Abstract
The present studies determined in greater detail the molecular mechanisms upstream of the CD95 death receptor by which geldanamycin HSP90 inhibitors and MEK1/2 inhibitors interact to kill carcinoma cells. MEK1/2 inhibition enhanced 17AAG toxicity that was suppressed in cells deleted for mutant active RAS which were non-tumorigenic but was magnified in isogenic tumorigenic cells expressing H-RAS V12 or K-RAS D13. MEK1/2 inhibitor and 17AAG treatment increased intracellular Ca2+ levels and reduced GRP78/BiP expression in a Ca2+ -dependent manner. GRP78/BiP over-expression, however, also suppressed drug-induced intracellular Ca2+ levels. MEK1/2 inhibitor and 17AAG treatment increased ROS levels that were blocked by quenching Ca2+ or over-expression of GRP78/BiP. MEK1/2 inhibitor and 17AAG treatment activated CD95 and inhibition of ceramide synthesis; ROS or Ca2+ quenching blocked CD95 activation. In SW620 cells that are patient matched to SW480 cells, MEK1/2 inhibitor and 17AAG toxicity was significantly reduced that correlated with a lack of CD95 activation and lower expression of ceramide synthase 6 (LASS6). Over-expression of LASS6 in SW620 cells enhanced drug-induced CD95 activation and enhanced tumor cell killing. Inhibition of ceramide signaling abolished drug-induced ROS generation but not drug-induced cytosolic Ca2+ levels. Thus treatment of tumor cells with MEK1/2 inhibitor and 17AAG induces cytosolic Ca2+ and loss of GRP78/BiP function, leading to de novo ceramide synthesis pathway activation that plays a key role in ROS generation and CD95 activation.
Keywords: Geldanamycin, 17AAG, MEK1/2 inhibitor, CD95, c-FLIP-s, GRP78/BiP, autophagy, cell death, ASMase, de novo
Introduction
In the United States, colon cancer is diagnosed in ~150,000 patients per annum with ~50,000 deaths from the disease, with an overall 5 year survival rate of ~60% (1, 2). However, for patients with non-localized tumor at diagnosis, the 5 year survival is ~10%. Liver and pancreatic cancers have much lower overall 5 year survival rates than colon cancer, of ~10% and 5%, respectively (1, 2).
The RAF-MEK1/2-ERK1/2 pathway is frequently dysregulated in the neoplastic transformation of GI tumors, including mutational activation of RAS proteins in colorectal, liver and pancreatic carcinomas (3–5). There are three widely recognized isoforms of RAS: Harvey RAS (H-RAS), Kirsten RAS (K-RAS), and neuroblastoma RAS (N-RAS). GTP-RAS can interact with multiple downstream effector molecules, including the RAF-1 protein kinase and the phosphatidylinositol 3-kinase (PI3K) lipid kinase. Guanine nucleotide exchange of “RAS” proteins from their GDP bound state to their GTP-bound form permits RAF-1 and p110 PI3K to associate with the “RAS” protein resulting in kinase translocation to the plasma membrane environment where activation of these kinases, via complex mechanisms, takes place. RAS contains a GTPase activity that converts bound GTP to GDP resulting in inactivation of the RAS molecule. PI3K enzymes are also translocated to the plasma membrane environment via the p85 SH2 domain interaction with phosphorylated tyrosine residues on adaptor proteins and growth factor receptors (e.g., GAB2, IRS-1, and ERBB3). RAS proteins also can interact with RAL-GDS; RAL-GDS, via RAL small GTPases regulates multiple processes including altered metastatic spread and the regulation of ROS levels (6, 7; and references therein).
The MEK1/2-ERK1/2 module comprises, along with c-Jun NH2-terminal kinase (JNK1/2) and p38 MAPK, members of the MAPK super-family. These kinases are involved in responses to diverse mitogens and environmental stresses and have also been implicated in cell survival processes. Activation of the ERK1/2 pathway is often associated with cell survival whereas JNK1/2 and p38 MAPK pathway signaling often causes apoptosis. Although the mechanisms by which ERK1/2 activation promote survival are not fully characterized, a number of anti-apoptotic effector proteins have been identified, including increased expression of anti-apoptotic proteins such as c-FLIP-s (8–11). In view of the importance of the RAF-MEK1/2-ERK1/2 pathway in neoplastic cell survival, inhibitors have been developed that have entered clinical trials, including sorafenib (Bay 43-9006, Nexavar®; a Raf inhibitor), AZD6244 (MEK1/2 inhibitor) and 17AAG and 17DMAG (HSP90 inhibitors that facilitate lower expression of Raf kinases and other regulatory proteins) (12, 13).
Heat shock protein 90 (HSP90) and HSP70 are families of chaperone proteins involved in the proper folding and intracellular disposition of multiple proteins involved in cell signaling and survival (14, 15). Tumor cells generally have higher rates of protein synthesis than non-neoplastic cells and disruption of HSP90 family function in tumor cells, e.g., by benzoquinoid ansamycin antibiotics such as 17-allylamino-17-demethoxygeldanamycin (17AAG), which has both superior pharmacokinetic and reduced normal tissue toxicity characteristics compared with geldanamycin induces improper folding of diverse proteins, including RAF-1, B-RAF, AKT, ERBB family receptors, among numerous others, culminating in their proteasomal degradation (16–21). These events have been shown to induce apoptosis or, alternatively, to increase the susceptibility of tumor cells to established cytotoxic agents (18, 19). Many studies have argued that inhibition of the PI3 kinase – AKT pathway, rather than the RAF-MEK1/2-ERK1/2 pathway, represents a key component of 17AAG toxicity and sensitization effects in tumor cells (22, 23). Of particular note, however, has been the observation in some cell types that pre-treatment of tumor cells with geldanamycins, which leads to a compensatory increase in the expression of HSP70 family chaperone proteins, can protect tumor cells from geldanamycin toxicity (e.g. 24, and references therein). Because of this phenomenon, many laboratories are actively searching for approaches to suppress expression/function of HSP70 family chaperones.
Free plasma concentrations of 17AAG in patients have been noted to be in the low 1 to 5 μmol/L range for up to 12 h after drug infusion, which is significantly higher than the required concentration of drug to inhibit HSP90 function (25, 26). We recently published that pharmacologically achievable concentrations of 17AAG and MEK1/2 inhibitors interact to kill in hepatocellular carcinoma cells in vitro and in vivo via activation of the CD95 extrinsic apoptotic pathway, concomitant with drug-induced reduced expression of c-FLIP-s that was, in part, due to prolonged inactivation of ERK1/2 and AKT (27). Recent studies in which inhibitors of MEK1/2 and PI3K have been combined to achieve a tumoricidal effect in lung cancer, in principle, also confirm these findings (28). The mechanism by which CD95 was activated in hepatoma cells was dependent on ROS – induced activation of p38 MAPK (27).
