p <.001) and hippocampus (t45 = 5.96, p <.001) of suicide subjects (Fig. 4C). However, when the level of Ser380 phopshorylated PTEN was examined as a ratio to the total PTEN, we found that the level of p-PTEN was significantly lower in both PFC (t47 = 37.2, p <.001) and hippocampus (t45 = 42.1, p <.001) of suicide subjects (Fig. 4D).
Effects of Confounding Variables
No significant effects of age, PMI, brain pH (Table S3 in Supplement 1), gender or race (Table S4 in Supplement 1) were observed on any of the measures in which we found significant differences between normal controls and suicide subjects, except that PTEN mRNA was negatively correlated with PMI and brain pH and level of p-Akt1 (Ser473) was greater in females than males in hippocampus (Table S4 in Supplement 1). The level of p-PTEN was slightly but significantly lower in hippocampus of Black subjects.
Effect of Major Depression
To examine whether the changes in various measures in suicide subjects were related to depression, we divided the suicide subjects into those who had major depression and those who had other psychiatric disorders or no mental illnesses. Of the 28 suicide subjects, 11 had major depression, whereas in the suicide group with other psychiatric disorders (n=17), there were 5 with adjustment disorder, 2 with schizoaffective disorder, 1 with bipolar disorder, 1 with schizophrenia, 1 with polysubstance abuse, and 3 with drug/alcohol abuse; there was no diagnosed psychiatric illness in 2 subjects, and the diagnosis was not available in 2 subjects. We found that none of the variables was significantly different between suicide subjects with major depression and suicide subjects with other psychiatric disorders in both the PFC (Table 2) and the hippocampus (Table S2 in Supplement 1).
Table 2.
Effect of major depression (MDD) on various measures in the PFC of subjects in the suicide groupa
| Variables | Normal controls (n=21) 1 |
Subjects in the suicide group (n=28) |
Overall ANOVA |
P value for Multiple comparison by groupb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| With a history of MDD (n=11) 2 |
With a history of other psychiatric disorders (n=17) 3 |
||||||||||||
| Mean | SD | Mean | SD | Mean | SD | df | F | P | 1 vs 2 | 1 vs 3 | 2 vs 3 | ||
| Akt1 studies | |||||||||||||
| Akt1 catalytic activityb Immunolabelingb |
100 | 15 | 55 | 16 | 59 | 15 | 2,46 | 44.78 | <.001 | <.001 | <.001 | .49 | |
| p-Akt1 (Thr308) | 100 | 11 | 59 | 19 | 65 | 17 | 2,46 | 36.89 | <.001 | <.001 | <.001 | .23 | |
| p-Akt1 (Ser473) | 100 | 13 | 63 | 18 | 59 | 13 | 2,46 | 46.29 | <.001 | <.001 | <.001 | .41 | |
| PDK1 studies | |||||||||||||
| Catalytic activityb | 100 | 19 | 68 | 10 | 62 | 14 | 2,46 | 32.32 | <.001 | <.001 | <.001 | .29 | |
| p-PDK1 immunolabelingb | 100 | 17 | 73 | 24 | 69 | 13 | 2,46 | 16.19 | <.001 | <.001 | <.001 | .58 | |
| PTEN studies | |||||||||||||
| mRNAc | 495 | 82 | 932 | 131 | 958 | 177 | 2,46 | 69.85 | <.001 | <.001 | <.001 | .61 | |
| Total PTEN immunolabelingb | 100 | 19 | 145 | 26 | 139 | 33 | 2,46 | 15.33 | <.001 | <.001 | <.001 | .60 | |
| p-PTENd | |||||||||||||
| 1.02 | .22 | .72 | .12 | .71 | .21 | 2,46 | 14.53 | <.001 | <.001 | <.001 | .89 | ||
Data were analyzed using univariate analysis of variance (ANOVA). Bonferroni-adjusted P values were considered significant (P = .05/7 = .007).
