Abstract
Interleukin (IL)-15 contributes to the immunopathogenesis of Celiac disease (CD). However, it is not clear how IL-15 affects APC that shape adaptive immune responses to the dietary antigen, gliadin. Using PBMC from healthy individuals, we show that monocytes differentiated with IL-15 (IL15-DC) produced IL-1β, IL-6, IL-15, IL-23, TNFα and CCL20 in response to pepsin-trypsin digested gliadin (PTG) and activated contact-dependent Th17 and Th1 responses from autologous CD4+ T cells. Lower concentrations of IL-15 augmented IFNγ responses to PTG in PBMC from CD patients compared to controls. Thus, IL-15 supports Th17 and Th1 responses to a dietary antigen that is normally well-tolerated in healthy individuals by generating IL15-DC. These potentially pathogenic immune responses may result in CD patients and not healthy individuals as a consequence of IL-15 hypersensitivity. Therefore, genetic and/or environmental factors that control IL-15 expression and responsiveness in the intestine likely participate in the pathogenesis of CD.
Keywords: IL-15, IL-23, innate immunity, Th17, Th1, Celiac Disease, autoimmunity
Introduction
Th17 cells are memory CD4+ T cells implicated in the pathogenesis of an increasing number of tissue-specific autoimmune diseases [1–6]. Circulating human Th17 cells are characterized by the expression of the surface markers CD45RO, CD161, IL-23R, CCR6 and CCR4 [7, 8]. Th17 cells are thought to perpetuate inflammation by secreting the proinflammatory mediators IL-17, IL-21, IL-22 and IFNγ that act on immune and nonimmune cells within the local tissue [8]. Although it is not yet clear how human Th17 cells develop from naïve CD4+ T cells in vivo, in vitro studies indicate that memory Th17 responses are induced by interactions with activated monocytes or their CD14+ tissue progeny and the proinflammatory mediators IL-1β, IL-6, IL-15, IL-23, TNFα and CCL20 that are produced in response to danger signals [3, 9–13]. Surprisingly, the relationship between Th17 cells and IL-15 was identified in rheumatoid arthritis (RA) before the discovery of IL-23 and the Th17 hypothesis, and yet the role of IL-15 in the generation, activation and/or expansion of this T helper subset remains to be determined [1, 14].
IL-15 is a pleiotropic cytokine related to innate immunity, which has been associated with numerous inflammatory conditions, including Celiac disease (CD), Crohn s disease, psoriasis, RA, type 1 diabetes and multiple sclerosis [3, 15–19]. IL-15 provides activating and survival signals to CTL and is thought to indirectly modulate CD4+ T cell responses by acting on APC [20, 21]. During inflammation, monocytes infiltrate the affected tissue and differentiate into various types of inflammatory APC depending on the cytokine milieu. In the presence of IL-15, monocytes differentiate into potent APC that exhibit a unique phenotype, coexpressing surface markers thought to distinguish monocytes/macrophages (CD14) from myeloid (CD86, CD209) and plasmacytoid (BDCA2, CD123) DC [21, 22]. Therefore, IL-15 in the inflamed tissues of individuals with autoimmune diseases may promote Th17 responses by generating this unique subset of CD14+ APC [10, 23, 24].
Among the spectrum of autoimmune disorders associated with IL-15 and the Th17 axis, CD is the only one in which the major genetic (HLA-DQ2/8) and environmental factors (dietary glutens) are known [2, 6, 25]. CD is an enteropathy generally considered to be mediated by gliadin-specific IFNγ-producing CD4+ T cells that are restricted by the disease associated alleles, HLA-DQ2 and HLA-DQ8 [26]. Still, the majority of HLA-DQ2+/8+ individuals (~35% of the general U.S. population) tolerate dietary glutens [27] and “humanized mice” engineered to express HLA-DQ2 did not develop the intestinal pathology that is seen in CD even though gliadin-specific CD4+ T cell responses occurred in these animals [28]. Thus, factors in addition to CD4+ T cells that recognize peptide restricted HLA alleles are likely required for CD. Accumulating evidence indicate that IL-15 is one of these factors.
Increased levels of IL-15 have been detected in the intestinal epithelium and lamina propria of patients with untreated CD compared to patients in remission and healthy controls [29]. Furthermore, it has been shown that the p31–43 peptide in α-gliadin rapidly induced CD68+ APC to produce IL-15 in intestinal explants from CD patients and not healthy controls. Importantly, this IL-15 response was found to modulate IEL-mediated epithelial destruction and Th1 activation ex vivo [25]. More recently, the pathologic features of CD were recapitulated in the proximal small intestine of mice engineered to over express IL-15 in enterocytes in vivo [30]. Together, these findings indicate that production of IL-15 in an unidentified subset of CD68+ (monocyte-derived) APC in the mucosa is fundamental to the pathogenesis of CD.
Although augmented levels of IL-15, increased numbers of CD14+ cells and higher expression of Th17 and Th1 promoting factors have been described in the intestine of untreated CD by independent reports, a direct relationship between these separate observations has not been examined [2, 6, 24, 29, 31].