The studies in the present manuscript were designed to understand, upstream of CD95, how ROS was generated by the 17AAG + MEK1/2 inhibitor combination; the putative primary target of the drug combination permitting ROS generation; whether other GI tumor cell types could be killed by this drug combination; and to determine if the tumorigenicity of a tumor cell (with specific reference to RAS signaling) impacted upon drug combination lethality (27). We found that the drug-induced generation of intracellular Ca2+; the generation of ROS; and ceramide signaling, were all causal in CD95 activation. We ordered the sequence of induction of these signaling processes: drug treatment promoted Ca2+ generation which facilitated ceramide dependent increases in ROS levels. We noted that the ER resident HSP70 family chaperone, GRP78/BiP, played a key role in regulating the induction of Ca2+ by drug treatment but that also the drug treatment resulted in lower GRP78/BiP expression in a Ca2+ dependent manner. This argues that GRP78/BiP function/expression is one key primary effector protein upon which the drug combination acts. In prior studies it was shown that deletion of mutant active K-RAS D13 in HCT116 cells abolished their tumorigenicity, and we have noted that introduction of H-RAS V12 into such cells lacking a mutant active RAS restores their tumorigenic potential. Loss of K-RAS D13 expression abolished the lethal interaction between 17AAG and MEK1/2 inhibitors that was restored by expression of H-RAS V12. This data demonstrates that tumorigenic cells are selectively killed by the 17AAG+MEK inhibitor drug combination over transformed cells that cannot form tumors.
Materials and Methods
Materials
Total BAX, cleaved caspase 3, Phospho-/total-ERK1/2/5, Phospho-/total-JNK1-3, Phospho-/total-p38 MAPK, Anti-S473 AKT and total AKT antibodies were purchased from Cell Signaling Technologies (Worcester, MA). Active BAX specific antibody (6A7) for immunoprecipitation was purchased from Sigma (St. Lois, MO). The JNK inhibitor peptide (JNK IP), caspase inhibitors (zVAD, IETD, LEHD) and 17AAG was supplied by Calbiochem (San Diego, CA) as powder, dissolved in sterile DMSO, and stored frozen under light-protected conditions at −80°C. Trypsin-EDTA, RPMI medium, penicillin-streptomycin were purchased from GIBCOBRL (GIBCOBRL Life Technologies, Grand Island, NY). HuH7, HEPG2 and HEP3B (hepatoma), pancreatic (PANC1, Mia Paca2), and colorectal (SW480, SW620, DLD1, HCT116) cancer cells were obtained from the ATCC (Rockville, MD). Commercially available validated short hairpin RNA molecules to knock down RNA/protein levels were from Qiagen (Valencia, CA): CD95 (SI02654463; SI03118255); ATG5 (SI02655310); Beclin 1 (SI00055573, SI00055587); BAX (SI02662401; SI02654533); BAK (SI00299376; SI02654512). We also made use for confirmatory purposes of the short hairpin RNA construct targeting ATG5 (pLVTHM/Atg5) that was a gift from Dr. Yousefi, Department of Pharmacology, University of Bern, Switzerland. BAK −/−, BAK −/−, BAX+BAK −/−, fibroblasts were kindly provided by Dr. S. Korsmeyer (Harvard University, Boston, MA). The dominant negative p38 MAPK and activated MEK1 EE recombinant adenoviruses were kindly provided by Drs. K. Valerie, VCU and J. Moltken (University of Cincinnati), respectively. The plasmids to express LC3-GFP, GRP78/BiP and Calbindin D28 were from Dr. S. Spiegel (VCU), Dr. A. Lee (UCLA) and Dr. Y.J. Oh (Yonsei University, Seoul, South Korea), respectively. Other reagents were of the highest quality commercially available (11, 27, 29–32).
Methods
Cell culture and in vitro exposure of cells to drugs
All established cell lines were cultured at 37 °C (5% (v/v CO2) in vitro using RPMI supplemented with 5% (v/v) fetal calf serum and 10% (v/v) Non-essential amino acids. For short-term cell killing assays and immunoblotting, cells were plated at a density of 3 × 10 3 per cm2 and 36 h after plating were treated with various drugs, as indicated. In vitro small molecule inhibitor treatments were from a 100 mM stock solution of each drug and the maximal concentration of Vehicle (DMSO) in media was 0.02% (v/v). For adenoviral infection, cells were infected 12 h after plating and the expression of the recombinant viral transgene allowed to occur for 24 h prior to any additional experimental procedure. Cells were not cultured in reduced serum media during any study.
Generation of Rho 0 HuH7 Cells
HuH7.Ntcp human hepatoma cells (kindly provided by Dr. G. Gores, Mayo Clinic, Rochester, MN) were cultured in DMEM containing 10% (v/v) FCS. To generate HuH7.Ntcp Rho 0 cells, HuH7.Ntcp cells were cultured in DMEM containing 10% (v/v) FCS, 50 μg/mL uridine, 1 mmol/L sodium pyruvate, and the growth medium supplemented(for Rho 0 cell generation) with 10 μg/mL ethidium bromide. Cells were cultured in this medium or in parallel in growth medium without ethidium bromide for 8 weeks before any further experimentation. Removal of uridine and pyruvate from the growth medium of established HuH7 Rho 0 cells resulted in rapid (~ 24–48h) growth arrest and cell death (data not shown).
Cell treatments, SDS-PAGE and Western blot analysis
Unless otherwise indicated in the Figure Legend, cells were treated with either vehicle (VEH, DMSO), or the combination of MEK1/2 inhibitor PD184352 (PD184; 1 μM) or PD98059 (PD98; 25μM) as indicated, and geldanamycin (17AAG; 0.1–1.0 μM or 17DMAG; 0.25 μM) or both agents combined. For SDS PAGE and immunoblotting, cells were lysed in either a non-denaturing lysis buffer, and prepared for immunoprecipitation as described in (refs. 27, 29–32) or in whole-cell lysis buffer (0.5 M Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 1% β-mercaptoethanol, 0.02% bromophenol blue), and the samples were boiled for 30 min. After immunoprecipitation, samples were boiled in whole cell lysis buffer. The boiled samples were loaded onto 10–14% SDS-PAGE and electrophoresis was run overnight. Proteins were electrophoretically transferred onto 0.22 μm nitrocellulose, and immunoblotted with indicated primary antibodies against the different proteins. All immunoblots were visualized by an Odyssey Infra red imager. For presentation, blots were imported into Adobe PhotoShop 8.0, and their color removed and Figures generated in MicroSoft PowerPoint.
