Abstract
Presbycusis can be considered a slow age-related peripheral and central deterioration of auditory function which manifests itself as deficits in speech comprehension, especially in noisy environments. The present study examined neural correlates of a simple broadband noise stimulus in primary auditory cortex (A1) of young and aged Fisher-Brown Norway (FBN) rats. Age-related changes in unit responses to broadband noise-bursts and spontaneous activity were simultaneously recorded across A1 layers using a single shank, 16-channel electrode. Noise bursts were presented contralateral to the left A1 at 80 dB SPL. Aged A1 units displayed increased spontaneous (29%), peak (24%), and steady state response rates (38%) than did young A1 units. This was true across all A1 layers, although age-related differences were significantly greater for layers I–III (43% vs 18%) than lower layers. There was a significant age-related difference in the depth and duration of post-onset suppression between young and aged upper layer A1 units. The present functional differences across layers were consistent with studies showing greatest losses of gamma-aminobutyric acid (GABA) markers in superficial layers of A1 and with anatomic studies showing highest levels of inhibitory neurons located in superficial cortical layers. The present findings were also consistent with aging studies suggesting loss of functional inhibition in other cortical sensory systems.
Keywords: aging, inhibition, noise, rats
Introduction
Presbycusis is associated with both peripheral and central deficits that make speech difficult to process in noisy or complex environments (Willott, 1991). This inability to clearly comprehend speech is one possible reason that a number of elderly individuals withdraw from society (Jones et al., 1984; Lutman, 1990; Mulrow et al., 1990). Although hearing aids can be of assistance, they do not compensate for the central nervous system components of presbycusis (Frisina and Frisina, 1997; Gordon-Salant and Fitzgibbons, 1993; Hayes and Jerger, 1979; Mulrow et al., 1990; Thomas and Herbst, 1980; Weinstein and Ventry, 1982). An age-related loss in the ability to accurately process stimulus onset in a complex acoustic environment may, in part, underpin age-related loss of accurate speech processing in humans (see Frisina and Frisina, 1997). Functional and neurochemical age-related changes at the level of primary auditory cortex (A1) are suggestive of a significant loss of synaptic inhibition and include increased amplitude of auditory cortical evoked potentials in animals and man, as well as a significant decrease in markers of GABAergic inhibition in animal models of aging (for review see Caspary et al., 2008; Tremblay et al., 2002). Subcortical auditory aging neurochemical studies show age-related loss of inhibition throughout the auditory neuraxis (Caspary et al., 2008 for review).
The major ascending and descending projections, basic laminar organization, neuronal organization and morphology of rat A1 is consistent with the organization seen in other species and in other sensory cortices (Games and Winer, 1988; Roger and Arnault, 1989; Sally and Kelly, 1988; Webster, 1995; Winer and Larue, 1989; Zilles, 1985). Rat auditory cortex is organized into belt and core regions based, in part, on segregation of inputs from the medial geniculate body (MGB). Major descending projections originate from layer V and terminate in the dorsal and external nuclei of the inferior colliculus (IC) (Coleman and Clerici, 1987; Faye-Lund, 1985; Herbert et al., 1991). Descending projections from IC, project to most major brainstem auditory nuclei (Saldaña et al., 1996; Weedman and Ryugo, 1996). Descending projections that originate from layer VI are considered reciprocal to ascending ventral MGB projections (Barbour and Callaway, 2008; LeDoux et al., 1985; Roger and Arnault, 1989; Winer and Larue, 1989). Cells located in cortical layers II–III primarily give rise to contralateral commissural connections but have some descending projections as well (Prieto et al., 1994). The major ascending auditory input into rat A1 is from the ventral MGB terminating primarily in layer IV (for review see Webster, 1995).
The present study examined the neural correlates of stimulus onset and sustained activity across A1 layers in young and aged FBN rats.
Materials and Methods
Subjects
Fifteen young (4–6 mos.) and thirteen aged (30–32 mos.) Fischer-Brown Norway rats were obtained from Harlan Sprague-Dawley, Inc. under a contract with the Office of Biological Resources of the National Institute on Aging (NIA). Prior to the recording of electrophysiological data, all animals underwent auditory brainstem response (ABR) assessment for thresholds at 8, 12, 16, 20, 24, & 32 kHz. Thresholds for animals included in the study were consistent with the recent published values of Wang et al. (2009). Animals were used in accordance with a protocol approved by the SIU School of Medicine Laboratory Animal Care and Use Committee.
Surgery
Young rats were anesthetized with a 3:1 mixture of Ketamine (100mg/ml): Xylazine (20 mg/ml) (1.4 ml/kg IM) and maintained for the remainder of the experiment, typically 10–12 hours, with i.p. injections of urethane (500 mg/kg body weight). Aged animals were given 80% of the young dosage to account for altered metabolism (see Palombi and Caspary, 1996 a,b,c; Turner et al., 2005 a,b). Urethane was selected for this study based on prior experience in single unit studies from primary auditory cortex (Turner et al., 2005a,b). Primary auditory cortex neurons recorded from urethane-anesthetized animals were more responsive to acoustic stimuli than cells under ketamine/ xylazine or barbiturate (Turner, Hughes, and Caspary, unpublished observations). Urethane was also reported to have relatively minor affects on the GABAergic systems (Hara and Harris 2002; Maggi and Meli 1986). Rats were placed in a stereotaxic apparatus within an IAC sound-attenuating booth. Dental acrylic was used to secure the skull to a post held by the stereotaxic frame. A craniotomy (3mm ovoid) centered 5mm caudal and 4mm ventral to Bregma was made (Figure 1), exposing the majority of the left A1 (Turner et al., 2005 a,b). Overlying dura was removed and a 16 channel single shank multichannel (Michigan) probe (100 micron node separation) was advanced perpendicular to the pial surface, while a 50 msec broadband search stimulus was presented to the contralateral ear. The electrode was advanced until responses disappeared from the bottom two channels and no responses appeared on the top four channels. This allowed the electrode to span all 6 layers of A1 making placement of the electrode channels as consistent as possible across the layers of A1 (Figure 2).