Group 1 indicates normal controls; group 2, subjects in the suicide group with a history of MDD; and group 3, subjects in the suicide group with a history of other psychiatric disorders. bPercent of control;
attomoles/µg total RNA;
ratio to total PTEN
Effect of Antidepressant Toxicology
We did not find significant differences in any of the measures between suicide subjects who tested positive for antidepressants at the time of death (n= 5) and those who did not (n= 23) (Table S5 in Supplement 1).
Discussion
Recently, we demonstrated that the expression and activation of PI 3-K, a key component of PI 3-K signaling, are compromised in brains of suicide subjects (26). The present study further investigates whether abnormalities in PI 3-K signaling occur at the levels of downstream substrate and/or upstream regulatory molecules.
Akt is one of the most important substrates of PI 3-K. It has been shown that the PH domain of Akt mediates lipid-protein and protein-protein interactions and directly interacts with PIP3 to recruit Akt at the plasma membrane. This brings Akt to close proximity to regulatory PI 3-K, which phosphorylates and activates Akt (33). When expression levels of two abundantly expressed isoforms of Akt (i.e., Akt1 and Akt3) were examined, no significant changes were observed in PFC and hippocampus of suicide subjects. Interestingly, when isoform-specific catalytic activities of Akts were determined, we found that the activity of only Akt1 was decreased, without any change in the activity of Akt3. These results explicitly implicate selective Akt1 in suicide. Our finding are supported by previous studies showing similar results in brains of suicide subjects (34,35), although, these studies do not specify changes in the selective isoform of Akt.
We next examined the mechanisms of Akt1 activation in suicide brain. The major mechanism of Akt1 activation is through its phosphorylation at Thr308 and Ser473 residues located in the C-terminal regulatory domain and activation loop, respectively. Akt1 can be partially activated through phosphorylation of Ser308 alone (36); however, phosphorylation of both Ser473 and Thr308 residues is necessary for its full activation (37,38). Interestingly, when the Thr308 residue in the activation loop of Akt is unphosphorylated, it negatively regulates the activity of Akt. When examined, we found that phopshorylation of Akt1 at both Thr308 and Ser473 was significantly reduced in PFC and hippocampus of suicide subjects, suggesting that decreased activation of Akt1 in suicide brain may be associated with reduced phosphorylation of Akt1 at Ser473 and Thr308 residues.
To further examine the regulation of Akt1 activation, we studied PDK1, an upstream regulatory kinase, whose PH domain binds to the PI 3-K product PIP3 with high affinity (16). Binding of PIP3 targets PDK1 to the plasma membrane, where it phosphorylates Akt at the Thr308 residue (39). It has been shown that overexpression of PDK1 is sufficient to activate Akt; however, for maximal activity, the presence of PIP3 is essential (15). Conversely, PDK1 itself is activated by phosphorylation at the Ser241 residue in the activation loop (40). When expression of PDK-1 was examined, we did not find any significant change in mRNA level of PDK1 in PFC or hippocampus of suicide subjects. However, using antibodies that detect total or phosphorylated PDK1 (Ser241), we found less expression of phosphorylated PDK1 in both brain areas of suicide subjects, without any change in the expression of total PDK1. This was accompanied by reduction in catalytic activity of PDK1. Given that PDK1 specifically phosphorylates Akt1 at the Thr308 residue, and that Thr308 phosphorylation is lower in suicide brain, there is a strong possibility that decreased phosphorylation of Thr308 is due to diminished phosphorylation and activation of PDK1. In the present study, we carefully matched the subjects based on PMI and correlated each measure with this variables. We did not find any significant correlations, suggesting that the observed results were not influenced by this variable. Nonetheless, one should be cautions in interpreting the phopshorylation data in postmortem brain, as there are studies suggesting that phopshorylation of certain proteins may decrease within the few hours of death.