Recombinant adenoviral vectors; infection in vitro
We generated and purchased previously noted recombinant adenoviruses to express constitutively activated and dominant negative MEK1 proteins, dominant negative p38 MAPK, dominant negative caspase 9, the caspase 9 inhibitor XIAP, the endogenous caspase 8 inhibitor c-FLIP-s, the polyoma virus caspase 8 inhibitor CRM A, and mitochondrial protective protein BCL-XL (Vector Biolabs, Philadelphia, PA). Unless otherwise stated, cells were infected with these adenoviruses at an approximate multiplicity of infection (m.o.i.) of 50 that results in > 80% infection of tumor cells. As noted above, cells were further incubated for 24 h to ensure adequate expression of transduced gene products prior to drug exposures (27). In confirmatory studies, and in agreement with published studies using these reagents, we noted that activated and dominant negative MEK1 proteins activated and reduced ERK1/2 phosphorylation in cells, and that dominant negative p38 MAPK suppressed sorbitol – induced p38 MAPK activation (27).
siRNA transfection in vitro
Approximately 10 nM of a defined pre-validated siRNA (Ambion technologies) was diluted into 50 μl growth media lacking FBS and pen-strep. Based on the Manufacturer’s instructions, an appropriate amount of Lipofectamine 2000 reagent (usually 1 μl) (Invitrogen, Carlsbad, CA) was diluted into a separate vial containing media with lacking FBS or pen-strep. The two solutions were incubated separately at room temperature for 5 min, then mixed together (vortexed) and incubated at room temperature for 30 min. The mixture was added to each well (slide or 12-well plate) containing an appropriate amount (~ 0.5 ml) of pen-strep- and FBS-free medium. Cells were incubated for 2–4 h at 37 deg C with gentle rocking. Media was then replaced with 1 ml of 1x pen-strep and FBS containing media. In the Figures we present representative immunoblots showing knock down of CD95, ATG5 and Beclin 1 in the various cell lines used. Regardless of cell line, knock down of protein by siRNA was at least 70% in all cell lines used (data not shown) (27).
Detection of cell death by Trypan Blue, Hoechst, TUNEL and flow cytometric assays
Cells were harvested by trypsinization with Trypsin/EDTA for ~10 min at 37 °C. As some apoptotic cells detached from the culture substratum into the medium, these cells were also collected by centrifugation of the medium at 1,500 rpm for 5 min. The pooled cell pellets were resuspended and mixed with trypan blue dye. Trypan blue stain, in which blue dye incorporating cells were scored as being dead was performed by counting of cells using a light microscope and a hemacytometer. Five hundred cells from randomly chosen fields were counted and the number of dead cells was counted and expressed as a percentage of the total number of cells counted. For confirmatory purposes the extent of apoptosis was evaluated by assessing Hoechst and TUNEL stained cytospin slides under fluorescent light microscopy and scoring the number of cells exhibiting the “classic” morphological features of apoptosis and necrosis. For each condition, 10 randomly selected fields per slide were evaluated, encompassing at least 1500 cells. Alternatively, the Annexin V/propidium iodide assay was carried to determine cell viability out as per the manufacturer’s instructions(BD PharMingen) using a Becton Dickinson FACScan flow cytometer (Mansfield, MA) (29, 30).
Plasmid transfection
Plasmid DNA (0.5 μg/total plasmid transfected) was diluted into 50 μl of RPMI growth media that lacked supplementation with FBS or with penicillin-streptomycin. Lipofectamine 2000 reagent (1 μl) (Invitrogen, Carlsbad, CA) was diluted into 50 μl growth media that lacked supplementation with FBS or with penicillin-streptomycin. The two solutions were then mixed together and incubated at room temperature for 30 min. The total mix was added to each well (4-well glass slide or 12-well plate) containing 200 μl growth media that lacked supplementation with FBS or with penicillin-streptomycin. The cells were incubated for 4 h at 37°C, after which time the media was replaced with RPMI growth media containing 5% (v/v) FBS and 1x pen-strep.
Assessment of ROS (Reactive Oxygen Species) Generation
Hepatoma and Colon cancer cells were plated in 96 well plates. Cells were pre-incubated with dihydro-DCF (5mM for 30 minutes) which is non-fluorescent in its di-hydro form but upon reaction with ROS (peroxide) becomes highly fluorescent. Di-hydro-DCFis sensitive to oxidation by hydroxyl radicals and peroxy-nitrite directly and hydrogen peroxide in the presence of oxidases. Fluorescence measurements were obtained 0–30 minutes after drug addition with a Vector 3 plate reader. Data are presented corrected for basal fluorescence of vehicle-treated cells at each time point and expressed as a –Fold increase in ROS levels. Each time point represents the mean of six data points per experiment and of a total of three independent experiments.
Assessment of Cytosolic Ca2+ Levels
A high-speed wavelength switching fluorescence image analysis system (Vector 3 plate reader) was used to determine [Ca2+]i in the carcinoma cells, seeded in 96 well plates (20,000 cells per well), with fura-2 acetoxymethylester (fura-2) as an indicator. After loading with 10 μmol/L fura-2 at room temperature for 50 min, the cells were washed thrice with Hanks’ buffer (pH 7.4). Then vehicle (DMSO), 17AAG, PD184352, or the drug combination was added into the media solution to stimulate the cells, respectively. A fluorescence ratio of excitation at 340 nm to that at 380 nm (F340/F380) was determined after background subtraction. The ratio of fura-2 emissions, when excited at the wavelengths of 340 and 380 nm, was recorded and analysis software were used to process and statistical analyze data.
Microscopic assessment of autophagy
For autophagy studies, cells transfected in duplicate with LC3-GFP in glass chambered slides were applied after drug exposure to high powered light/confocal microscopes (Zeiss LSM 510 Meta-confocal scanning microscope; Zeiss HBO 100 microscope with Axio Cam MRm camera) at 40× magnification and 40 cells per well assessed for formation of punctate autophagic vesicles.
Colony formation assays
Tumor cells growing in log phase were re-plated as single cells in 60 mm dishes. Cells were treated 12h after plating (250–1500 cells/well) in sextuplicate with vehicle (VEH, DMSO), 17AAG (μM) or PD184352 (μM), or with both drugs combined, as indicated at a fixed concentration ratio to perform median dose effect analyses for the determination of synergy. After drug exposure (48h), the media was changed and cells cultured in drug free media for an additional 10–14 days. Cells were fixed, stained with crystal violet and colonies of > 50 cells/colony counted. Colony formation data were entered into the Calcusyn for Windows program and combination index (CI) values determined. Fa: Fraction affected. A CI value of < than 1.00 indicates synergy.
Data analysis
Comparison of the effects of various treatments was performed using one way analysis of variance and a two tailed Student’s t-test. Differences with a p-value of < 0.05 were considered statistically significant. These values were determined using the statistical programming within Sigma Stat and Sigma Plot. Median dose effect isobologram analyses to determine synergism of drug interaction were performed according to the Methods of T-C Chou and P Talalay using the Calcusyn program for Windows (BIOSOFT, Cambridge, UK). Data points from all experiments shown are the mean of multiple individual data points summated from the stated number of multiple experiments i.e. (mean data shown = Σ n, all data points, ± SEM).