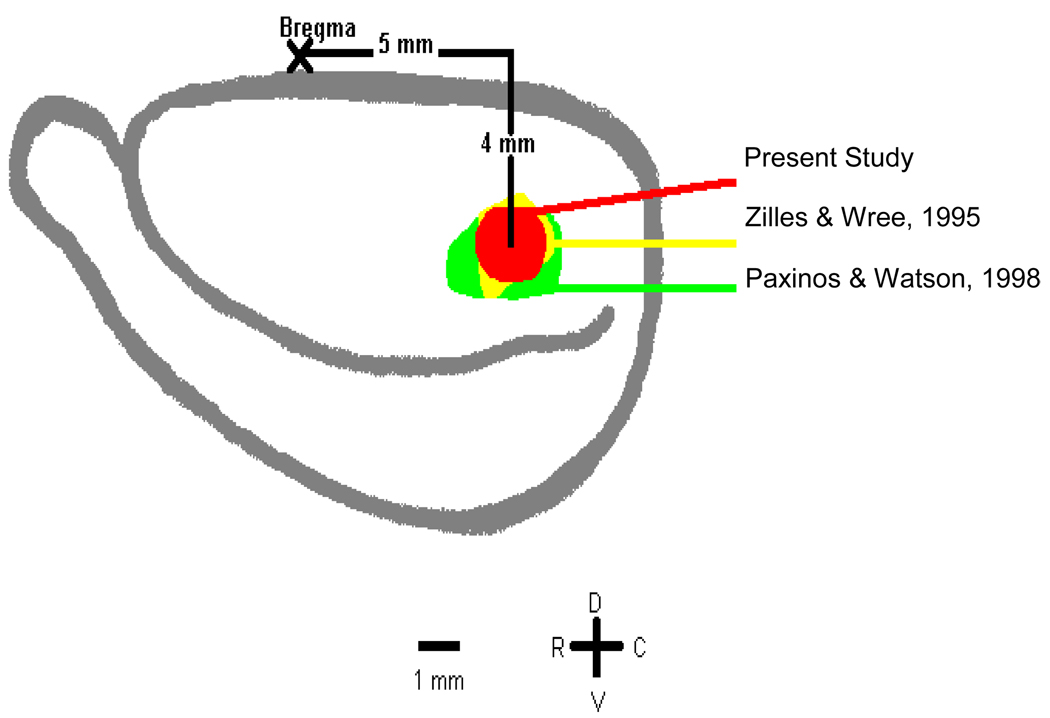
Figure 1.
Diagram of the lateral view of rat neocortex showing the location of recordings in relation to commonly used boundaries of A1. The center of the recording field was usually 4 mm ventral and 5 mm caudal to bregma.
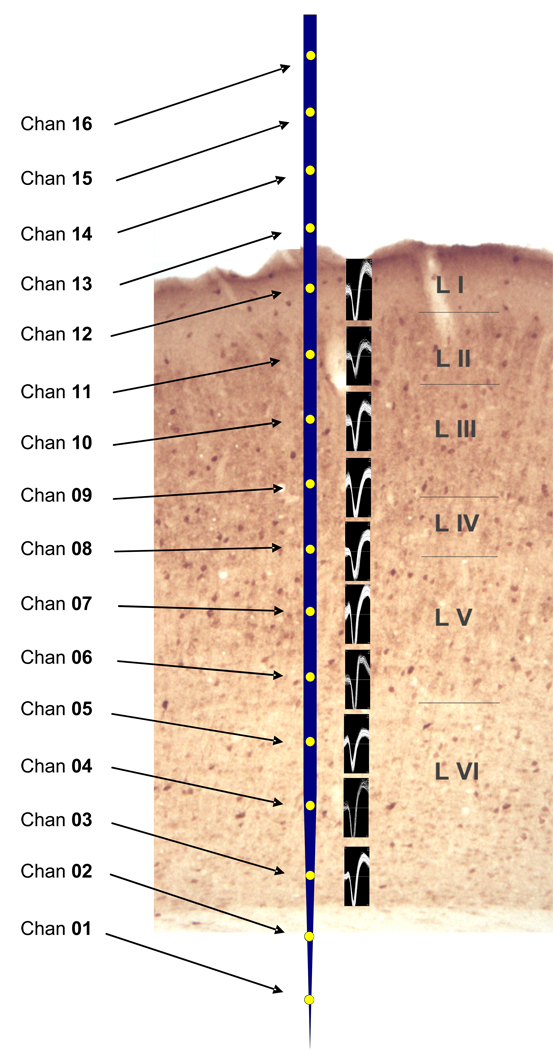
Figure 2.
GAD immunolabeled primary auditory cortex from a young FBN rat, rotated such that the normally laterally located superficial layer I, is at the top of the figure. A scaled cartoon of the 16 channel Neuronexus probe placed over A1 used in the present study with 10 nodes/channels within the six layers of rat A1 (see methods). Examples of off-line sorted spikes placed next to their appropriate nodes from a single penetration in an aged rat A1 (see methods for spike sorting).
Stimulus
The stimulus consisted of a 500 msec broadband noise burst with a rise/fall of 0.5 msec presented to the right ear at 80 dB SPL. Data were collected for 150 repetitions with an ISI (onset to onset) of 2 sec.
Spike Sorting
No more than three penetrations per animal were attempted. 1) Cross-channel artifacts (appeared on 80% or more of the channels) were eliminated using tools in Offline Sorter (Plexon Inc.). 2) Units were aligned on global minimums while ignoring waveform assignments. 3) Maximum alignment shift was set at 50 clock ticks (25 µsec) and Principal Components Analyses were recalculated. 4) Obvious “invalid” waveforms were eliminated prior to sorting. 5) Spikes were clustered, using Valley-Seeking and T-Distribution algorithms. 6) Spike templates, that were established using the automated clustering algorithms, were used as guides to further sort waveforms with the template sort function. 7) Manual sort template tolerances were set to 200 (medium); occasionally, tolerances were set at higher levels to optimize sorting outcomes. 8) Invalid waveforms were again eliminated. 9) Remaining unsorted waveforms were examined for timestamp regularity before being eliminated. More than 1.5 million spikes from 1100 units remained, and were included in the analysis.
