Abstract
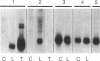
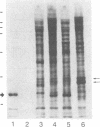
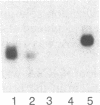
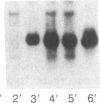
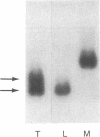
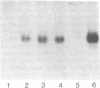
Serum amyloid A (SAA) is a small (12 kDa) acute-phase apoprotein of high density lipoprotein found in mammals. It is also the precursor to amyloid protein A, the main protein constituent of fibrils found in amyloidosis secondary to chronic or recurrent inflammation--e.g., rheumatoid arthritis. However, rats do not develop amyloidosis and SAA is not an apoprotein of rat high density lipoprotein; thus rats appear to be an exception in regard to expression of SAA genes. We report here that rats do have representatives of the SAA gene family and express two distinct SAA mRNAs. Moreover, the pattern of genes expressed among tissues, and their induction by inflammatory agents, is similar to that of related mouse genes. RNA from various tissues of normal and injured rats was examined by RNA blot hybridization with SAA cDNA and complementary RNA probes for the three murine SAA genes. A SAA mRNA of approximately 400 nucleotides related to mouse SAA1 and SAA2 mRNAs reached a high level in liver 24 hr after injection of bacterial lipopolysaccharide. No extra-hepatic tissues were found to express the SAA1/SAA2-related mRNA. Turpentine induced two hepatic SAA1/SAA2-related mRNAs of approximately 400 and approximately 500 nucleotides in length. Liver SAA1/SAA2-related mRNA hybrid selected and translated in a wheat germ protein-synthesizing system, from lipopolysaccharide- and turpentine-injected rats, produced a single protein with an estimated molecular mass of 8 kDa. This rat liver SAA-related mRNA appears to lack a highly conserved coding region for portions of two amphipathic helical domains and the joining sequence. An mRNA related to mouse SAA3 was found expressed at a high level in lung after lipopolysaccharide but not following turpentine injection. This mRNA was also expressed at high levels in ileum and large intestine of control rats and was not found in the liver of control or challenged rats. These observations show that the SAA gene family is present and expressed in rats and that its expression is found under situations similar to those found in mice. This lends support for the importance of the SAA gene family in the response to injury by vertebrates.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amasino R. M. Acceleration of nucleic acid hybridization rate by polyethylene glycol. Anal Biochem. 1986 Feb 1;152(2):304–307. doi: 10.1016/0003-2697(86)90413-6. [DOI] [PubMed] [Google Scholar]
- Baltz M. L., Rowe I. F., Caspi D., Turnell W. G., Pepys M. B. Acute-phase high-density lipoprotein in the rat does not contain serum amyloid A protein. Biochem J. 1987 Feb 15;242(1):301–303. doi: 10.1042/bj2420301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausserman L. L., Herbert P. N., McAdam K. P. Heterogeneity of human serum amyloid A proteins. J Exp Med. 1980 Sep 1;152(3):641–656. doi: 10.1084/jem.152.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Amyloid protein SAA is associated with high density lipoprotein from human serum. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4025–4028. doi: 10.1073/pnas.74.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N., Hanson R. H. Amyloid protein SAA is an apoprotein of mouse plasma high density lipoprotein. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4092–4096. doi: 10.1073/pnas.76.8.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N., Hermodson M. A., Ericsson L. H. The major proteins of human and monkey amyloid substance: Common properties including unusual N-terminal amino acid sequences. FEBS Lett. 1971 Dec 1;19(2):169–173. doi: 10.1016/0014-5793(71)80506-9. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N., Meek R. L. Serum amyloid A protein. Methods Enzymol. 1988;163:510–523. doi: 10.1016/0076-6879(88)63047-3. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Dwulet F. E., Benson M. D. Primary structure of amyloid fibril protein AA in azocasein-induced amyloidosis of CBA/J mice. J Lab Clin Med. 1987 Sep;110(3):322–329. [PubMed] [Google Scholar]