Results
Treatment of pancreatic and liver cancer cells that express mutant active RAS proteins and other membrane-proximal oncogenic signaling proteins with the geldanamycin HSP90 inhibitor 17AAG and the MEK1/2 inhibitor PD184352 resulted in greater than additive increases in tumor cell killing within 48h (Figure 1A). PD184352 and 17AAG were shown to synergistically interact to kill multiple cancer cell types in long-term colony formation assays (Table S1). As MiaPaca2, PANC-1 and HEPG2 cells express mutated active K-RAS, K-RAS and N-RAS proteins, respectively, and we next examined whether other GI tumor cell types that express mutated active RAS proteins, or activated downstream effectors of RAS such as B-RAF and PI3K, also present with a greater than additive toxic interaction between 17AAG and MEK1/2 inhibitor.
Figure 1. PD184352 and 17AAG interact to kill multiple human GI tumor cell lines.
Panel A. Human hepatoma cells (HEPG2, HEP3B) and human pancreatic tumor cells (PANC1, MiaPaca2) were treated 24 h after triplicate plating with vehicle, PD184352 (PD, 1 μM), 17AAG (17AAG, 1 μM), or with both drugs combined. Cell viability was determined 48 h after drug treatment by trypan blue exclusion (± SEM, n = 3 independent studies, * p< 0.05 value greater amount of cell killing compared to VEH or individual drug treated cells). Panel B. Human colon cancer cells (SW620, SW480, HCT116, DLD1) were treated 24 h after triplicate plating with vehicle, PD184352 (PD, 1 μM), 17AAG (17AAG, 1 μM), or with both drugs combined. Cell viability was determined 48 h and 96 h after drug treatment by trypan blue exclusion (± SEM, n = 3 independent studies, * p< 0.05 value greater amount of cell killing compared to VEH or individual drug treated cells). Panel C. HCT116 cells (WT parental expressing K-RAS D13; deleted for K-RAS D13; deleted for K-RAS D13 expressing H-RAS V12 and effector mutants of H-RAS V12), 24h after plating in triplicate, were treated with vehicle (DMSO), 17AAG (1 μM), PD184352 (1 μM) or 17AAG+PD184352. Forty eight h and 96h after exposure, cells were isolated. Cell viability was determined 48 h and 96 h after drug treatment by trypan blue exclusion (± SEM, n = 3 independent studies, * p< 0.05 value greater amount of cell killing compared to VEH or individual drug treated cells).
HT-29 colon cancer cells express a mutated active B-RAF protein, but do not express a mutated RAS allele. SW620 and SW480 cells are patient matched colon cancer lines that express two alleles of mutated active K-RAS: SW620 has also been noted to be refractory to death receptor-induced lethality in part by lacking ceramide synthase 6 (LASS6) expression (33). HCT116 cells express a single mutated active allele of K-RAS as well as a mutated active PI3K p110α protein (34). In HCT116, SW480 and HT29 cells, PD184352 and 17AAG interacted in an additive to greater than additive manner to cause tumor cell death (Figure 1B). However, SW620 cells were refractory to drug-induced killing.
To determine the most important pathways downstream of activated RAS proteins in GI tumor cells that define 17AAG+PD184352 toxicity, we made use of HCT116 cells, and variants of HCT116 cells that are genetically deleted for K-RAS D13 and transfected with H-RAS V12 and with effector mutants of H-RAS V12 (27, 35). In short term viability assays, PD184352 and 17AAG interacted in a greater than additive manner to kill parental HCT116 cells (Figure 1C). Knock out of K-RAS D13 significantly suppressed drug toxicity and transfection of H-RAS V12 into these cells restored drug lethality. Of note, HCT116 cells lacking K-RAS D13 expression are non-tumorigenic (35). Transfection of H-RAS V12 G37 into tumor cells lacking K-RAS D13; a RAS effector domain mutant that specifically activates the RAL-GDS pathway, profoundly enhanced the toxicity of 17AAG, and of 17AAG+MEK1/2 inhibitor treatment (Figure 1C and Figure S1).
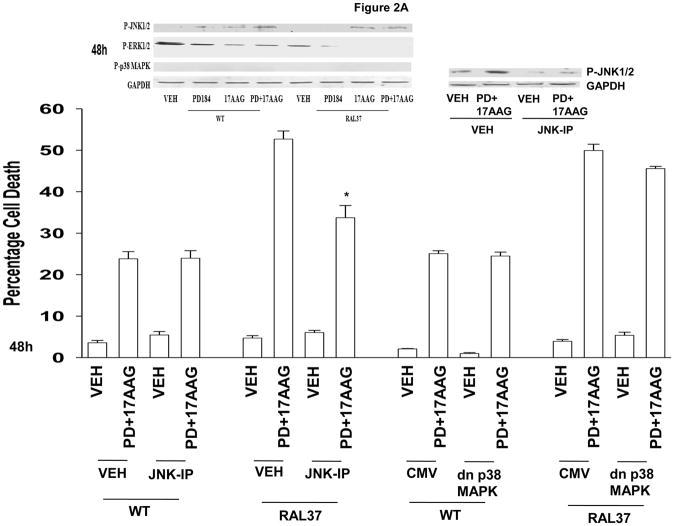
Expression of H-RAS V12 G37 enhanced 17AAG- and 17AAG+PD184352 – induced activation of JNK1/2 and inactivation of ERK1/2 (Figure 2A, upper blots). Inhibition of JNK1/2 signaling suppressed the toxicity of 17AAG+PD184352 in cells expressing H-RAS V12 G37, but not in parental HCT116 cells (Figure 2A, lower graph). This is of particular note because in hepatoma cells inhibition of p38 MAPK and not that of JNK1/2 was responsible for drug combination lethality (27).
Figure 2. Signaling by RAL-GDS downstream of H-RAS V12 promotes 17AAG toxicity.
Panel A. Upper blotting: Parental HCT116 (WT) cells and HCT116 H-RAS V12 G37 were treated with vehicle (DMSO), 17AAG, PD184352 or 17AAG+PD184352. Cells were isolated 24h and 48h after exposure and cell lysates subjected to SDS PAGE and immunoblotting to determine the phosphorylation of ERK1/2, p38 MAPK and JNK1/2, and the expression of GAPDH. In parallel blots, 24 h after plating cells were treated with vehicle (DMSO) or JNK-IP (10 μM) and 30 min later with vehicle (DMSO) or 17AAG+PD184352 and cells isolated 24h after drug exposure. Lower Graphical: Parental HCT116 (WT) cells and HCT116 H-RAS V12 G37 were infected with recombinant adenoviruses to express: nothing (CMV) or dnp38 MAPK. Twenty four h after infection cells were treated with vehicle (DMSO) or JNK-IP (10 μM) and 30 min later with vehicle (DMSO), 17AAG, PD184352 or 17AAG+PD184352. Forty eight h after exposure, cells were isolated. Cell viability was determined 48h after drug treatment by trypan blue exclusion (± SEM, n = 3 independent studies, * p< 0.05 value lower amount of cell killing compared to parallel treated WT cells). Panel B. Parental HCT116 and HCT116 H-RAS V12 G37 cells were infected with recombinant adenoviruses to express: nothing (CMV) or caMEK1. Twenty four h after infection cells were treated with vehicle (DMSO), 17AAG, PD98059 (25 μM) or 17AAG+PD98059. Forty eight h after exposure, cells were isolated. Cell viability was determined 48h after drug treatment by trypan blue (± SEM, n = 3 independent studies, * p< 0.05 value lower amount of cell killing compared to parallel drug treated CMV infected cells). Panel C. Upper Images: Parental HCT116 cells and HCT116 H-RAS V12 G37 cells 24h after plating in glass-chambered slides were treated with vehicle (DMSO), 17AAG, PD184352 or 17AAG+PD184352. Six h after exposure, cells were fixed and stained for plasma membrane localization of CD95 (± SEM, n = 2). Lower Graphical: Parental HCT116 cells and HCT116 H-RAS V12 G37 were transfected with a scrambled (siSCR) siRNA molecule, or an siRNA molecule to suppress expression of CD95 (siCD95). Twenty four h after transfection cells were treated with vehicle (DMSO), 17AAG, PD184352 or 17AAG+PD184352. Forty eight h after exposure, cells were isolated. Cell viability was determined 48h after drug treatment by trypan blue exclusion (± SEM, n = 3 independent studies, * p< 0.05 lower amount of cell killing compared to siSCR treated cells).