Temporal Segmentation of response
Peri-stimulus time-histograms (PSTH) were constructed for each sorted neuron on each recording channel, of each penetration for each animal. PSTHs had a bin width of 1 msec and were 500 msec long and consisted of the data from 150 stimulus repetitions. Visually, an onset burst of activity (onset), followed by a period of suppressed activity (suppression), was followed by steady activity period (steady state), was apparent in the PSTHs. Virtually all of the responses were of the phasic type and exhibited this form of the PSTH. For quantitative data analysis, bins 1–50 were designated as onset, 51–248 as suppression, and 251–448 as steady state. Recording channels were grouped into homogeneous subgroups by selecting channels which produced an absence of a recording channel by time interaction (relatively homogeneous pattern of firing across the 500 msec). This resulted in two distinct clusters; channels in cortical layers I–III (Upper layers) dominated by cortical commissural pathways, and channels located in cortical layers IV–VI (Lower layers) dominated by extracortical ascending or descending connections.
Statistical Analyses
Data files generated during the experiments were imported to Excel spreadsheets and assessed for integrity and distributional characteristics. The raw data for analysis consisted of spike counts/bin (1 msec width) for a duration of 500 bins summed across the 150 repetitions. These data were gathered for each sorted neuron on each of the 10 channels for each of the penetrations for all of the animals in each of the age groups. A mixed-effects, repeated measures Analysis of variance (ANOVA) was used to assess changes in response characteristics as a function of age and cortical layers (Upper vs Lower). Statistical procedures were implemented with SPSS 17.0.
Results
Spontaneous activity
Spontaneous activity recorded during the 2 minute period prior to the presentation of the noise burst (see Table 1) revealed a significant increase in firing rates for aged neurons relative to young (p=.001). Both young and aged neurons showed higher firing rates in the upper layers compared to the lower layers (p<.001). However, the age-related increase in spontaneous activity was larger in the Upper layers than in the Lower layers (age × layer interaction p=.029). Analysis of the simple effect of age indicated a significant age effect for the Upper layers (43.6% increment: p=.003), but age effect failed to achieve significance in the Lower layers (14.3% increment).
Table 1.
Spontaneous Activity for a 2 minute Period
| Layers | Mean | SD | N | |
|---|---|---|---|---|
| Aged | Lower | 7.50 | 10.21 | 321 |
| Upper | 14.06 | 18.41 | 216 | |
| Young | Lower | 6.57 | 10.68 | 358 |
| Upper | 9.78 | 12.74 | 268 |
General Effects
Aged A1 units also displayed increased stimulus driven discharge rates compared to the young A1 units (p<.001). This was true in both Upper (I–III) and Lower layers (IV–VI), although the difference was significantly greater in the upper layers (age × layer interaction p<.05). Both spontaneous and driven rates were higher in the Upper layers than in the Lower layers (p<.001). This was observed for both the Young and Aged A1, although these differences were larger in Aged A1 (p<.05).
Segmental Effects
(onset, suppression, steady state)
Onset
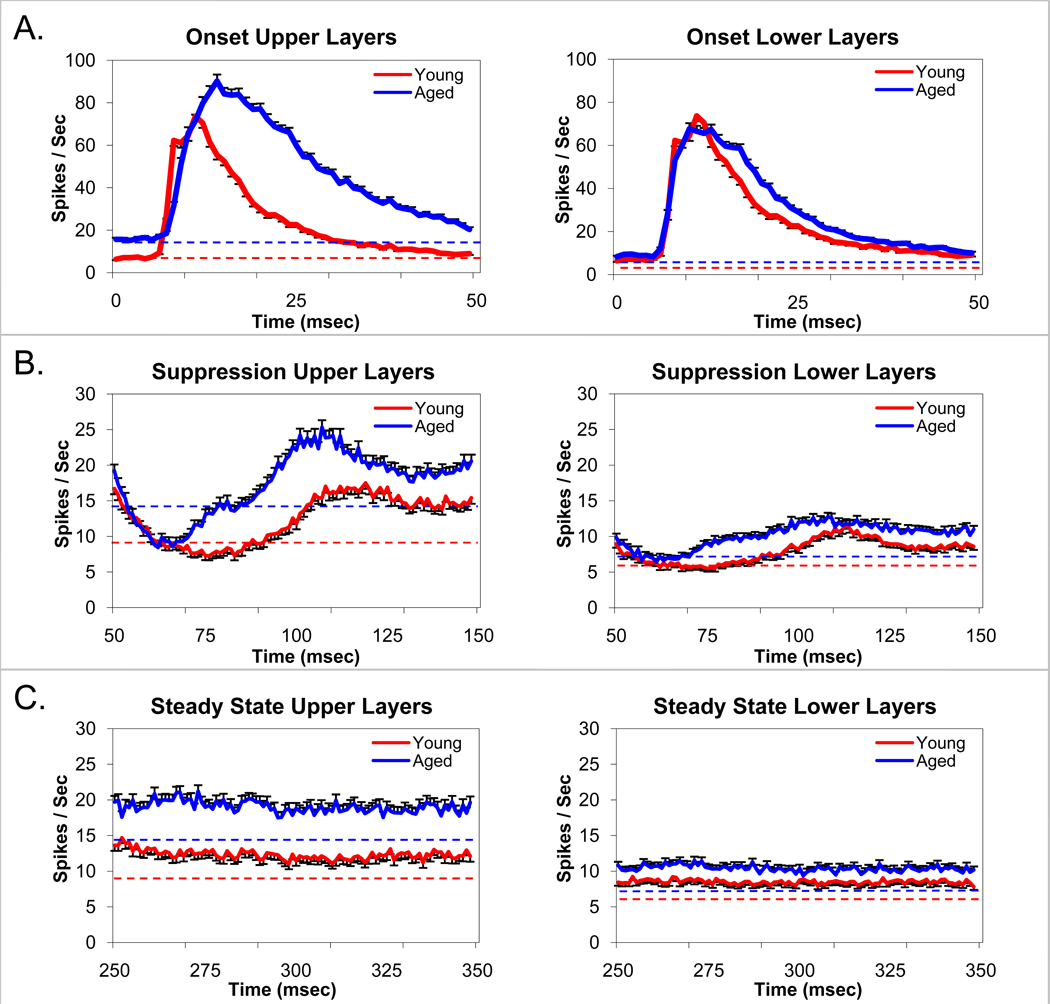
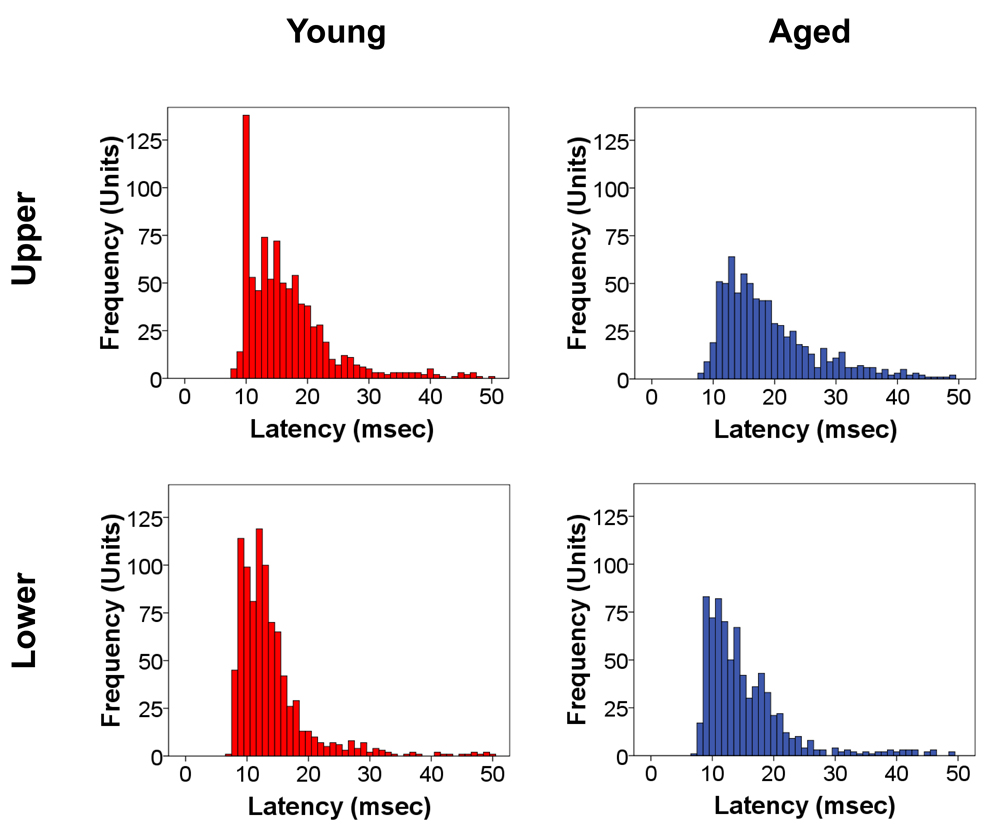
Onset responses (Figure 3, panel A) were layer and age dependent (age × layer interaction, p=.002). In A1 Upper layers, Aged A1 units displayed increased (30.9%) peak onset response rates compared to Young A1 units (p<.001) while in Lower layers the increment in peak A1 unit onset response rates (17.8%) failed to reach significance. In the lower portions of layer V and the upper portions of layer VI the onset peak firing rate was slightly greater for Young A1 units although the peak width was not significantly different between age groups. Onset peaks were significantly broader for Aged A1 units in both