To examine whether 3’phosphorylated phospholipids play any role in less activation of Akt, we studied the status of PTEN, a lipid phosphatase that converts PIP3 into phosphatidylinositol (4,5) biphosphate, thus antagonizing the functions of PI 3-K (41,42,43). PTEN is widely expressed in the brain and preferentially expressed in neurons (44). It has been shown that overexpression of wild-type PTEN decreases Akt activity, whereas dominant-negative PTEN increases Akt activity in neurons (45). On the other hand, phosphorylation of PTEN prevents its interaction with PIP3, thus inactivating its activity (21,46). In the present study, we found that expression of PTEN was significantly increased in PFC and hippocampus of suicide subjects, which suggests that there is an added mechanism of regulation of PI 3-K/Akt through PTEN in brain of suicide subjects. An increased level of PTEN may result in less availability of PIP3 and, thus, less recruitment of Akt1 and PDK1 to the plasma membrane. Because Akt1 is active only when it is in close proximity to PDK1, a decrease in the PIP3 pool via PTEN may hinder the facilitation of Akt activation. Interestingly, we observed that the level of p-PTEN, when calculated as a ratio to total PTEN, was lower in brains of suicide subjects. This suggests that PTEN may be less active in suicide brain, which may have occurred as a compensatory response to increased expression of PTEN. Nevertheless, our findings of increased expression of PTEN has relevance to less activation of Akt in suicide brain, as expression of PTEN is directly correlated with increased PTEN activity, and increased expression of PTEN causes decrease in PIP3 pool. Our results thus suggest that PTEN may be playing an important role in regulating Akt1 activity in suicide brain. Our findings are supported by a recent study showing similar changes in the level of total PTEN in brain of suicide subjects (34).
The reason as well as significance of reduced activation of selective Akt1 in suicide brain is not clear at the present time, although both Akt1 and Akt3 share a high degree of amino acid identity and substrate specificity. Interestingly, there are some marked differences regarding the involvement of Akt isoforms in behavioral phenotypes and physiological functions. Akt1-deficient mice show reduced working memory performance (47), whereas Akt3-deficient mice exhibit reduced brain size (48). On the other hand, there is a lack of functional compensation between these two Akt isoforms in Akt-deficient mice, which suggests that not all Akt isoforms perform equally. In addition, recent studies suggest that functions of Akt isoforms are fine tuned by Akt-interacting proteins such as inosine monophosphatase, histone H3 methyltransferase, leucine-rich repeat protein phosphatase, c-Jun N-terminal kinase (JNK), JNK interacting proteins, RasGap, and Ca2+-calmodulin kinase (49). Further studies will be required to examine how these interacting proteins delineate specific functions elucidated by each Akt isoform.
Regardless of the specificity, PI 3-K/Akt signaling is recognized as one of the most critical pathways for many physiological functions in the brain. For example, PI 3-K/Akt plays a crucial role in cell survival mediated by neurotrophins and GSK-3β (50,51). Similarly, Akt-mediated phosphorylation of CREB and NF-κB is critical in inducing expression of survival genes such as Bcl-xL, IAPs, and Bcl-2, whereas, Akt-mediated phosphorylation of Forkhead family of transcription factors diminishes death promoting signals (9). In addition, Akt blocks the functioning of p75 neurotrophin receptor, which activates pro-apoptotic JNK/p53/Bad pathway (52). Moreover, components of the PI 3-K/Akt pathway are also involved in a variety of other neuronal functions. For example, TrkB-mediated axonal growth and spatial memory formation and BDNF-mediated dendritic remodeling occur via PI 3-K/Akt activation (53,54). PI 3-K/Akt signaling also regulates neuronal polarity and appropriate integration of neurons into functional circuits (55). In addition, translational machinery is regulated via Akt-mediated phosphorylation of p706K (56). Important roles for PTEN have also been ascribed such that PTEN controls neuronal size (57) and knockdown of PTEN induces dendritic growth (58). It is pertinent to mention that suicide brains exhibit reduced availability of neurotrophins (28,32,59), TrkB (28,60), CREB (61,62) and GSK-3β (63), and show induction of p75 neurotrophin receptor (26). Given that abnormalities occur in structure (64,65,66,67,68,69,70) and neural plasticity (71) in suicide brains, our findings of reduced activation of PI 3-K, PDK1, and Akt1 and increased expression of PTEN, are quite relevant and suggest that these abnormalities may be critical in aberrant structure and functioning of suicide brains. Interestingly, it has recently been demonstrated that stress-induced downregulation of Akt in the ventral tegamental area cause vulnerability to develop depression in mice and rats (72) and that Akt and PDK1 activities are regulated by antidepressants, mood-stabilizing agents (73), and electroconvulsive shock (74). These studies further substantiate the role of PI 3-k/Akt signaling in depression/suicide.