Expression of activated MEK1 significantly suppressed the toxicity of 17AAG and the MEK1/2 inhibitor PD98059 (that does not inhibit MEK1 EE) in both parental cells and in cells expressing H-RAS V12 G37 (Figure 2B). Knock down of CD95 in parental HCT116 cells blocked the potentiation of 17AAG lethality by PD184352 however knock down of CD95 in cells expressing H-RAS V12 G37 had no effect on the toxicity of the drug interaction (Figure 2C). In a similar manner to parental HCT116 cells, inhibition of CD95/extrinsic pathway signaling blocked 17AAG+PD184352 – induced cell killing in pancreatic and liver cancer cells, and in these cells the drug combination also caused CD95 activation (Figures 3A and 3B; Figure S2). CD95 activation could be mediated by either FAS-L or by ligand independent processes e.g. increased ROS or ceramide levels (36). Use of a neutralizing anti-FAS ligand antibody CD95 did not block either drug-induced CD95 activation or drug-induced cell killing (Figure 3C).
Figure 3. MEK1/2 inhibitor and 17AAG toxicity in GI cancer cells is mediated by CD95-caspase 8 signaling and does not require FAS-L.
Panel A. MiaPaca2 cells were infected with recombinant adenoviruses (empty vector CMV; caspase 8 inhibitor CRM A; caspase 8 inhibitor c-FLIP-s; BCL-XL; dominant negative caspase 9) at a multiplicity of infection of 50. Twenty four h after infection, cells were treated with vehicle (DMSO), 17AAG (1 μM), PD184352 (1 μM) or both drugs together. Forty eight hours after exposure, cells were isolated and stained with Annexin V – propidium iodide and viability determined by flow cytometry (± SEM, n = 3;* p< 0.05 value greater amount of cell killing compared to VEH or individual drug treated cells). Panel B. Upper Images: MiaPaca2 cells 24h after plating in glass-chambered slides were treated with vehicle (DMSO), 17AAG, PD184352 or 17AAG+PD184352. Six h after exposure, cells were fixed and stained for plasma membrane localization of CD95 (± SEM, n = 2). Lower graphical panel: HEP3B and MiaPaca2 cells were transfected with either a scrambled siRNA (siSCR, 20 nM) or an siRNA to knock down CD95 expression. Twenty four h after transfection, cells were treated with vehicle (DMSO), 17AAG (1 μM), PD184352 (1 μM) or both drugs together. Forty eight hours after exposure, cells were isolated and cell viability determined via trypan blue exclusion (± SEM, n = 3; * p< 0.05 value lower amount of cell killing compared to corresponding value in siSCR cells). Panel C. HEPG2 and HEP3B cells 24h after plating in either 8 well chamber slides or 12 well plates were treated with a non-specific IgG or an IgG that acts to neutralize FAS-L (NOK-1, 10 μg/ml). Cells were treated with vehicle (DMSO), 17AAG (1 μM), PD184352 (1 μM) or both drugs together. In the slides, cells were fixed 6h after drug exposure and stained for surface localization of CD95 (± SEM, n = 3 independent studies). In the 12 well plates, cells were isolated 48h after exposure and cell viability determined via trypan blue exclusion (± SEM, n = 3 independent studies).
Prior studies from this group have demonstrated in hepatoma cells that agents which quench ROS block 17AAG + MEK1/2 inhibitor toxicity, though in those analyses we did not measure actual alterations in ROS generation (27). Treatment of hepatoma cells with 17AAG weakly increased ROS levels that were significantly enhanced by inhibition of MEK1/2 (Figure 4A). As CD95 signaling was not responsible for enhanced morbidity of HCT116 cells expressing H-RAS V12 G37, we determined whether a differential induction of reactive oxygen species comparing parental and H-RAS V12 G37 HCT116 cells could explain the altered levels of cell survival after drug exposure and the CD95 –independent nature of drug-induced killing in cells expressing H-RAS V12 G37. As was noted in hepatoma cells, in parental HCT116 cells PD184352 rapidly enhanced 17AAG -induced ROS levels (Figure 4B). In cells expressing H-RAS V12 RAL 37, however, both 17AAG and 17AAG+PD184352 treatment enhanced ROS production, ~2.5-fold above that observed in parental cells, and in addition prolonged drug-induced ROS generation. Quenching of ROS production blocked CD95 activation and drug –induced cell killing (Figure 4C, data not shown). Molecular quenching of ROS production in cells expressing H-RAS V12 RAL 37, via over-expression of thioredoxin (TRX), suppressed drug-induced JNK pathway activation; JNK pathway signaling playing a key role in drug-induced killing (Figure S3; Figure 2A).
Figure 4. Mitochondrial-dependent generation of ROS plays a key role in CD95 activation.