the Upper and Lower layers, showing less post-onset damping (age × time interactions p<.001). Analysis of peak onset latencies (latencies of peak firing rate during the onset burst) revealed shorter mean latencies for the Young A1 units (Upper = 16.8, Lower = 13.9) than the Aged A1 units (Upper = 19.2, Lower = 15.4) in both the Upper and Lower layers (p<.001). Shorter latencies were found in the Lower layers than the Upper layers for both Young and Aged A1 units (p<.001). Age-related differences were larger in the Upper layers (age × layer interaction (p=.05). Figure 4 shows age-related changes in the latency histograms for the variance. The mean Aged peak latencies were longer than those for the Young in the Upper layers (p<.003) and in the Lower layers (p<.001). A comparison of peak firing rates relative to the average steady state level revealed an age-related increase in the onset peak relative to Steady State responses in the Upper Layers (p=.049), but not in the Lower layers. Thus, even when taking into account the overall firing rate differences between age groups, Aged A1 units showed higher onset peak firing rates in the Upper Layers than Young A1 units.
Figure 3.
Unit responses to broadband noise bursts at 80dB SPL parsed into Onset (1–50msec) (A), Suppression (51–248msec) (B), and Steady-state (251–448msec) (C) segments. The impact of aging vs layers in FBN rats are compared between young (red) and aged (blue) and upper (layers I–III) and lower (layers IV–VI). Each trace contains data from at least 215 sorted A1units. There was a significant age-related increase in the amplitude and duration of the onset peak in upper cortical layers (I–III) but not in the lower cortical layers (IV–VI) (A). Dotted line represents spontaneous activity for young (red) and aged (blue) units. Note that the increase in onset activity cannot be accounted for by a simple shift in baseline (see text). Temporal dynamics of unit responses to the suppressive period following the onset response to the noise burst were significantly altered in upper A1 layers of aged units but less so in the lower A1 layers (B). There was a significant age-related decrease in depth and duration of the post-onset suppression across A1 (B). There was a significant age-related increase in steady-state responses to broadband noise burst stimuli in upper A1 layers but not in lower layers (C). Error bars represent the standard error. The dashed line represents the level of spontaneous activity.
Figure 4.
Frequency distribution of response latency of sorted units to the noise burst stimulation in young (red) and aged (blue) A1 located in upper and lower layers. As expected, young and aged sorted units showed significantly shorter onset latencies for lower (IV–VI) than upper (I–III) A1 layers. There was a significant age-related rightward shift of onset latencies throughout A1 with the largest shift seen in the upper A1 layers. The age-related increase in latency is suggestive of an age-related increase in temporal uncertainty. Bin widths are 1 msec.
Suppression
Units in A1 displayed a distinct period of suppression following the onset peak (Figure 3, panel B). This segment of the response displayed age and layer dependent differences (age × layer interaction, p=.002). Upper layer A1 units in Aged animals showed a period of suppression that was biphasic (suppression followed by overshoot). This was supported by a trend analysis revealing a significant 4th order component (p<.001) accounting for 21% of the variance as measured by partial eta squared. Young A1 units displayed a significant 3rd order component (p<.001) also accounting for 21% of the variance. Thus Aged A1 units showed a firing rate by time function that had 3 maximum/minimum points while Young A1 units showed 2 maximum/minimum points indicating significant overshoot for the Aged A1 units but not for the Young A1 units. Young A1 units showed a longer period of suppression than the Aged (age × time interaction for the initial phase of suppression, p<.001).