We previously reported that a pepsin-trypsin digest of gliadin (PTG) induced PBMC from patients with CD as well as PBMC from healthy controls to secrete cytokines associated with the generation of Th17 cell responses [32]. The difference between PBMC from healthy individuals compared to PBMC from CD patients was significantly higher levels of proinflammatory cytokines produced by the latter. In addition, we determined that circulating monocytes (CD14+) were the source of the observed cytokine profile induced by PTG. These observations together with the data cited above regarding IL15-DC and the key role of IL-15 in the pathogenesis of CD, prompted us to test the hypothesis that IL-15 promotes Th17 and Th1 responses by differentiating monocytes into IL15-DC (CD14+) that produce activating signals upon encounter with PTG. For practical reasons, the majority of these studies were carried out using PBMC from healthy individuals since we found similar qualitative responses with these cells to PTG as that found in PBMC from CD patients. The rationale for using monocytes is justified by the accumulation of monocyte-derived cells that has been described in the inflamed intestine of CD patients on a gluten-containing diet [24, 31].
Monocytes differentiated into CD11c+CD14+CD209+HLA-DQ+ cells in the presence of GM-CSF and IL-15 (IL15-DC) and produced IL-15, IL-23 and other memory Th17 and Th1 promoting cytokines in response to PTG. Conversely, monocytes that were differentiated into immature DC (CD11c+CD14−CD209+) with GM-CSF and IL-4 (IL4-DC) did not. The IL15-DC loaded with PTG induced secretion of IL-17 and IFNγ in autologous CD4+ T cells from healthy individuals, which normally tolerate this common dietary antigen. Secretion of IL-17 and IFNγ was cell-contact dependent and in addition, IFNγ production required surface expression of IL-15, similar to the mechanism reported in RA by Miranda-Carus et al [19]. Notably, PBMC from CD patients had significantly higher IFNγ responses to PTG compared to PBMC from controls when exposed to 10-fold lower levels of IL-15. Together, these results indicate that IL-15 supports Th17 and Th1 responses by skewing monocytes into IL15-DC (CD14+ APC) and in addition, offer insight as to how IL-15 expression and responsiveness may orchestrate the immune response to gliadin in individuals with and without CD.
Materials and methods
Cells
PBMC from CD patients and healthy donors were isolated by density gradient centrifugation in Lymphocyte Separation Medium (ICN Biomedicals Inc.) according to manufacturer s instructions. Purified lymphocytes and monocytes were obtained from healthy donors as above followed by countercurrent centrifugal elutriation. All individuals gave informed consent. The study protocol was approved by the Institutional Review Board at the University of Maryland School of Medicine.
DNA Extraction and HLA Typing
DNA was extracted from PBMC using the QIAamp DNA Mini Kit (Qiagen). HLA alleles were determined by One Lambda Micro SSP™ ABDR Typing Kit (One Lambda) and DQA1 and DQB1 SSP UniTray® Kits (Dynal Biotech) per manufacturers instructions.
Reagents
PTG was prepared by enzymatic digestion as follows. 50g of crude gliadin (Sigma) was dissolved in 500ml of 0.2 N HCl for 2h at 37°C with 1g of pepsin (Sigma). The resulting product was further digested by addition of 1g of trypsin (Sigma) and the pH adjusted to 7.4 with 2 M NaOH. The solution was stirred vigorously at 37°C for 4h, boiled (100°C) for 30min, freeze-dried, lyophilized in 10mg aliquots, and stored at −20°C. Endotoxin contamination was ruled out using the Limulus amebocyte assay and treatment of PTG with endotoxin neutralizing protein (Sigma).
Flow Cytometry
Cells were stained with the following fluorochrome-conjugated antibodies per standard flow cytometry methods. Anti-human CD11c, CD14, CD16, CD23, CD32, CD40, CD64, CD80, CD83, CD86, CD89, HLA-DQ, HLA-DR, TLR2 (BD Pharmingen), TLR4 (eBiosciences), CD209, IL-15 (R&D systems) or appropriate isotype controls (BD Pharmingen).
Cell cultures
CD4+ T cells (95% pure) were isolated by magnetic cell separation using the CD4+ T cell Isolation Kit II (Miltenyi). To generate IL4-DC and IL15-DC, elutriated monocytes were cultured in cRPMI (RPMI-1640, 10% FBS, 1%L-glutamine, 1%Pen-Strep and 20mM Hepes) (Invitrogen) with 100ng/ml GM-CSF and 20ng/ml IL-4 or GM-CSF and 200ng/ml IL-15 (R&D Systems) for 72h. Cells were phenotyped by flow cytometry or incubated with and without 100μg/ml PTG with and without autologous CD4+ T cells at a 1:1 ratio for 24–72h. 0.4μm transwell inserts (Sigma) or human αIL-15 or mIgG1 (R&D Systems) were also included in cocultures with IL15-DC and PTG for 72h. PBMC were incubated in cRPMI with and without 100μg/ml PTG in the presence and absence of 10–100ng/ml IL-15 (R&D Systems) for 72h.
Cytokine & chemokine analysis
Cell-free culture fluids were analyzed for IL-1β, IL-6, IL-12p70, IL-17, IL-21, IL-23, IFNγ, TNFα (eBiosciences ELISA kit) and IL-22 (Quantikine ELISA kit, R & D Systems) following the manufacturers protocols.