Panel A. HEPG2 and HEP3B cells 24h after plating in 96 well plates were treated with vehicle (DMSO), 17AAG (1 μM; 5 μM), PD184352 (1 μM; 5 μM) or both drugs combined and where indicated treated in parallel with vehicle (PBS); N-acetyl cysteine (10 mM) or MnTBAP (1 μM). The generation of reactive oxygen species (ROS) in cells was determined 15 min after drug addition using a Vector 3 plate reader (± SEM, n = 3; * p< 0.05 value lower amount of ROS compared to corresponding Vehicle treated cells). Panel B. Parental HCT116 and HCT116 H-RAS V12 G37 cells 24h after plating in 96 well plates were treated with vehicle (DMSO), 17AAG (1 μM), PD184352 (1 μM) or both drugs combined. The generation of reactive oxygen species (ROS) in cells was determined 5 and 10 min after drug addition using a Vector 3 plate reader (± SEM, n = 3 independent studies). Panel C. Upper IHC: HEPG2, parental HCT116 and HCT116 H-RAS V12 G37 cells 24h after plating in 8 well chamber slides were treated with vehicle (DMSO), 17AAG (1 μM), PD184352 (1 μM) or both drugs combined and where indicated treated in parallel with vehicle (PBS) or MnTBAP (1 μM). Cells were fixed 6h after drug exposure and stained for surface localization of CD95 (± SEM, n = 3; * p< 0.05 value lower amount of CD95 activation compared to corresponding value in Vehicle treated cells). Lower graph: HEPG2, MiaPaca2, parental HCT116 and HCT116 H-RAS V12 G37 cells 24h after plating were treated with vehicle (DMSO) or with 17AAG (1 μM) and PD184352 (1 μM) combined and where indicated treated in parallel with vehicle (PBS) or MnTBAP (1 μM). Cells were isolated 48h after exposure and survival determined by trypan blue exclusion (± SEM, n = 3; * p< 0.05 value lower amount of cell killing compared to corresponding value in Vehicle treated cells). Panel D. HuH7 parental and Rho zero cells 24h after plating in 96 well plates were treated with vehicle (DMSO), 17AAG (1 μM; 5 μM), PD184352 (1 μM; 5 μM) or both drugs combined. As indicated, cells were treated with vehicle (DMSO), Cyclosporine A (Csa) or Bongkrekic acid (Bong). The generation of reactive oxygen species (ROS) in cells was determined 15 min after drug addition using a Vector 3 plate reader (± SEM, n = 3; * p< 0.05 value lower amount of ROS compared to corresponding Vehicle treated cells).
A significant source of ROS generation induced by this drug combination was dependent on functioning mitochondria, as judged by use of mitochondria deficient Rho zero HuH7 cells (Figure 4D). Similar data arguing for mitochondrial-dependent induction of ROS by this drug combination was also obtained in HEPG2 cells (Figure S4). It is known that activation of CD95 can generate ROS via activation of NADPH oxidases; as HuH7 cells lack CD95 our data with 17AAG and PD184352 thus tend to support an NADPH oxidase independent form of ROS generation (37–39). As previously stated, the small GTP-binding protein RAL is activated by RAL-GDS, one of the effector molecules for RAS. Active RAL binds to a GTPase activating protein for CDC42 and RAC, and the activity of NADPH oxidase is regulated by RAC. Thus of note was that while inhibition of p47phox –dependent NADPH oxidase in parental HCT116 cells did not alter drug-induced ROS levels, knock down of p47phox in HCT116 cells expressing H-RAS V12 RAL 37 significantly reduced the induction of ROS and the enhanced levels of tumor cell killing (Figure S5). Thus enhanced drug toxicity in H-RAS V12 G37 cells was due to high levels of NADPH oxidase –dependent ROS generation.
In prior studies we have noted in a stimulus specific manner that ligand-independent activation of CD95 could be dependent on the actions of acidic sphingomyelinase and/or the de novo ceramide synthesis pathway (29, 30). Knock down of acidic sphingomyelinase expression or treatment with an inhibitor of the de novo ceramide synthesis pathway blocked 17AAG + PD184352 –induced CD95 activation (Figure 5A, upper IHC). Inhibition of acidic sphingomyelinase or the de novo ceramide synthesis pathway significantly reduced 17AAG + MEK1/2 inhibitor –induced ROS levels (Figure 5A, lower graph). SW620 cells, that lack LASS6 expression, were resistant to 17AAG + MEK1/2 inhibitor toxicity however stable expression of LASS6 in SW620 cells significantly enhanced the lethality of 17AAG and of 17AAG + MEK1/2 inhibitor treatment, and facilitated drug-induced CD95 activation (Figures 5B and 5C).
Figure 5. Ceramide-dependent generation of ROS plays a key role in CD95 activation.

Panel A. HEPG2 cells were transfected with scrambled siRNA (siSCR, 20 nM) or an siRNA to knock down acidic sphingomyelinase expression (siASMase). Cells were then plated: in 8 well chambered slides for determination of CD95 surface localization; in 96 well plates for determination of ROS levels. Unless otherwise indicated, 24h after re-plating cells were pre-treated with vehicle or myriocin (1 μM) and 30 min later treated with vehicle (DMSO), 17AAG (1 μM), PD184352 (1 μM) or both drugs combined. For CD95 plasma membrane localization, cells were fixed 6h after drug exposure and surface CD95 detected by IHC in unpermeabilized cells. A representative study from 3 experiments is shown. The generation of reactive oxygen species (ROS) in cells was determined 15 min after drug addition using a Vector 3 plate reader (± SEM, n = 3 independent studies). Panel B. SW620 cells stably transfected with either vector control plasmid of a plasmid to express LASS6, 24h after plating, were exposed to vehicle (DMSO), 17AAG (1 μM), PD184352 (1 μM) or both drugs combined. Forty eight hours after exposure, cells were isolated and viability determined by trypan blue (± SEM, n = 2). * p< 0.05 greater than corresponding value in empty vector cells. The toxicity of PD184352 did not alter between vector control and LASS6 expressing cells (data not shown). Panel C. SW620 cells 24h after plating in 8 well chamber slides as above, and exposed to vehicle (DMSO) or to 17AAG (1 μM) and PD184352 (1 μM). Cells were fixed 6h after exposure and the amount of plasma membrane associated CD95 determined by immunohistochemistry (± SEM, n = 2). Panel D. HEPG2 cells were transfected with scrambled siRNA (siSCR, 20 nM) or an siRNA to knock down acidic sphingomyelinase expression (siASMase) and in parallel with a vector control plasmid or a plasmid to express Calbindin D28. Cells were then plated in 96 well plates for determination of ROS and Ca2+ levels, as indicated. Twenty-four h after re-plating cells were pre-treated with vehicle or myriocin (1 μM) and 30 min later were loaded with either fura-2 or loaded with Di-hydro-DCF. After 30 min, cells treated with vehicle (DMSO), 17AAG (1 μM; 5 μM), PD184352 (1 μM; 5 μM) or both drugs combined. The generation of ROS and Ca2+ in cells was determined 15 min after drug addition using a Vector 3 plate reader (± SEM, n = 3).
Treatment with 17AAG causes mitochondrial-dependent ROS generation and also promotes an unfolded protein response/endoplasmic reticulum stress. As Ca2+ homeostasis plays a central role in the functions of both organelles, we next investigated whether drug-induced ROS generation was dependent on changes in the levels of cytosolic Ca2+. Knock down of acidic sphingomyelinase or inhibition of de novo ceramide synthesis in hepatoma cells did not alter the induction of cytosolic Ca2+ levels by 17AAG+PD184352 treatment (Figure 5D, left panel). However, quenching of cytosolic Ca2+ using Calbindin D28 suppressed drug-induced ROS (Figure 5D, right panel). In SW620 cells regardless of LASS6 expression 17AAG+PD184352 treatment increased cytosolic Ca2+ levels however in vector control transfected SW620 cells, lacking LASS6 expression, drug exposure only weakly increased ROS levels (Figures S6 and S7). Expression of LASS6 restored drug-induced ROS generation in SW620 cells. Hence, 17AAG and MEK1/2 inhibitors increase cytosolic Ca2+ levels that activate ceramide synthetic/regulatory pathways which in turn lead to the generation of ROS. Increased Ceramide and ROS levels play an essential role in promoting mitochondrial dysfunction and CD95 activation.