In the lower A1 layers (IV–VI), age-related changes in suppression were qualitatively similar to what was seen in the Upper layers (I–III). The depths of the post-onset suppressive effects were shallower in Lower layers than Upper layers (p<.001), but proportional, given the overall lower firing rates in the Lower layers. Trend analysis results for the suppression were the same as in the Upper layers except that the effect size was reduced to 7% for each age group. Again, the suppression was prolonged for Young A1 units, more gradual recovery from maximum suppression, than for Aged A1 units (p<.001).
Steady state
There was an age-related increase in steady state driven activity (Figure 3, panel C) recorded during the last 200 msec of the stimulus (p<.001). Aged A1 units displayed higher firing rates than the Young A1 units. Both Young and Aged A1 units showed higher firing rates in the Upper layers compared to the Lower layers (p<.001). However, the age effect was larger (54.3% vs 22.4%) in the Upper layers than in the Lower layers (age × layer interaction p<.001). Additionally, during the last 200 msec there was a gradual decrease in unit firing rate to approximate the spontaneous level. This was significant for the Aged A1 units in both the Upper and Lower layers (p’s<.001). The gradual decline was significant for the Young units in the Upper A1 Layers (p<.001), but failed to reach significance in the Lower A1 layers (p=.077).
Discussion
The present in vivo study examined functional age-related changes in the ability of sorted single units recorded simultaneously across the layers of rat primary auditory cortex (A1) in response to noise burst stimuli. Findings of an age-related increase in discharge rate and reduced damping are consistent with previous studies suggestive of an age-related loss of inhibition in the central auditory system. Although it is likely that inhibitory changes observed in the present study may, in part, reflect age-related inhibitory down-regulation throughout the brain stem, both A1 layer differences in discharge rates and damping suggest a de novo differential age-related loss of inhibitory function in A1. Ling et al., (2005) described differential layer specific age-related changes in glutamic acid decarboxylase (GAD) levels, in rat primary auditory cortex. Significant age-related decreases in GAD message levels for GAD isoforms showed the greatest changes (GAD65: −26.6% and GAD67: −40.1%) in layer II. Electrophysiologic A1 findings in the present study of a layer dependency for the age-related changes are consistent with the GAD changes seen in A1 (Ling et al., 2005). The increased peak firing rate and duration of the onset burst, the less intense post-onset burst suppression, and the subsequent higher steady state firing rates for the Aged A1 units are suggestive of losses in both phasic and tonic inhibition. Recent single unit aging and iontophoretic studies in primate and feline visual cortex also show age-related changes in the response properties of visual cortical neurons consistent with a selective down-regulation of gamma-aminobutyric acid (GABA) inhibition (Hua et al., 2006; Leventhal et al., 2003; Schmolesky et al., 2000). Similar age-related central visual changes have also been observed in humans (Betts et al., 2007).
The present age-related changes, for driven and spontaneous activity, were differential with respect to A1 layers whose laminar structure has been elegantly described by Winer and his colleagues (Games and Winer, 1988; Prieto et al., 1994; Winer, 1992; Winer and Larue, 1989). Unit responses were simultaneously recorded from all layers of A1, with results parsed into two major divisions; cortical layers I–III (Upper layers) dominated by cortical commissural pathways and interneurons, and cortical layers IV–VI (Lower layers) dominated by midbrain and thalamic ascending or descending connections. Prieto et al., (1994) found a three-tiered numerical distribution of puncta, that were immunoreactive for GABA or its synthesizing enzyme, GAD, with the highest density in layer Ia, and intermediate numbers in layers Ib–IVb. The lowest concentrations of markers for GABA were found in layers V and VI, respectively (Prieto et al., 1994). Briefly, Winer and colleagues found Layer I to be largely acellular containing some small GAD-positive horizontal cells while Layer II contains relatively small polymorphic cells with small or medium-sized GAD-positive cells (Games and Winer, 1988; Prieto et al., 1994; Winer and Larue, 1989). Layer III, was found to contain pyramidal and non-pyramidal neurons with larger neurons typically located in the deeper portion of the layer. Layer IV receives the major input projection from ventral MGB and is characterized by small cells which give it its granular appearance while Layer V is characterized by large pyramidal cells which are the origin of major output projections to auditory midbrain and contralateral A1 (Games and Winer, 1988). Layer VI, the thickest layer in rat A1, contains pyramidal and non-pyramidal neurons (Games and Winer, 1988).
The present results support a role for GABA inhibition in shaping A1 response to a simple broadband noise burst, particularly the magnitude and duration of the onset burst and the post-onset recovery. The greater variability seen in the latencies of the Aged peak firing rate for A1 neurons during the onset burst is consistent with the interpretation of increased temporal uncertainty that is age dependent and may also be a function of the loss of inhibition with aging. Elegant studies which directly and indirectly assess synaptic responses properties in vivo indicate that the interaction of synaptic excitation and inhibition shapes temporal and spectral responses of A1 neurons (Atencio and Schreiner, 2008; Tan et al., 2004). Turner et al., (2005 a,b) found age-related increases in the discharge rates of layer V neurons showing complex spectral properties. These same in vivo recordings found that complex layer V A1 neurons, which are thought to receive significant inhibition in young adults, were more readily driven by current pulses delivered to the soma in aged A1 neurons (Turner et al., 2005 a,b). A significant role for GABA inhibition in shaping A1 response properties to complex stimuli has been described from in vitro and in vivo studies (Foeller et al., 2001; Hefti and Smith, 2000; Pernberg et al., 1998; Shevelev et al., 1998; Wang et al., 2000). Muscimol, a GABAA receptor agonist, was found to disrupt coding of certain complex signals in bat A1 (Riquimaroux et al., 1992). GABAA receptor blockade alters A1 neuronal responses across A1 layers, lowering thresholds and reducing the complexity of frequency response maps (Chen and Jen, 2000; Foeller et al., 2001; Horikawa et al., 1996; Wang et al., 2000). GABAA receptor blockade altered Sinusoidal Amplitude Modulation (AM) coding in gerbil A1 neurons (Schulze and Langner; 1997 a,b). Directional selectivity of A1 neurons to Frequency Modulation (FM) stimuli and selective responses to low AM modulation frequencies are indicative of de novo inhibitory processing in A1 (Eggermont, 1998; Giraud et al., 2000; Kilgard and Merzenich, 1999; Mendelson et al., 1993; Mendelson and Ricketts, 2001; Schulze and Langner , 1997a,b).