Statistical analyses
Data are presented as mean values +SD Paired two-tailed Student s t tests were used to calculate p values within the same individuals and unpaired two-tailed Student s t tests were used to calculate p values between study groups. p values < 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Distinct phenotype of monocytes differentiated with IL-15
Increased numbers of monocyte-derived cells that express CD11c, CD14, CD16, CD209 and HLA-DQ have been detected in the gut of untreated CD patients compared to treated patients in remission and healthy controls [24, 31]. Since higher levels of IL-15 have been reported in these tissues, we tested the hypothesis that an environment rich in IL-15 would generate a population of CD11c+CD14+CD16+CD209+HLA-DQ+ APC from circulating monocytes. To test this, elutriated monocytes from healthy individuals were cultured with GM-CSF and IL-15 (IL15-DC) or GM-CSF and IL-4 (IL4-DC) for 72h and harvested for phenotypic analysis.
Similar to their monocyte precursors, IL15-DC expressed CD14, CD16, CD32, CD64, CD89 and TLR2, whereas IL4-DC down regulated all of these surface receptors (Fig. 1a) and displayed lower levels of TLR4 than IL15-DC (Fig. 1b). Both types of DC upregulated CD40, CD209 and HLA-DQ compared to their monocyte precursors, however, higher densities of CD40 and HLA-DQ and reduced frequencies of CD209 were detected on IL15-DC compared to IL4-DC (Fig. 1b). Expression of CD80, CD86 and HLA-DR was similar in IL15-DC and IL4-DC and both expressed the monocyte marker CD68 (data not shown). These data suggest that IL-15 may be responsible for the accumulation of CD11c+CD14+CD68+ cells that has been reported in the gut of untreated CD patients [24, 31] and in addition, may offer insight into the CD14+ APC that have been implicated in other chronic inflammatory disorders, such as Crohn s disease [12], psoriasis [33] and RA [10].
Figure 1. IL15-DC exhibit features of DC and their monocyte precursors.
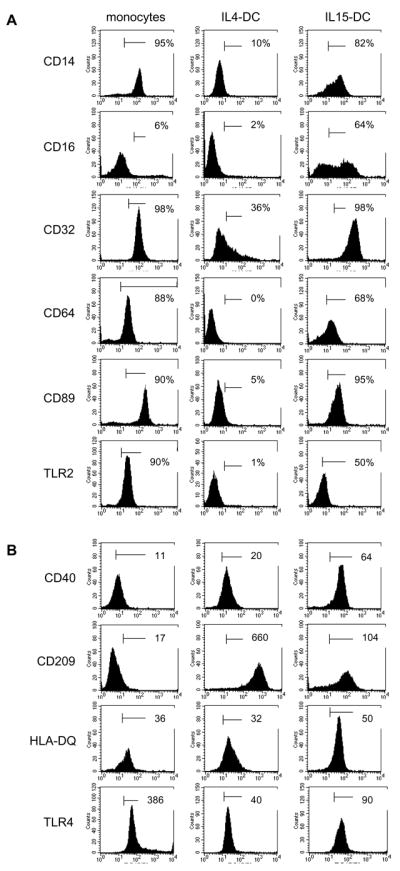
Elutriated monocytes were stained immediately or were cultured with GM-CSF plus IL-4 or IL-15 for 72h. Cells were labeled with anti-human mAbs or appropriate isotype controls for flow cytometric analysis. Markers were set by isotype overlay. A, Numbers indicate % positive cells. B, Numbers are MFI because the % of cells staining positive for these markers were similar between IL4DC and IL15-DC. Histograms are representative of 5 independent experiments.
IL15-DC produce mediators related to the Th17 axis in response to PTG
We were the first to suggest that the IL-23 response pathway may be involved in the pathogenesis of CD [32]. Moreover, we showed that monocytes and not their IL4-DC progeny have the capacity to secrete IL-23 and other Th17 promoting factors in response to PTG [32]. Subsequent studies have reported increased levels of IL-23 and other proinflammatory mediators that polarize Th17 in the intestine of untreated CD patients, providing in vivo evidence that the IL-23 response pathway is involved in the disease process [2]. Still, the cell types and receptor-ligand interactions that initiate this response in the gut of patients have not been established. Since IL15-DC retain many features of their monocyte precursors and have been reported to express IL-1β and IL-15 in response to danger signals [21, 22], we predicted that IL15-DC would produce IL-23 and related proinflammatory mediators upon exposure to PTG. To test this, IL15-DC or IL4-DC were incubated with or without PTG for 20h. Cells and the culture supernatants were harvested for flow cytometric and cytokine analysis, respectively.
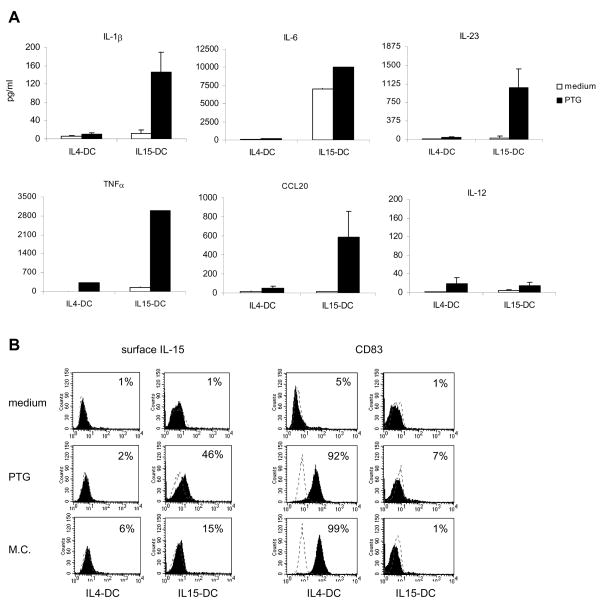
As anticipated, PTG stimulated higher levels of IL-1β, IL-6, IL-23, TNFα and CCL20 secretion from IL15-DC as compared to that produced by IL4-DC (Fig. 2a). In addition, IL-12 was not detected in any of the culture fluids (Fig. 2a). Further analysis revealed that PTG also stimulated IL15-DC to produce IL-15 on the cell surface and IL4-DC to upregulate the maturation marker CD83 (Fig. 2b). Based on these findings, we speculate that IL15-DC may preferentially induce memory Th17 and Th1 responses [14, 34].