In addition to linking ceramide in CD95 signaling, we have also shown in several prior studies that activation of CD95 increased autophagic vesicle formation, an event that was “protective” against CD95-induced apoptosis (29, 30). Treatment of hepatoma cells with 17AAG + MEK1/2 inhibitor enhanced autophagic vesicle formation 6h-24h after exposure that was blocked by knock down of ATG5 or CD95, or by expression of dominant negative PERK (Figure 6A). HuH7 hepatoma cells do not express CD95 and did not produce a significant autophagic response following 17AAG and MEK1/2 inhibitor exposure (Figure 6B). However, expression of CD95-YFP in HuH7 cells increased the number of drug-induced puncate LC3-GFP vesicles, and in agreement with CD95 mediating the toxic actions of this drug combination. Knock down of ATG5 or Beclin1 enhanced 17AAG+MEK1/2 inhibitor toxicity whereas expression of dominant negative PERK reduced drug lethality (Figure 6C). Similar data were obtained in ATG5 null SV40 transformed MEFs (Figure S8). Deletion of PERK or expression of dominant negative eIF2α (S51A) in SV40 transformed MEFs suppressed the toxicity of 17AAG+MEK1/2 inhibitor exposure (Figure 6D).
Figure 6. MEK1/2 inhibitor + 17AAG treatment increases the levels of protective autophagic vesicles in a CD95-, PERK- and ATG5-dependent fashion.
Panel A. HEPG2 cells in 8 well chambered glass slides 24h after plating were transfected with a plasmid to express LC3-GFP. As indicated, cells were co-transfected with: siRNA molecules to knock down expression of ATG5, CD95 or with a siScramble control (20 nM); or with an empty vector control plasmid or a plasmid to express dominant negative PERK. Twenty four h after transfection, cells were treated with vehicle (DMSO), 17AAG (1 μM), PD184352 (1 μM) or both drugs combined. Six hours after drug exposure, cells were visualized at 40× using an Axiovert 200 fluorescent microscope under fluorescent light using the FITC filter. The mean number of autophagic vesicles per cell from random fields of 40 cells were counted (± SEM, n = 3). Panel B. graph to left: HuH7 cells in 8 well chambered glass slides 24h after plating were transfected with LC3-GFP and with either an empty vector control plasmid (CMV) or a plasmid to express CD95.Twenty four h after transfection, cells were treated with vehicle (DMSO), 17AAG (1 μM), PD184352 (1 μM) or both drugs combined. Six hours after drug exposure, cells were visualized at 40× using an Axiovert 200 fluorescent microscope under fluorescent light using the FITC filter. The mean number of autophagic vesicles per cell from random fields of 40 cells were counted (± SEM, n = 2); graph to right: HuH7 cells in 12 well plates, 24h after plating were transfected with either an empty vector control plasmid (CMV) or a plasmid to express CD95. Twenty four h after transfection, cells were treated with vehicle (DMSO), 17AAG (1 μM), PD184352 (1 μM) or both drugs combined. Forty eight h after drug exposure cells were isolated cells and cell viability determined via trypan blue exclusion (± SEM, n = 2 independent studies). Panel C. Lower graphical panel: HEPG2 cells 24h after plating were transfected with: siRNA molecules to knock down expression of ATG5 or Beclin1, or with a siScramble control (20 nM); or with an empty vector control plasmid or a plasmid to express dominant negative PERK. Twenty four h after transfection, cells were treated with vehicle (DMSO), 17AAG (1 μM), PD184352 (1 μM) or both drugs combined. Cells were isolated 48 h later and cell viability determined via trypan blue exclusion assay (± SEM, n = 3 independent studies). Upper inset blot: HEPG2 cells were isolated 6h and 24h after 17AAG+PD184352 exposure and immunoblotting performed to determine the expression and phosphorylation of the indicated proteins. (n = 2). Panel D. Lower graphical panel: SV40 Large T antigen transformed mouse embryonic fibroblasts with specific genomic homozygous deletions (BAK/BAX −/−; BID −/−; PERK −/−) or expressing dominant negative eIF2α, were, 24h after plating, treated with vehicle (DMSO), 17AAG (1 μM), PD184352 (2 μM) or both drugs combined. Forty eight hours after drug exposure, cells were isolated and cell viability determined via trypan blue exclusion (± SEM, n = 3 independent studies). Upper inset: HEPG2 cells were transfected with siRNA molecules to knock down expression of CD95 or with a siScramble control (20 nM). Twenty four h after transfection cells were treated with vehicle (DMSO) or with 17AAG (1 μM) and PD184352 (1 μM). Cells were isolated 6h after drug exposure and the phosphorylation of PERK determined by immunoblotting (n = 2).
In addition to promoting phosphorylation of PERK and eIF2α, treatment with 17AAG+MEK1/2 inhibitor increased the levels of CHOP and of IRE1 without altering the levels of ATF6 and, surprisingly, decreased the levels of the ER resident HSP70 family chaperone GRP78/BiP (Figure 6C, upper inset panel). Over-expression of GRP78/BiP protected cells from drug-induced killing (Figure S9). Over-expression of GRP78/BiP suppressed drug induced autophagy and CD95 activation, and drug-induced ROS generation (Figures S10 and S11). Molecular quenching of Ca2+ signaling suppressed basal levels of GRP78/BiP but also inhibited drug-induced loss of GRP78/BiP levels (Figure S12). In contrast, over-expression of GRP78/BiP suppressed drug-induced Ca2+ signaling (Figure S13). Collectively these findings argue that the primary increase in Ca2+ levels following geldanamycin + MEK1/2 inhibitor exposure is responsible for loss of protective HSP70 family chaperone function facilitating ROS generation and CD95 activation.
Discussion
The present studies were designed to examine in greater detail the molecular mechanisms by which 17AAG and MEK1/2 inhibitors interact to kill GI tumor cells, and in particular the role RAS mutational status played in regulating drug sensitivity. In short term viability assays, 17AAG and MEK1/2 inhibitors caused an additive to greater than additive increase in cell death that was dependent on CD95 activation. In long-term colony formation assays, 17AAG and MEK1/2 inhibitor treatment synergized to kill multiple GI tumor cell types with CI values of ≤ 0.70, indicating a strong level of synergy.