Findings in the present study from rat A1 units in response to a noise burst stimuli found increased mean on-set latency across all A1 layers. Aging IC studies show conflicting results. Studies in CBA mouse IC found age-related decreases/shortening of first spike latency in response to simple and modulated tonal stimuli over a range of stimulus intensity (Simon et al., 2004). In rat IC, Palombi and Caspary (1996a) found no significant age-related changes in first spike latency in response to superthreshold (30dB SL) BF tone bursts. The same study using 80dB SPL stimuli for both young and aged F344 rat IC units, found significant age-related increases in first spike latency in the central nucleus of the inferior colliculus (CIC). Consistent with the present findings, Mendleson and Rickets (2001) working in rat auditory cortex, found small age-related increases in first spike latency.
The firing rates of the vast majority of A1 neurons are modulated only at the onset and/or offset of long duration steady stimuli, and quickly habituate to their spontaneous levels (Brugge et al., 1969; Chimoto et al. 2002; Eggermont, 1991; Phillips and Hall, 1987, 1990; Phillips and Sark 1991). While this statement is based primarily on results from anesthetized preparations, it has also been reported in the results of awake/behaving animals (Pfingst and O’Connor, 1981; Schulze and Langner, 1997a,b; Suga, 1977). Given the use of a long duration stimulus with a relatively sharp rise time, 150 repetitions of the same stimulus within a 5 minute interval, and an anesthetized preparation, nearly all of the responses in our study were of the phasic type. Therefore the findings of this study are limited to the population of phasic neurons.
It is likely that peripheral, age-related changes result in a partial deafferentation of the central auditory processor. This, in turn, results in a series of plastic/pathologic compensatory changes including a down regulation of inhibitory function (Caspary et. al., 1990; Caspary et al., 2008; Eggermont and Roberts, 2004; Sörös et al., 2009). The change in inhibitory function, at the level of A1, has a negative impact on the processing of simple and complex stimuli in the elderly.
Acknowledgements
Supported in part by Grants (SIU Excellence in Academic Medicine grant to LFH, NIH DC00151 to DMC, NIH AG023910 to JGT). The authors wish to express their sincere thanks to Loren Zuiderveld and Andrew K. James for their help with spike sorting.
Abbreviations
- A1
primary auditory cortex
- ABR
auditory brainstem response
- ANOVA
analysis of variance
- AM
amplitude modulation
- BF
best frequency
- CIC
central nucleus of the inferior colliculus
- FBN
Fisher-Brown Norway
- FM
frequency modulation
- GABA
gamma-aminobutyric acid
- GAD
glutamic acid decarboxylase
- IAC
industrial acoustics company
- IC
inferior colliculus
- MGB
medial geniculate body
- PSTH
peri-stimulus time-histograms
- SL
sensation level
- SPL
sound pressure level
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atencio CA, Schreiner CE. Spectrotemporal processing differences between auditory cortical fast-spiking and regular-spiking neurons. J Neurosci. 2008;28(15):3897–3910. doi: 10.1523/JNEUROSCI.5366-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour DL, Callaway EM. Excitatory local connections of superfical neurons in rat auditory cortex. J Neurosci. 2008;28(44):11174–11185. doi: 10.1523/JNEUROSCI.2093-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts LR, Sekuler AB, Bennett PJ. The effects of aging on orientation discrimination. Vision Res. 2007;47(13):1769–1780. doi: 10.1016/j.visres.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Brugge JF, Dubrovsky NA, Aitkin LM, Anderson DJ. Sensitivity of single neurons in auditory cortex of cat to binaural tonal stimulation; effects of varying interaural time and intensity. J Neurophysiol. 1969;32(6):1005–1024. doi: 10.1152/jn.1969.32.6.1005. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Raza A, Lawhorn Armour BA, Pippen J, Arneri SP. Immunocytochemical and Neurochemical Evidence for Age-related Loss of GABA in the Inferior Colliculus: Implications for Neural Presbycusis. J Neurosci. 1990;10(7):2363–2372. doi: 10.1523/JNEUROSCI.10-07-02363.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211(Pt 11):1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QC, Jen PH. Bicuculline application affects discharge patterns, rate-intensity functions, and frequency tuning characteristics of bat auditory cortical neurons. Hear Res. 2000;150(1–2):161–174. doi: 10.1016/s0378-5955(00)00197-0. [DOI] [PubMed] [Google Scholar]
- Chimoto S, Kitama T, Qin L, Sakayori S, Sata Y. Tonal response patterns of primary auditory cortex neurons in alert cats. Brain Res. 2002;934:34–42. doi: 10.1016/s0006-8993(02)02316-8. [DOI] [PubMed] [Google Scholar]
- Coleman JR, Clerici WJ. Sources of projections to subdivisions of the inferior colliculus in the rat. J Comp Neurol. 1987;262(2):215–226. doi: 10.1002/cne.902620204. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Rate and synchronization measures of periodicity coding in cat primary auditory cortex. Hear Res. 1991;56(1–2):153–167. doi: 10.1016/0378-5955(91)90165-6. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Representation of spectral and temporal sound features in three cortical fields of the cat. Similarities outweigh differences. J Neurophysiol. 1998;80(5):2743–2764. doi: 10.1152/jn.1998.80.5.2743. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The Neuroscience of Tinnitus. Trends in Neurosciences. 2004;27(11):676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Faye-Lund H. The neocortical projection to the inferior colliculus in the albino rat. Anat Embryol. (Berl) 1985;173(1):53–70. doi: 10.1007/BF00707304. [DOI] [PubMed] [Google Scholar]