Figure 2. PTG stimulates IL15-DC to secrete IL-1β, IL-6, IL-23, TNFα and CCL20 and produce surface IL-15.
IL15-DC or IL4-DC were incubated with and without 100μg/ml PTG for 20h. A, Cell-free culture fluids were collected and analyzed for secreted IL-1β, IL-6, IL-23, TNFα, CCL20 and IL-12 by ELISAs. IL-1β, IL-23, CCL20 and IL-12 data are the means of 3–5 independent experiments tested in duplicate, IL-6 and TNFα data are from 1 of the donors tested. B, Cells were harvested and labeled with anti-human IL-15, CD83 or isotype controls (dashed line) and analyzed by flow cytometry. M.C. maturation cocktail (IL-1β, IL-6, TNFα and PGE2) served as a positive control for CD83 on IL4-DC. Histograms are representative of 3 different donors tested in duplicate and numbers signify % positive cells.
We would like to point out that these same responses, which occurred in subsets derived from healthy donor monocytes, have been demonstrated in intestinal explants from CD patients, wherein the p31-43 α-gliadin peptide induced expression of IL-15 in CD68+ APC and CD83 in CD86+ APC [25]. Given that CD68+ cells that coexpress CD14 have also been described in the active celiac lesion [24, 31], we propose that IL15-DC are representative of the CD68+ subset of intestinal APC in untreated CD. Such inflammatory APC could drive intestinal inflammation by; 1) stimulating further production of IL-15 by intestinal epithelial cells or APC [22]; 2) activating or maturing neighboring APC [25]; 3) recruiting, activating and/or maintaining cytotoxic IEL [25]; 4) inhibiting the suppressive function of regulatory T cells [35–37] or 5) driving memory subsets of Th17 and Th1 cells [9, 14, 38].
IL15-DC exposed to PTG stimulate Th1 and Th17 responses
In attempt to gain a better understanding of the relationship between IL-15 and Th17 responses, our investigation focused on the capacity of IL15-DC to generate Th17 and/or Th1 responses upon encounter with PTG. Untouched CD4+ T cells were purified from PBMC of healthy individuals and analyzed by flow cytometry for Th17 markers or cultured with or without autologous IL15-DC or IL4-DC at a 1:1 ratio in the presence or absence of PTG for 3 days. The supernatants were assessed for IL-17, IL-21, IL-22, IFNγ as well as the cytokines that promote Th17 and Th1 responses.
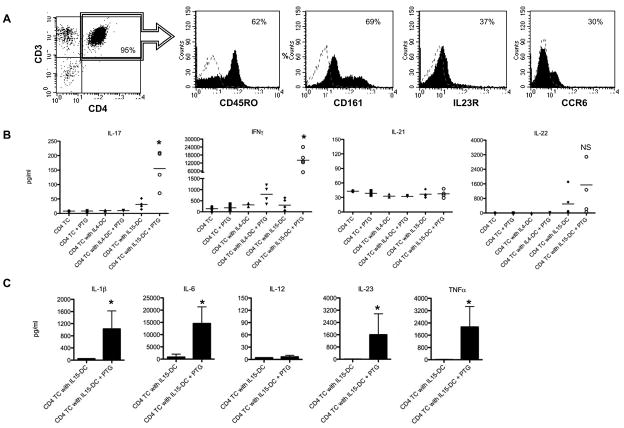
Importantly, the CD4+ T cells isolated from all donors tested contained cells expressing the Th17 markers CD45RO, CD161, IL-23R and CCR6 [7, 8] (Fig. 3a). PTG triggered secretion of IL-17 and IFNγ in T cell cultures with IL15-DC (Fig. 3b) as well as the related mediators IL-1β, IL-6, IL-23 and TNFα (Fig. 3c). IL-21 secretion was not induced by PTG in any of the donors tested (Fig. 3b). This is not surprising, given that IL-12 was not increased in any of the conditions tested and IL-21 is thought to be involved in the IL-12-dependent differentiation of naïve CD4+ T cells [39] (Fig. 3c and 2a). Surprisingly, IL-22 was spontaneously produced in some of the cocultures with IL15-DC and in others, PTG stimulation was required (Fig. 3b). This may be explained by the relative amounts of IL-6 and TNFα in the respective supernatants, since these two cytokines were recently demonstrated to induce IL-22 secretion from human CD4+ T cells [40]. Of the healthy individuals tested, only one expressed the HLA-DR3/DQ2 haplotype that is associated with the disease, illustrating that the IL-17 and IFNγ responses that were induced by activated IL15-DC were not restricted by HLA-DQ2. There are several possible explanations for this, which we are currently exploring; 1) the individuals tested have gliadin-specific T cells that are restricted by other HLA class II alleles, which has been reported previously [41], 2) Th17 and Th1 responses occurred independent of TCR specificity, similar to what has been demonstrated for memory CTL [20, 21], or 3) PTG acted as an adjuvant similar to TLR agonists, providing the necessary danger signals to induce antigen-specific Th17 and Th1 responses to self or other foreign antigens already loaded in the HLA molecules of IL15-DC.