Some studies demonstrate that Akt is a candidate gene for schizophrenia (reviewed in 75) and a recent study shows that Akt activity is decreased in postmortem brain of both depressed suicide and non-suicide depressed subjects (35). In the present study, we observed that changes in Akt and other measures were not related to depression but were present in all suicide subjects. Since we used Axis-I life time diagnosis for depression, whether the changes in PI 3-K/Akt occur in context of depression need to be further confirmed.
Overall, our present study demonstrates reduced activation and expression of Akt1 in suicide brain, which appears to be regulated by PDK1 and PTEN. Although, it is difficult to correlate the functional assays with a specific PI 3-K subclass, however, given that activity of PDK1 is dependent on the availability of PIP3 generated in response to PI 3-K and catabolized through activation of PTEN, and that abnormalities occur in PI 3-K activation in suicide brain (23), our present study indicates the possibility that functionality of PI 3-K signaling is decreased in suicide brain. These abnormalities in PI 3-K signaling may be crucial in the pathophysiologic mechanisms of suicide.
Supplementary Material
Acknowledgements
This work was supported by the NIMH (R0MH168777, R21MH081099, R01MH082802), NARSAD, and the American Foundation for Suicide Prevention grants to Dr. Y. Dwivedi; NIMH grant RO1MH48153 to Dr. G. N. Pandey; and MH60744 and MH66123 grants to Dr. R. Roberts. We thank Miljana Petkovic, Barbara Brown, and Joy K. Roche for their help in organizing the brain tissue. We also thank the members of the Maryland Brain Collection for their efforts, particularly in family interviews and dissection. We are grateful for the cooperation of the Office of the Chief Medical Examiner in Baltimore, Maryland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Wymann MP, Zvelebil M, Laffargue M. Phosphoinositide 3-kinase signaling - which way to target? Trends Pharmacol Sci. 2003;24:366–376. doi: 10.1016/S0165-6147(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 2.Downes CP, Gray A, Lucocq JM. Probing phosphoinositide functions in signaling and membrane trafficking. Trends Cell Biol. 2005;15:259–268. doi: 10.1016/j.tcb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinase as regulators of growth and metabolism. Nature. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 5.Segal RA. Selectivity in neurotrophin signaling: theme and variations. Annu Ev Neurosci. 2003;26:299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- 6.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 7.Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 9.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murthy SS, Tosolini A, Taguchi T, Testa JR. Mapping of AKT3, encoding a member of the Akt/protein kinase B family, to human and rodent chromosomes by fluorescence in situ hybridization. Cytogenet Cell Genet. 2000;88:38–40. doi: 10.1159/000015481. [DOI] [PubMed] [Google Scholar]
- 11.Cheng JQ, Godwin AK, Bellacosa A, Taguchi T, Franke TF, Hamilton TC, et al. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci USA. 1992;89:9267–9271. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staal SP, Huebner K, Croce CM, Parsa NZ, Testa JR. The AKT1 proto-oncogene maps to human chromosome 14, band q32. Genomics. 1988;2:96–98. doi: 10.1016/0888-7543(88)90114-0. [DOI] [PubMed] [Google Scholar]