- Ericsson L. H., Eriksen N., Walsh K. A., Benditt E. P. Primary structure of duck amyloid protein A. The form deposited in tissues may be identical to its serum precursor. FEBS Lett. 1987 Jun 22;218(1):11–16. doi: 10.1016/0014-5793(87)81008-6. [DOI] [PubMed] [Google Scholar]
- Hoffman J. S., Benditt E. P. Changes in high density lipoprotein content following endotoxin administration in the mouse. Formation of serum amyloid protein-rich subfractions. J Biol Chem. 1982 Sep 10;257(17):10510–10517. [PubMed] [Google Scholar]
- Husebekk A., Skogen B., Husby G., Marhaug G. Transformation of amyloid precursor SAA to protein AA and incorporation in amyloid fibrils in vivo. Scand J Immunol. 1985 Mar;21(3):283–287. doi: 10.1111/j.1365-3083.1985.tb01431.x. [DOI] [PubMed] [Google Scholar]
- Kluve-Beckerman B., Dwulet F. E., Benson M. D. Human serum amyloid A. Three hepatic mRNAs and the corresponding proteins in one person. J Clin Invest. 1988 Nov;82(5):1670–1675. doi: 10.1172/JCI113779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Levin M., Franklin E. C., Frangione B., Pras M. The amino acid sequence of a major nonimmunoglobulin component of some amyloid fibrils. J Clin Invest. 1972 Oct;51(10):2773–2776. doi: 10.1172/JCI107098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell C. A., Potter D. A., Stearman R. S., Morrow J. F. Structure of the murine serum amyloid A gene family. Gene conversion. J Biol Chem. 1986 Jun 25;261(18):8442–8452. [PubMed] [Google Scholar]
- Lowell C. A., Stearman R. S., Morrow J. F. Transcriptional regulation of serum amyloid A gene expression. J Biol Chem. 1986 Jun 25;261(18):8453–8461. [PubMed] [Google Scholar]
- Meek R. L., Benditt E. P. Amyloid A gene family expression in different mouse tissues. J Exp Med. 1986 Dec 1;164(6):2006–2017. doi: 10.1084/jem.164.6.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek R. L., Hoffman J. S., Benditt E. P. Amyloidogenesis. One serum amyloid A isotype is selectively removed from the circulation. J Exp Med. 1986 Mar 1;163(3):499–510. doi: 10.1084/jem.163.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. F., Stearman R. S., Peltzman C. G., Potter D. A. Induction of hepatic synthesis of serum amyloid A protein and actin. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4718–4722. doi: 10.1073/pnas.78.8.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmelee D. C., Titani K., Ericsson L. H., Eriksen N., Benditt E. P., Walsh K. A. Amino acid sequence of amyloid-related apoprotein (apoSAA1) from human high-density lipoprotein. Biochemistry. 1982 Jul 6;21(14):3298–3303. doi: 10.1021/bi00257a008. [DOI] [PubMed] [Google Scholar]
- Ramadori G., Sipe J. D., Colten H. R. Expression and regulation of the murine serum amyloid A (SAA) gene in extrahepatic sites. J Immunol. 1985 Dec;135(6):3645–3647. [PubMed] [Google Scholar]
- Schreiber G., Aldred A. R., Thomas T., Birch H. E., Dickson P. W., Tu G. F., Heinrich P. C., Northemann W., Howlett G. J., de Jong F. A. Levels of messenger ribonucleic acids for plasma proteins in rat liver during acute experimental inflammation. Inflammation. 1986 Mar;10(1):59–66. doi: 10.1007/BF00916041. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Morrisett J. D., Gotto A. M., Jr A molecular theory of lipid-protein interactions in the plasma lipoproteins. FEBS Lett. 1974 Jan 15;38(3):247–258. doi: 10.1016/0014-5793(74)80064-5. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Pownall H. J., Jackson R. L., Glenner G. G., Pollock P. S. Amyloid A: amphipathic helixes and lipid binding. Biochemistry. 1976 Jul 27;15(15):3187–3191. doi: 10.1021/bi00660a005. [DOI] [PubMed] [Google Scholar]
- Sletten K., Husebekk A., Husby G. The amino acid sequence of an amyloid fibril protein AA isolated from the horse. Scand J Immunol. 1987 Jul;26(1):79–84. doi: 10.1111/j.1365-3083.1987.tb02237.x. [DOI] [PubMed] [Google Scholar]
- Stearman R. S., Lowell C. A., Peltzman C. G., Morrow J. F. The sequence and structure of a new serum amyloid A gene. Nucleic Acids Res. 1986 Jan 24;14(2):797–809. doi: 10.1093/nar/14.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Migita S. Complete primary structures of two major murine serum amyloid A proteins deduced from cDNA sequences. Proc Natl Acad Sci U S A. 1985 May;82(9):2915–2919. doi: 10.1073/pnas.82.9.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]