Figure 3. IL15-DC induce IL-17 and IFNγ secretion in cultures with autologous CD4+ T cells and PTG.
A, CD4+ T cells were purified by magnetic cell separation and analyzed by flow cytometry for the presence of Th17 surface markers. Dashed histograms are isotype controls and numbers are % positive cells. Histograms from 1 of 4 independent experiments are shown. B, CD4+ T cells were cultured alone or together with autologous IL15-DC or IL4-DC with and without 100μg/ml PTG for 72h. Cell-free supernatants were tested for secreted IL-17, IFNγ IL-21 and IL-22 by ELISA. Data are the means of 4–5 different donors tested in duplicate. C, The levels of IL-1β, IL-6, IL-12, IL-23 and TNFα were analyzed in the culture fluids from IL15-DC and CD4+ T cells incubated with and without 100μg/ml PTG for 72h by ELISA. Shown are the means of 4 independent experiments tested in duplicate. *, p<0.05 between T cell cultures with IL15-DC +/− PTG
Taken together, these results indicate that IL15-DC are likely responsible for the Th17 and Th1 cytokine profiles that have been described in the intestine of untreated CD patients [2, 6]. To our knowledge, IL-22 has not yet been described in CD; however, our data suggest that it may be involved in the disease process. Increased levels of IL-22 have been detected in Crohn s disease and psoriasis, which target the intestine and skin, respectively [8, 40]. Given that CD effects the small intestine and can include the skin manifestation, dermatitis herpetiformis, it will be particularly interesting to compare the relative contributions of IFNγ, IL-17 and IL-22 producing Th subsets in these cases.
Th17 and Th1 responses induced by PTG require contact with IL15-DC
In addition to the release of proinflammatory cytokines, we discovered that IL15-DC express surface IL-15 upon encounter with PTG (Fig 2b). Since transpresentation of IL-15 is a potent stimulator of IFNγ-producing memory CTL [20, 21], we considered the possibility that the Th1 and/or Th17 response to PTG required CD4+ T cell contact with IL-15 on the surface of IL15-DC similar to what has recently been described for fibroblast-like cells isolated from the synovium of RA patients [36]. Thus, we set up the same experimental conditions described above and added a blocking antibody to IL-15 or the isotype control to cocultures incubated with PTG. In addition, CD4+ T cells were plated in 0.4μm semi-permeable transwell inserts to prevent cell contact with IL15-DC stimulated with PTG. In other experiments, CD4+ T cells were cultured with conditioned media from IL15-DC incubated with or without PTG to further assess the role of soluble cytokines in the activation process. As before, culture fluids were collected after 3 days and assessed for IL-17 and IFNγ.
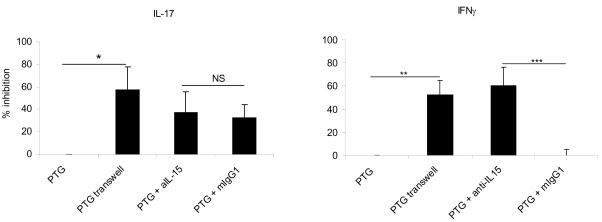
Transwell inserts significantly reduced the levels of IL-17 and IFNγ demonstrating that cell contact with IL15-DC was necessary for IL-17 and IFNγ responses (Fig. 4). Moreover, the conditioned media from IL15-DC stimulated with PTG did not recapitulate the IL-17 or IFNγ responses, providing further evidence that cell-cell interactions are important for induction of these cytokine responses (negative data not shown). However, soluble factors may enhance IL-17 and IFNγ responses since they were not totally blocked by transwell inserts. We are currently investigating the possibility that this may be attributed to IL-23, since this cytokine is an important mediator of memory Th1 and Th17 responses [14, 34]. Antibody blockade of IL-15 was as equally effective at inhibiting IFNγ production as transwell inserts in all individuals tested, strongly suggesting that surface IL-15 stimulates secretion of this cytokine (Fig. 4). In contrast, anti-IL-15 treatment only partially reduced the IL-17 response induced by PTG in two of the donors tested; indicating that surface expression of IL-15 may not be required for IL-17 secretion under all circumstances. Therefore, separate, though potentially overlapping, contact-dependent mechanisms appear to control the production of IL-17 and IFNγ in these cocultures. Studies are underway to sort out whether the observed IL-17 and IFNγ responses are being produced by the same subset or distinct Th17 and Th1 cells, respectively. We anticipate that the results will expand upon the current knowledge of the immunobiology of Th17 cells and hopefully resolve some of the controversy regarding the function of memory Th1 vs. Th17 in human autoimmune diseases.
Figure 4. Th17 and Th1 responses induced by PTG are contact-dependent; IFNγ requires contact with IL-15 on the surface of IL15-DC.