- 13.Franke TF. PI3K/Akt: getting it right matters. Oncogene. 2008;27:6473–6488. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- 14.Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 15.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 16.Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- 17.Wick MJ, Ramos FJ, Chen H, Quon MJ, Dong LQ, Liu F. Mouse 3-phosphoinositide-dependent protein kinase-1 undergoes dimerization and trans-phosphorylation in the activation loop. J Biol Chem. 2003;278:42913–42919. doi: 10.1074/jbc.M304172200. [DOI] [PubMed] [Google Scholar]
- 18.Yin Y, Shen WH. PTEN: a new guardian of the genome. Oncogene. 2008;27:5443–5453. doi: 10.1038/onc.2008.241. [DOI] [PubMed] [Google Scholar]
- 19.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. PNAS. 1998;95:15587–15591. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vazquez F, Sellers WR. The PTEN tumor suppressor protein: an antagonist of phosphoinositide 3-kinase signaling. Biochim Biophys Acta. 2000;1470:M21–M35. doi: 10.1016/s0304-419x(99)00032-3. [DOI] [PubMed] [Google Scholar]
- 22.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 23.Dwivedi Y, Rizavi HS, Teppen T, Zhang H, Mondal A, Roberts RC, et al. Lower phosphoinositide 3-kinase (PI 3-kinase) activity and differential expression levels of selective catalytic and regulatory PI 3-kinase subunit isoforms in prefrontal cortex and hippocampus of suicide subjects. Neuropsychopharmacology. 2008;33:2324–2340. doi: 10.1038/sj.npp.1301641. [DOI] [PubMed] [Google Scholar]
- 24.Salzman S, Endicott J, Clayton P, Winokur G. Diagnostic Evaluation After Death (DEAD) Rockville, MD: National Institute of Mental Health, Neuroscience Research Branch; 1983. Rockville, MD: [Google Scholar]
- 25.Spitzer RL, Williams JBW, Gibbon M, First MD. Structural Clinical Interview for DSM-IV (SCID) New York, NY: New York State Psychiatric Institute Biometrics Research; 1995. [Google Scholar]
- 26.Dwivedi Y, Rizavi HS, Zhang H, Mondal AC, Roberts RC, Conley RR, et al. Neurotrophin receptor activation and expression in human postmortem brain: effect of suicide. Biol Psychiatry. 2009;65:319–328. doi: 10.1016/j.biopsych.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. mRNA and protein expression of selective alpha subunits of G proteins are abnormal in prefrontal cortex of suicide victims. Neuropsychopharmacology. 2002;27:499–517. doi: 10.1016/S0893-133X(02)00335-4. [DOI] [PubMed] [Google Scholar]
- 28.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 29.Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res. 2005;136:29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 31.Kozicz T, Tilburg-Ouwens D, Faludi G, Palkovits M, Roubos E. Gender-related urocortin 1 and brain-derived neurotrophic factor expression in the adult human midbrain of suicide victims with major depression. Neuroscience. 2008;152:1015–1023. doi: 10.1016/j.neuroscience.2007.12.050. [DOI] [PubMed] [Google Scholar]
- 32.Pandey GN, Ren X, Rizavi HS, Conley RR, Roberts RC, Dwivedi Y. Brain-derived neurotrophic factor and tyrosine kinase B receptor signalling in post-mortem brain of teenage suicide victims. Int J Neuropsychopharmacol. 2008;11:1047–1061. doi: 10.1017/S1461145708009000. [DOI] [PubMed] [Google Scholar]
- 33.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 34.Hsiung SC, Adlersberg M, Arango V, Mann JJ, Tamir H, Liu KP. Attenuated 5-HT1A receptor signaling in brains of suicide victims: involvement of adenylyl cyclase, phosphatidylinositol 3-kinase, Akt and mitogen-activated protein kinase. J Neurochem. 2003;87:182–194. doi: 10.1046/j.1471-4159.2003.01987.x. [DOI] [PubMed] [Google Scholar]