- Foeller E, Vater M, Kössl M. Laminar analysis of inhibition in the gerbil primary auditory cortex. Assoc Res Otolaryngol. 2001;2(3):279–296. doi: 10.1007/s101620010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hear Res. 1997;106:95–104. doi: 10.1016/s0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Games KD, Winer JA. Layer V in rat auditory cortex: projections to the inferior colliculus and contralateral cortex. Hear Res. 1988;34(1):1–25. doi: 10.1016/0378-5955(88)90047-0. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Lorenzi C, Ashburner J, Wable J, Johnsrude I, Frackowiak R, Kleinschmidt A. Representation of the temporal envelope of sounds in the human brain. J Neurophysiol. 2000;84(3):1588–1598. doi: 10.1152/jn.2000.84.3.1588. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ. Temporal factors and speech recognition performance in young and elderly listeners. J Speech Hear Res. 1993;36:1276–1285. doi: 10.1044/jshr.3606.1276. [DOI] [PubMed] [Google Scholar]
- Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg. 2002;94(2):313–318. doi: 10.1097/00000539-200202000-00015. [DOI] [PubMed] [Google Scholar]
- Hayes D, Jerger J. Low-frequency hearing loss in presbycusis. A central interpretation. Arch. Otolaryngol. 1979;105(1):9–12. doi: 10.1001/archotol.1979.00790130013003. [DOI] [PubMed] [Google Scholar]
- Hefti BJ, Smith PH. Anatomy, physiology, and synaptic responses of rat layer V auditory cortical cells and effects of intracellular GABA(A) blockade. J Neurophysiol. 2000;83(5):2626–2638. doi: 10.1152/jn.2000.83.5.2626. [DOI] [PubMed] [Google Scholar]
- Herbert H, Aschoff A, Ostwald J. Topography of projections from the auditory cortex to the inferior colliculus in the rat. J Comp Neurol. 1991;304(1):103–122. doi: 10.1002/cne.903040108. [DOI] [PubMed] [Google Scholar]
- Horikawa J, Hosokawa Y, Kubota M, Nasu M, Taniguchi I. Optical imaging of spatiotemporal patterns of glutamatergic excitation and GABAergic inhibition in the guinea-pig auditory cortex in vivo. J Physiol. 1996;497(Pt 3):629–638. doi: 10.1113/jphysiol.1996.sp021795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua T, Li X, He L, Zhou Y, Wang Y, Leventhal AG. Functional degradation of visual cortical cells in old cats. Neurobiol Aging. 2006;27(1):155–162. doi: 10.1016/j.neurobiolaging.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Jones DA, Victor CR, Vetter NJ. Hearing difficulty and its psychological implications for the elderly. J Epidemiol Community Health. 1984;38(1):75–78. doi: 10.1136/jech.38.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Distributed representation of spectral and temporal information in rat primary auditory cortex. Hear Res. 1999;134(1–2):16–28. doi: 10.1016/s0378-5955(99)00061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Ruggiero DA, Reis DJ. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J Comp Neurol. 1985;242(2):182–213. doi: 10.1002/cne.902420204. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300(5620):812–815. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- Ling LL, Hughes LF, Caspary DM. Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neuroscience. 2005;132(4):1103–1113. doi: 10.1016/j.neuroscience.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Lutman ME. Hearing disability in the elderly. Acta Otolaryngol Suppl. 1990;476:239–248. doi: 10.3109/00016489109127285. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations. Part 3: Other systems and conclusions. Experientia. 1986;42(5):531–537. doi: 10.1007/BF01946692. [DOI] [PubMed] [Google Scholar]
- Mendelson JR, Schreiner CE, Sutter ML, Grasse KL. Functional topography of cat primary auditory cortex: responses to frequency-modulated sweeps. Exp Brain Res. 1993;94(1):65–87. doi: 10.1007/BF00230471. [DOI] [PubMed] [Google Scholar]
- Mendelson JR, Ricketts C. Age-related temporal processing speed deterioration in auditory cortex. Hear Res. 2001;158(1–2):84–94. doi: 10.1016/s0378-5955(01)00294-5. [DOI] [PubMed] [Google Scholar]
- Mulrow CD, Aguilar C, Endicott JE, Tuley MR, Velez R, Charlip WS, Rhodes MC, Hill JA, DeNino LA. Quality-of-life changes and hearing impairment: a randomized trial. Ann Intern Med. 1990;113:188–194. doi: 10.7326/0003-4819-113-3-188. [DOI] [PubMed] [Google Scholar]
- Palombi PS, Caspary DM. GABA inputs control discharge rate primarily within frequency receptive fields of inferior colliculus neurons. J Neurophysiol. 1996a;75(6):2211–2219. doi: 10.1152/jn.1996.75.6.2211. [DOI] [PubMed] [Google Scholar]
- Palombi PS, Caspary DM. Physiology of the young adult Fischer 344 rat inferior colliculus: responses to contralateral monaural stimuli. Hear Res. 1996b;100(1–2):41–58. doi: 10.1016/0378-5955(96)00115-3. [DOI] [PubMed] [Google Scholar]
- Palombi PS, Caspary DM. Responses of young and aged Fischer 344 rat inferior colliculus neurons to binaural tonal stimuli. Hear Res. 1996c;100(1–2):59–67. doi: 10.1016/0378-5955(96)00113-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. New York, NY: Academic Press; 1998. [Google Scholar]
- Pernberg J, Jirmann KU, Eysel UT. Structure and dynamics of receptive fields in the visual cortex of the cat (area 18) and the influence of GABAergic inhibition. Eur J Neurosci. 1998;10(12):3596–3606. doi: 10.1046/j.1460-9568.1998.00364.x. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, O'Connor TA. Characteristics of neurons in auditory cortex of monkeys performing a simple auditory task. J Neurophysiol. 1981;45(1):16–34. doi: 10.1152/jn.1981.45.1.16. [DOI] [PubMed] [Google Scholar]
- Phillips DP, Hall SE. Responses of single neurons in cat auditory cortex to time-varying stimuli: linear amplitude modulations. Exp Brain Res. 1987;67(3):479–492. doi: 10.1007/BF00247281. [DOI] [PubMed] [Google Scholar]
- Phillips DP, Hall SE. Response timing constraints on the cortical representation of sound time structure. J Acoust Soc Am. 1990;88(3):1403–1411. doi: 10.1121/1.399718. [DOI] [PubMed] [Google Scholar]