IL15-DC were cultured with autologous CD4+ T cells in the presence or absence of 100μg/ml PTG for 72h. In addition, IL15-DC and autologous CD4+ T cells were cultured using 0.4μm semi-permeable transwell inserts to separate the cell populations with 100μg/ml PTG or were incubated together with PTG in the presence and absence of 10μg/ml anti-human IL-15 or mIgG1 for 72h. All culture fluids were harvested and analyzed for IL-17 and IFNγ production by ELISA. The percent inhibition was calculated for each of the 5 different donors tested. Shown are the means of 5 independent experiments tested in duplicate. *, p<0.05 **, p<0.005 ***, p<0.0005
PBMC from CD patients are hypersensitive to IL-15
Besides the increased levels of IL-15 associated with untreated CD, there is also evidence that significantly less IL-15 is required to enhance production of nitrites and IFNγ in intestinal leukocytes from CD patients compared to that from healthy controls [15, 29]. Since a relatively high concentration of IL-15 (200ng/ml) was used to generate IL15-DC from healthy individuals, we predicted that lower levels of IL-15 might support proinflammatory cytokine responses in PBMC from CD patients exposed to PTG. PBMC from individuals with and without CD were exposed to PTG in the presence or absence of increasing doses of IL-15 for 72h and the culture fluids analyzed for IFNγ.
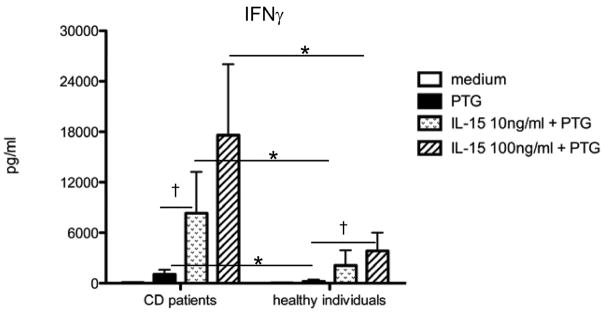
In the absence of IL-15, PTG stimulated IFNγ secretion from CD patients and not healthy individuals, which is in agreement with other reports [42]. Interestingly, IL-15 enhanced the IFNγ response induced by PTG in all individuals tested, however, the IL-15 response threshold from CD patients proved to be 10-fold lower than that of individuals without the disease (Fig. 5). The importance of these findings are 2-fold; 1) they indicate that significantly less IL-15 drives potentially pathogenic immune responses in CD patients exposed to PTG compared to individuals that tolerate PTG and 2) they show that PBMC are capable of recapitulating the IL-15-dependent IFNγ response that has been reported for intestinal leukocytes from CD patients and controls [15]. Since there is currently no animal model of CD and in vivo studies of CD are quite restricted, our results provide a feasible alternative for examining the dynamics that control IL-15 responsiveness in HLA-DQ2+/8+ individuals with and without CD [15, 29]. Given the fundamental role of regulatory T cells in peripheral tolerance and the ability of IL-15 to inhibit the suppressive function of these cells [36, 37], we are currently examining the relationship between IL-15 and IL-15R expression, effector T cell responses and regulatory T cell function in this in vitro system.
Figure 5. PBMC from CD patients are hyperresponsive to IL-15 compared to PBMC from healthy individuals.
PBMC from 5 individuals with CD and 5 healthy individuals (HD) were incubated with and without 100μg/ml gliadin (PTG) in the presence and absence of 10 or 100ng/ml IL-15 for 72h. Cell-free culture supernatants were collected and analyzed for IFNγ by ELISA. Background levels of IFNγ stimulated by IL-15 without PTG were subtracted from IL-15 conditions with PTG. †, p<0.05 differences between conditions within CD or HD *, p<0.05 differences between CD and HD.
Concluding remarks
In summary, our studies demonstrate for the first time that IL-15 promotes Th17 and Th1 responses to PTG, a dietary antigen that is normally well-tolerated in healthy individuals by differentiating monocytes into IL15-DC (CD14+CD68+ APC). These novel findings may help explain why healthy individuals (including HLA-DQ2+/8+), which transiently express IL-15 upon enteric exposure to this dietary antigen [15], have few mucosal CD14+CD68+ APC [24, 31], do not express Th1 and Th17 profiles in their intestine [2, 6] and do not develop CD. Moreover, it is conceivable that the difference in IL-15 responsiveness, which we and others have observed, magnifies the proinflammatory responses in the gut of CD patients beyond the point of control by the counteractive immunoregulatory mechanisms that restore intestinal homeostasis to PTG and related dietary antigens in healthy individuals, such as regulatory T cells, IL-10 and TGFβ [35, 37]. Collectively, these observations indicate that genetic and/or etiologic factors that determine IL-15 sensitivity may provide attractive new targets for the treatment and/or prevention of this disease and potentially other autoimmune disorders involving IL-15 and cell-mediated tissue destruction.