- 35.Karege F, Perroud N, Burkhardt S, Schwald M, Ballmann E, La Harpe R, et al. Alteration in kinase activity but not in protein levels of protein kinase B and glycogen synthase kinase-3beta in ventral prefrontal cortex of depressed suicide victims. Biol Psychiatry. 2007;61:240–245. doi: 10.1016/j.biopsych.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 36.Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- 37.Andjelković M, Maira SM, Cron P, Parker PJ, Hemmings BA. Domain swapping used to investigate the mechanism of protein kinase B regulation by 3-phosphoinositide-dependent protein kinase 1 and Ser473 kinase. Mol Cell Biol. 1999;19:5061–5072. doi: 10.1128/mcb.19.7.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheid MP, Huber M, Damen JE, Hughes M, Kang V, Neilsen P, et al. Phosphatidylinositol (3,4,5)P3 is essential but not sufficient for protein kinase B (PKB) activation; phosphatidylinositol (3,4)P2 is required for PKB phosphorylation at Ser-473: studies using cells from SH2-containing inositol-5-phosphatase knockout mice. J Biol Chem. 2002;277:9027–9035. doi: 10.1074/jbc.M106755200. [DOI] [PubMed] [Google Scholar]
- 39.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 40.Wick MJ, Wick KR, Chen H, He H, Dong LQ, Quon MJ, et al. Substitution of the autophosphorylation site Thr516 with a negatively charged residue confers constitutive activity to mouse 3-phosphoinositide-dependent protein kinase-1 in cells. J Biol Chem. 2002;277:16632–16638. doi: 10.1074/jbc.M112402200. [DOI] [PubMed] [Google Scholar]
- 41.Sulis ML, Parsons R. PTEN: from pathology to biology. Trends Cell Biol. 2003;13:478–483. doi: 10.1016/s0962-8924(03)00175-2. [DOI] [PubMed] [Google Scholar]
- 42.Leslie NR, Downes CP. PTEN function: how normal cells control it and tumour cells loseit. Biochem J. 2004;382:1–11. doi: 10.1042/BJ20040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 44.Lachyankar MB, Sultana N, Schonhoff CM, Mitra P, Poluha W, Lambert S, et al. A role for nuclear PTEN in neuronal differentiation. J Neurosci. 2000;20:1404–1413. doi: 10.1523/JNEUROSCI.20-04-01404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gary DS, Mattson MP. PTEN regulates Akt kinase activity in hippocampal neurons and increases their sensitivity to glutamate and apoptosis. Neuromolecular Med. 2002;2:261–269. doi: 10.1385/NMM:2:3:261. [DOI] [PubMed] [Google Scholar]
- 46.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 47.Lai WS, Xu B, Westphal KG, Paterlini M, Olivier B, Pavlidis P, et al. Akt1 deficiency affects neuronal morphology and predisposes to abnormalities in prefrontal cortex functioning. Proc Natl Acad Sci U S A. 2006;103:16906–16911. doi: 10.1073/pnas.0604994103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans. 2007;35:231–235. doi: 10.1042/BST0350231. [DOI] [PubMed] [Google Scholar]
- 49.Franke TF. Intracellular signaling by Akt: bound to be specific. Sci Signal. 2008;1:e29. doi: 10.1126/scisignal.124pe29. [DOI] [PubMed] [Google Scholar]
- 50.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 51.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 52.Miller FD, Kaplan DR. Neurotrophin signaling pathways regulating neuronal apoptosis. Cell Mol Life Sci. 2001;58:1045–1053. doi: 10.1007/PL00000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yacoubian TA, Lo DC. Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth. Nat Neurosci. 2000;3:342–349. doi: 10.1038/73911. [DOI] [PubMed] [Google Scholar]
- 54.Dijkhuizen PA, Ghosh A. BDNF regulates primary dendrite formation in cortical neurons via the PI3-kinase and MAP kinase signaling pathways. J Neurobiol. 2005;62:278–288. doi: 10.1002/neu.20100. [DOI] [PubMed] [Google Scholar]
- 55.Yoshimura T, Arimura N, Kawano Y, Kawabata S, Wang S, Kaibuchi K. Ras regulates neuronal polarity via the PI3-kinase/Akt/GSK-3beta/CRMP-2 pathway. Biochem Biophys Res Commun. 2006;340:62–68. doi: 10.1016/j.bbrc.2005.11.147. [DOI] [PubMed] [Google Scholar]