- Phillips DP, Sark SA. Separate mechanisms control spike numbers and inter-spike intervals in transient responses of cat auditory cortex neurons. Hear Res. 1991;53(1):17–27. doi: 10.1016/0378-5955(91)90210-z. [DOI] [PubMed] [Google Scholar]
- Prieto JJ, Peterson BA, Winer JA. Morphology and spatial distribution of GABAergic neurons in cat primary auditory cortex (AI) J Comp Neurol. 1994;344(3):349–382. doi: 10.1002/cne.903440304. [DOI] [PubMed] [Google Scholar]
- Riquimaroux H, Gaioni SJ, Suga N. Inactivation of the DSCF area of the auditory cortex with muscimol disrupts frequency discrimination in the mustached bat. J Neurophysiol. 1992;68(5):1613–1623. doi: 10.1152/jn.1992.68.5.1613. [DOI] [PubMed] [Google Scholar]
- Roger M, Arnault P. Anatomical study of the connections of the primary auditory area in the rat. J Comp Neurol. 1989;287(3):339–356. doi: 10.1002/cne.902870306. [DOI] [PubMed] [Google Scholar]
- Saldaña E, Feliciano M, Mugnaini E. Distribution of descending projections from primary auditory neocortex to inferior colliculus mimics the topography of intracollicular projections. J Comp Neurol. 1996;371(1):15–40. doi: 10.1002/(SICI)1096-9861(19960715)371:1<15::AID-CNE2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Sally SL, Kelly JB. Organization of auditory cortex in the albino rat: sound frequency. J Neurophysiol. 1988;59(5):1627–1638. doi: 10.1152/jn.1988.59.5.1627. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Pu M, Leventhal AG. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nat Neurosci. 2000;3(4):384–390. doi: 10.1038/73957. [DOI] [PubMed] [Google Scholar]
- Schulze H, Langner G. Representation of periodicity pitch in the primary auditory cortex of the Mongolian gerbil. Acta Otolaryngol Suppl. 1997a;532:89–95. doi: 10.3109/00016489709126150. [DOI] [PubMed] [Google Scholar]
- Schulze H, Langner G. Periodicity coding in the primary auditory cortex of the Mongolian gerbil (Meriones unguiculatus): two different coding strategies for pitch and rhythm? J Comp Physiol. 1997b;181(6):651–663. doi: 10.1007/s003590050147. [DOI] [PubMed] [Google Scholar]
- Shevelev IA, Jirmann KU, Sharaev GA, Eysel UT. Contribution of GABAergic inhibition to sensitivity to cross-like figures in striate cortex. Neuroreport. 1998;9(14):3153–3157. doi: 10.1097/00001756-199810050-00006. [DOI] [PubMed] [Google Scholar]
- Simon H, Frisina RD, Walton JP. Age reduces response latency of mouse inferior colliculus neurons to AM sounds. J. Acoust. Soc/ Am/ 2004;116:469–477. doi: 10.1121/1.1760796. [DOI] [PubMed] [Google Scholar]
- Sörös P, Teismann IK, Manemann E, Lütkenhöner B. Auditory temporal processing in healthy aging: a magnetoencephalographic study. BMC Neurosci. 2009;7:10–34. doi: 10.1186/1471-2202-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N. Amplitude spectrum representation in the Doppler-shifted-CF processing area of the auditory cortex of the mustache bat. Science. 1977;196(4285):64–67. doi: 10.1126/science.190681. [DOI] [PubMed] [Google Scholar]
- Tan AY, Zhang LI, Merzenich MM, Schreiner CE. Tone-evoked excitatory and inhibitory synaptic conductances of primary auditory cortex neurons. J Neurophysiol. 2004;92(1):630–643. doi: 10.1152/jn.01020.2003. [DOI] [PubMed] [Google Scholar]
- Thomas A, Herbst KG. Social and psychological implications of acquired deafness for adults of employment age. Br J Audiol. 1980;14:76–85. doi: 10.3109/03005368009078906. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Piskosz M, Souza P. Aging alters the neural representation of speech cues. Neuroreport. 2002;13(15):1865–1870. doi: 10.1097/00001756-200210280-00007. [DOI] [PubMed] [Google Scholar]
- Turner JG, Hughes LF, Caspary DM. Effects of aging on receptive fields in rat primary auditory cortex layer V neurons. J Neurophysiol. 2005a;94(4):2738–2747. doi: 10.1152/jn.00362.2005. [DOI] [PubMed] [Google Scholar]
- Turner JG, Hughes LF, Caspary DM. Divergent response properties of layer-V neurons in rat primary auditory cortex. Hear Res. 2005b;202(1–2):129–140. doi: 10.1016/j.heares.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Wang J, Caspary D, Salvi RJ. GABA-A antagonist causes dramatic expansion of tuning in primary auditory cortex. Neuroreport. 2000;11(5):1137–1140. doi: 10.1097/00001756-200004070-00045. [DOI] [PubMed] [Google Scholar]
- Wang H, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Age-related changes in glycine receptor subunit composition and binding to dorsal cochlear nucleus. Neuroscience. 2009;160:227–239. doi: 10.1016/j.neuroscience.2009.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster WR. Auditory System. In: Paxinos G, editor. The Rat Nervous System. 2nd Ed. New York: Academic Press; 1995. [Google Scholar]
- Weedman DL, Ryugo DK. Projections from auditory cortex to the cochlear nucleus in rats: synapses on granule cell dendrites. J Comp Neurol. 1996;371(2):311–324. doi: 10.1002/(SICI)1096-9861(19960722)371:2<311::AID-CNE10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Weinstein BE, Ventry IM. Hearing impairment and social isolation in the elderly. J Speech Hear Res. 1982;25:593–599. doi: 10.1044/jshr.2504.593. [DOI] [PubMed] [Google Scholar]
- Willott JF. Aging and the Auditory System. San Diego: Singular; 1991. [Google Scholar]
- Winer JA. The fuctional architecture of the medial geniculate body and the primary auditory cortex. In: Webster DB, Popper AN, Fay RR, editors. Springer Handbook of Auditory Research, The Mammalian Auditory Pathways: Neuroanatomy. Vol. 1. New York: Springer-Verlag; 1992. pp. 222–409. [Google Scholar]
- Winer JA, Larue DT. Populations of GABAergic neurons and axons in layer I of rat auditory cortex. Neuroscience. 1989;33(3):499–515. doi: 10.1016/0306-4522(89)90402-8. [DOI] [PubMed] [Google Scholar]
- Zilles K. The cortex of the rat: a stereotaxic atlas. Berlin: Springer-Verlag; 1985. [Google Scholar]
- Zilles K, Wree A. Cortex: areal and laminar structure. In: Paxinos G, editor. The Rat Nervous System. 2nd Ed. New York: Academic Press; 1995. pp. 649–685. [Google Scholar]