Acknowledgments
We would like to thank the Center for Celiac Disease Research for providing PBMC from CD patients and healthy controls and the pepsin-trypsin digest of gliadin (PTG). This research was supported by an Internal Research Grant from the Department of Pathology at the University of Maryland School of Medicine to D.M. as well as a National Institutes of Health Grant DK078699 to A.F. K.H. and D.M. have no relationships to declare. A.F. is stock holder of ALBA Therapeutics (Baltimore, MD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ziolkowska M, Koc A, Luszczykiewicz G, Ksiezopolska-Pietrzak K, Klimczak E, Chwalinska-Sadowska H, Maslinski W. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol. 2000;164:2832–2838. doi: 10.4049/jimmunol.164.5.2832. [DOI] [PubMed] [Google Scholar]
- 2.Castellanos-Rubio A, Santin I, Irastorza I, Castano L, Carlos Vitoria J, Ramon Bilbao J. TH17 (and TH1) signatures of intestinal biopsies of CD patients in response to gliadin. Autoimmunity. 2009;42:69–73. doi: 10.1080/08916930802350789. [DOI] [PubMed] [Google Scholar]
- 3.Elder JT. IL-15 and psoriasis: another genetic link to Th17? J Invest Dermatol. 2007;127:2495–2497. doi: 10.1038/sj.jid.5700855. [DOI] [PubMed] [Google Scholar]
- 4.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sapone A, Lammers KM, Giuseppe M, Mikhailenko I, Carteni M, Casolaro V, Fasano A. Differential Mucosal IL-17 Expression in Two Gliadin-induced Disorders: Gluten Sensitivity and the Autoimmune Enteropathy Celiac Disease. Int Arch Allergy Immunol. 2010 doi: 10.1159/000260087. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 8.Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, Raskin L, Desai B, Faubion WA, de Waal Malefyt R, Pierce RH, McClanahan T, Kastelein RA. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoeve MA, Savage ND, de Boer T, Langenberg DM, de Waal Malefyt R, Ottenhoff TH, Verreck FA. Divergent effects of IL-12 and IL-23 on the production of IL-17 by human T cells. Eur J Immunol. 2006;36:661–670. doi: 10.1002/eji.200535239. [DOI] [PubMed] [Google Scholar]
- 10.Evans HG, Gullick NJ, Kelly S, Pitzalis C, Lord GM, Kirkham BW, Taams LS. In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. Proc Natl Acad Sci U S A. 2009;106:6232–6237. doi: 10.1073/pnas.0808144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc Natl Acad Sci U S A. 2007;104:17034–17039. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamada N, Hisamatsu T, Honda H, Kobayashi T, Chinen H, Kitazume MT, Takayama T, Okamoto S, Koganei K, Sugita A, Kanai T, Hibi T. Human CD14+ macrophages in intestinal lamina propria exhibit potent antigen-presenting ability. J Immunol. 2009;183:1724–1731. doi: 10.4049/jimmunol.0804369. [DOI] [PubMed] [Google Scholar]
- 13.Bradshaw EM, Raddassi K, Elyaman W, Orban T, Gottlieb PA, Kent SC, Hafler DA. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J Immunol. 2009;183:4432–4439. doi: 10.4049/jimmunol.0900576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 15.Bernardo D, Garrote JA, Allegretti Y, Leon A, Gomez E, Bermejo-Martin JF, Calvo C, Riestra S, Fernandez-Salazar L, Blanco-Quiros A, Chirdo F, Arranz E. Higher constitutive IL15R alpha expression and lower IL-15 response threshold in coeliac disease patients. Clin Exp Immunol. 2008;154:64–73. doi: 10.1111/j.1365-2249.2008.03743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kivisakk P, Matusevicius D, He B, Soderstrom M, Fredrikson S, Link H. IL-15 mRNA expression is up-regulated in blood and cerebrospinal fluid mononuclear cells in multiple sclerosis (MS) Clin Exp Immunol. 1998;111:193–197. doi: 10.1046/j.1365-2249.1998.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuczynski S, Winiarska H, Abramczyk M, Szczawinska K, Wierusz-Wysocka B, Dworacka M. IL-15 is elevated in serum patients with type 1 diabetes mellitus. Diabetes Res Clin Pract. 2005;69:231–236. doi: 10.1016/j.diabres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Geboes K, Colpaert S, D’Haens GR, Rutgeerts P, Ceuppens JL. IL-15 is highly expressed in inflammatory bowel disease and regulates local T cell-dependent cytokine production. J Immunol. 2000;164:3608–3615. doi: 10.4049/jimmunol.164.7.3608. [DOI] [PubMed] [Google Scholar]
- 19.Miranda-Carus ME, Balsa A, Benito-Miguel M, Perez de Ayala C, Martin-Mola E. IL-15 and the initiation of cell contact-dependent synovial fibroblast-T lymphocyte cross-talk in rheumatoid arthritis: effect of methotrexate. J Immunol. 2004;173:1463–1476. doi: 10.4049/jimmunol.173.2.1463. [DOI] [PubMed] [Google Scholar]
- 20.Sato N, Patel HJ, Waldmann TA, Tagaya Y. The IL-15/IL-15Ralpha on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc Natl Acad Sci U S A. 2007;104:588–593. doi: 10.1073/pnas.0610115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubsky P, Saito H, Leogier M, Dantin C, Connolly JE, Banchereau J, Palucka AK. IL-15-induced human DC efficiently prime melanoma-specific naive CD8+ T cells to differentiate into CTL. Eur J Immunol. 2007;37:1678–1690. doi: 10.1002/eji.200636329. [DOI] [PubMed] [Google Scholar]