- 56.Toker A. Protein kinases as mediators of phosphoinositide 3-kinase signaling. Mol Pharmacol. 2000;57:652–658. [PubMed] [Google Scholar]
- 57.Backman S, Stambolic V, Mak T. PTEN function in mammalian cell size regulation. Curr Opin Neurobiol. 2002;12:516–522. doi: 10.1016/s0959-4388(02)00354-9. [DOI] [PubMed] [Google Scholar]
- 58.Arevalo MA, Rodríguez-Tébar A. Activation of casein kinase II and inhibition of phosphatase and tensin homologue deleted on chromosome 10 phosphatase by nerve growth factor/p75NTR inhibit glycogen synthase kinase-3beta and stimulate axonal growth. Mol Biol Cell. 2006;17:3369–3377. doi: 10.1091/mbc.E05-12-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dwivedi Y, Mondal AC, Rizavi HS, Conley RR. Suicide brain is associated with decreased expression of neurotrophins. Biol Psychiatry. 2005;58:315–324. doi: 10.1016/j.biopsych.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 60.Ernst C, Deleva V, Deng X, Sequeira A, Pomarenski A, Klempan T, et al. Alternative splicing, methylation state, and expression profile of tropomyosin-related kinase B in the frontal cortex of suicide completers. Arch Gen Psychiatry. 2009;66:22–32. doi: 10.1001/archpsyc.66.1.22. [DOI] [PubMed] [Google Scholar]
- 61.Dwivedi Y, Rao JS, Rizavi HS, Kotowski J, Conley RR, Roberts RC, et al. Abnormal expression and functional characteristics of cyclic adenosine monophosphate response element binding protein in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:273–282. doi: 10.1001/archpsyc.60.3.273. [DOI] [PubMed] [Google Scholar]
- 62.Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Roberts RC, Conley RR. Cyclic AMP response element-binding protein in post-mortem brain of teenage suicide victims: specific decrease in the prefrontal cortex but not the hippocampus. Int J Neuropsychopharmacol. 2007;10:621–629. doi: 10.1017/S1461145706007231. [DOI] [PubMed] [Google Scholar]
- 63.Pandey GN, Dwivedi Y, Rizavi HS, Teppen T, Gaszner GL, Roberts RC, et al. GSK-3beta gene expression in human postmortem brain: regional distribution, effects of age and suicide. Neurochem Res. 2009;34:274–285. doi: 10.1007/s11064-008-9770-1. [DOI] [PubMed] [Google Scholar]
- 64.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 65.Sheline YI, Warry P, Gado MH, Csernansky JC, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 67.Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- 68.Rajkowska G. Morphometric methods for studying the prefrontal cortex in suicide victims and psychiatric patients. Ann NY Acad Sci USA. 1997;836:253–268. doi: 10.1111/j.1749-6632.1997.tb52364.x. [DOI] [PubMed] [Google Scholar]
- 69.Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- 70.Altschuler DL, Casanova MF, Goldberg TE, Kleinman JE. The hippocampus and parahippocampus in schizophrenia, suicide and control brains. Arch Gen Psychiatry. 1990;47:1029–1034. doi: 10.1001/archpsyc.1990.01810230045008. [DOI] [PubMed] [Google Scholar]
- 71.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 72.Krishnan V, Han MH, Mazei-Robison M, Iñiguez SD, Ables JL, Vialou V, et al. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- 74.Kang UG, Roh MS, Jung JR, Shin SY, Lee YH, Park JB, et al. Activation of protein kinase B (Akt) signaling after electroconvulsive shock in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:41–44. doi: 10.1016/S0278-5846(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 75.Arguello PA, Gogos JA. A signaling pathway AKTing up in schizophrenia. J Clin Invest. 2008;118:2018–2021. doi: 10.1172/JCI35931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