- 22.Regamey N, Obregon C, Ferrari-Lacraz S, van Leer C, Chanson M, Nicod LP, Geiser T. Airway epithelial IL-15 transforms monocytes into dendritic cells. Am J Respir Cell Mol Biol. 2007;37:75–84. doi: 10.1165/rcmb.2006-0235OC. [DOI] [PubMed] [Google Scholar]
- 23.Kamada N, Hisamatsu T, Okamoto S, Chinen H, Kobayashi T, Sato T, Sakuraba A, Kitazume MT, Sugita A, Koganei K, Akagawa KS, Hibi T. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008;118:2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raki M, Tollefsen S, Molberg O, Lundin KE, Sollid LM, Jahnsen FL. A unique dendritic cell subset accumulates in the celiac lesion and efficiently activates gluten-reactive T cells. Gastroenterology. 2006;131:428–438. doi: 10.1053/j.gastro.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Maiuri L, Ciacci C, Ricciardelli I, Vacca L, Raia V, Auricchio S, Picard J, Osman M, Quaratino S, Londei M. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet. 2003;362:30–37. doi: 10.1016/S0140-6736(03)13803-2. [DOI] [PubMed] [Google Scholar]
- 26.Shan L, Molberg O, Parrot I, Hausch F, Filiz F, Gray GM, Sollid LM, Khosla C. Structural basis for gluten intolerance in celiac sprue. Science. 2002;297:2275–2279. doi: 10.1126/science.1074129. [DOI] [PubMed] [Google Scholar]
- 27.Rakhimova M, Esslinger B, Schulze-Krebs A, Hahn EG, Schuppan D, Dieterich W. In vitro differentiation of human monocytes into dendritic cells by peptic-tryptic digest of gliadin is independent of genetic predisposition and the presence of celiac disease. J Clin Immunol. 2009;29:29–37. doi: 10.1007/s10875-008-9228-x. [DOI] [PubMed] [Google Scholar]
- 28.de Kauwe AL, Chen Z, Anderson RP, Keech CL, Price JD, Wijburg O, Jackson DC, Ladhams J, Allison J, McCluskey J. Resistance to celiac disease in humanized HLA-DR3-DQ2-transgenic mice expressing specific anti-gliadin CD4+ T cells. J Immunol. 2009;182:7440–7450. doi: 10.4049/jimmunol.0900233. [DOI] [PubMed] [Google Scholar]
- 29.Di Sabatino A, Ciccocioppo R, Cupelli F, Cinque B, Millimaggi D, Clarkson MM, Paulli M, Cifone MG, Corazza GR. Epithelium derived interleukin 15 regulates intraepithelial lymphocyte Th1 cytokine production, cytotoxicity, and survival in coeliac disease. Gut. 2006;55:469–477. doi: 10.1136/gut.2005.068684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokoyama S, Watanabe N, Sato N, Perera P, Filoski L, Tanaka T, Miyasaka M, Waldmann TA, Hiroi T, Perera LP. Antibody-mediated blockade of IL-15 reverses the autoimmune intestinal damage in trangenic mice that overexpress IL-15 in enterocytes. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0908834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ter Steege J, Buurman W, Arends JW, Forget P. Presence of inducible nitric oxide synthase, nitrotyrosine, CD68, and CD14 in the small intestine in celiac disease. Lab Invest. 1997;77:29–36. [PubMed] [Google Scholar]
- 32.Harris KM, Fasano A, Mann DL. Cutting edge: IL-1 controls the IL-23 response induced by gliadin, the etiologic agent in celiac disease. J Immunol. 2008;181:4457–4460. doi: 10.4049/jimmunol.181.7.4457. [DOI] [PubMed] [Google Scholar]
- 33.Yawalkar N, Tscharner GG, Hunger RE, Hassan AS. Increased expression of IL-12p70 and IL-23 by multiple dendritic cell and macrophage subsets in plaque psoriasis. J Dermatol Sci. 2009;54:99–105. doi: 10.1016/j.jdermsci.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benahmed M, Meresse B, Arnulf B, Barbe U, Mention JJ, Verkarre V, Allez M, Cellier C, Hermine O, Cerf-Bensussan N. Inhibition of TGF-beta signaling by IL-15: a new role for IL-15 in the loss of immune homeostasis in celiac disease. Gastroenterology. 2007;132:994–1008. doi: 10.1053/j.gastro.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 36.Benito-Miguel M, Garcia-Carmona Y, Balsa A, Perez de Ayala C, Cobo-Ibanez T, Martin-Mola E, Miranda-Carus ME. A dual action of rheumatoid arthritis synovial fibroblast IL-15 expression on the equilibrium between CD4+CD25+ regulatory T cells and CD4+CD25- responder T cells. J Immunol. 2009;183:8268–8279. doi: 10.4049/jimmunol.0900007. [DOI] [PubMed] [Google Scholar]
- 37.Ben Ahmed M, Belhadj Hmida N, Moes N, Buyse S, Abdeladhim M, Louzir H, Cerf-Bensussan N. IL-15 renders conventional lymphocytes resistant to suppressive functions of regulatory T cells through activation of the phosphatidylinositol 3-kinase pathway. J Immunol. 2009;182:6763–6770. doi: 10.4049/jimmunol.0801792. [DOI] [PubMed] [Google Scholar]
- 38.Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, Deenick EK, Tangye SG. Early commitment of naive human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol. 2009 doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- 40.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 41.Gjertsen HA, Sollid LM, Ek J, Thorsby E, Lundin KE. T cells from the peripheral blood of coeliac disease patients recognize gluten antigens when presented by HLA-DR, -DQ, or -DP molecules. Scand J Immunol. 1994;39:567–574. doi: 10.1111/j.1365-3083.1994.tb03414.x. [DOI] [PubMed] [Google Scholar]
- 42.Anderson RP, van Heel DA, Tye-Din JA, Barnardo M, Salio M, Jewell DP, Hill AV. T cells in peripheral blood after gluten challenge in coeliac disease. Gut. 2005;54:1217–1223. doi: 10.1136/gut.2004.059998. [DOI] [PMC free article] [PubMed] [Google Scholar]