Based on the concept of oncogene addiction, it could be postulated that expression of a mutated active RAS protein which in a cell type dependent fashion constitutively increases ERK1/2 and/or PI3K activities would in turn facilitate the cytotoxic actions of both 17AAG, whose HSP90 inhibitory actions will tend to reduce signaling through ERK1/2 and AKT, and of MEK1/2 inhibitors whose actions will reduce signaling through ERK1/2. In agreement with this concept, we found that the lethality of 17AAG or the drug combination was suppressed by deletion of K-RAS D13 from HCT116 colon cancer cells that correlated with lower constitutive levels of ERK1/2 or AKT activity. It is known that HCT116 cells deleted for K-RAS D13 are non-tumorigenic, which in turn argues that our drug combination will have less toxicity in non-transformed cells. In agreement with this hypothesis in primary rodent hepatocytes MEK1/2 inhibitors did not enhance 17AAG toxicity (Mitchell and Dent, Unpublished observations). We then investigated the relative importance of three of the best defined pathways downstream of RAS proteins that were likely to be involved in controlling drug toxicity: RAF-MEK1/2 (H-RAS V12 S35); PI3K-AKT (H-RAS V12 C40); RAL-GDS (H-RAS V12 G37). We have noted using these cells in vitro and in vivo that activation of RAS predicted resistance to PI3K inhibitors even in the presence of activating PI3K mutations or loss of PTEN, whereas H-RAS V12 (C40) – induced single activation of PI3K predicted for sensitivity to PI3K inhibitors (35) (Figure S14).
Hence, a priori based on the hypothesis of oncogene addiction and the inhibitory actions of 17AAG on AKT and ERK1/2 signaling, we would have predicted that expression of the “RAF-1 (S35)” or the “PI3K-AKT (C40)” activating RAS mutants would have been linked to elevated drug toxicity. Expression of H-RAS V12 but surprisingly not the S35 or the C40 point effector mutants of H-RAS V12 restored the toxicity of 17AAG and of the drug combination, to near those levels observed in wild type cells expressing K-RAS D13. In contrast to the other RAS effector mutants, activation of the RAL-GDS pathway (G37 mutant) profoundly enhanced 17AAG lethality and that of the drug combination. This correlated with a large increase in drug-combination – induced ROS levels. In HCT116 cells expressing H-RAS V12 G37 that were hyper-sensitive to 17AAG-induced ROS production and drug-induced lethality, the large enhanced levels of ROS were partially dependent on mitochondrial function and were largely reliant on NADPH oxidase signaling.
There are several studies that have linked RAS signaling to activation of NADPH oxidases and our data suggest that RAS-dependent activation of the RAL-GDS pathway, in the absence of survival signaling by the RAF and PI3K pathways, plays a key role in RAS dependent tumor cell killing (40, 41). In other GI tumor cell types, including parental HCT116 cells, the generation of ROS by 17AAG+PD184352 treatment was almost abolished by use of inhibitors of mitochondrial respiration and mitochondria deficient Rho zero HuH7 cells lacked an ROS response to 17AAG+PD184352 treatment. As HuH7 cells do not express CD95, our data would argue that drug-induced ROS levels through mitochondrial signaling are truly a “primary” signal and not part of an amplification loop wherein activated CD95 promotes ROS generation through the actions of, e.g. NADPH oxidase enzymes. Treatment of parental HCT116 cells with 17AAG or with 17AAG+MEK1/2 inhibitor reduced ERK1/2 and increased JNK activity, and treatment of HCT116 cells expressing H-RAS V12 G37 caused even greater activation of JNK1/2 activation and a greater reduction in ERK1/2 signaling; effects that were ROS dependent. This data argues, in agreement with other studies, that oncogenic RAS signaling can both promote cell survival but also under certain circumstances play an active role in pro-apoptotic signaling. These findings also emphasize the possibility that a combination of agents that disrupt PI3K and MEK1/2 signaling, plus the use of 17AAG, may represent a useful approach to treat RAS-dependent/addicted tumors.
In prior studies where hepatoma and pancreatic cancer cells were treated with the drugs sorafenib and vorinostat it was shown that cell killing was PERK- and CD95 dependent and the induction of protective autophagy was also PERK- and CD95 dependent (29, 30). In the present studies with geldanamycin and MEK1/2 inhibitor exposure, we found that drug combination lethality was also CD95 dependent. Furthermore, 17AAG+MEK1/2 inhibitor treatment increased the phosphorylation of PERK and eIF2α in a CD95-dependent fashion and knock out or knock down of PERK suppressed drug lethality. MEK1/2 inhibitor + 17AAG treatment also promoted LC3-GFP vesicularization in a CD95- and PERK-dependent fashion that was a “protective” signal. Several groups activating the TRAIL receptor have recently shown that death receptor signaling has both pro-apoptotic components via caspases 8 and 10, and pro-survival components via increased autophagy (42, 43). Our findings in GI tumor cells and primary hepatocytes treated with bile acids, sorafenib+vorinostat and now with 17AAG+MEK1/2 inhibitor provide further strong evidence to argue that activation of the CD95 death receptor produces conflicting survival signals in tumor cells (29, 30). This finding with respect to CD95 signaling could represent one mechanism by which tumor cells could escape immune surveillance killing and have a general resistance to toxic therapeutic agents.
In our studies using bile acids and sorafenib+ vorinostat we have shown that ceramide generation plays a central role in the ability of these agents to cause CD95 activation; however it has also been shown that ceramide generation can alter mitochondrial membrane fluidity and mitochondrial ROS production (44, 45). Inhibition of the acidic sphingomyelinase or the de novo ceramide generation pathways blocked 17AAG+MEK1/2 inhibitor – induced ROS, and blocked CD95 activation. This data was confirmed, in part, when LASS6 was expressed in LASS6 null SW620 cells that facilitated 17AAG+MEK1/2 inhibitor -induced ROS generation, CD95 activation and cell killing. Mitochondrial ROS production has been linked to changes in mitochondrial and cytosolic Ca2+ levels, and vice versa (46). Knock down of acid sphingomyelinase or inhibition of de novo ceramide synthesis did not significantly alter the induction of Ca2+ after drug exposure, however, quenching Ca2+ blocked drug-induced ROS production. These findings argue that 17AAG+MEK1/2 inhibitor treatment induces a primary alteration in cellular Ca2+ fluxes that result in increased ceramide-dependent signaling which acts to promote ROS production. As ceramide can also alter the protein composition of lipid rafts, including activation of CD95 and other growth factor receptors, our data argue that ceramide signaling plays multiple roles in the activation of CD95. Further analyses will be required to define the precise mechanisms, such as modulation of ER Ca2+ ATPase or mitochondrial NADPH oxidase function, by which 17AAG and MEK1/2 inhibitors enhance Ca2+/ceramide/ROS levels in tumor cells.
Treatment of cells with 17AAG+PD184352, but not the individual drugs, suppressed GRP78/BiP expression. GRP78/BiP is an ER localized HSP70 family member whose expression is increased in response to nutrient deprivation and ER stress. GRP78/BiP deletion is embryonic lethal in mice; over-expression of GRP78/BiP can prevent amyloid plaque formation i.e. Alzheimer’s disease; and GRP78/BiP has been extensively characterized with respect to its role in promoting cancer development and progression, tumor drug resistance and metastatic spread (47, 48). A cyto-protective role of GRP78/BiP activation has been argued via complex formation with pro-apoptotic proteins such BAX and pro-caspase 7 and GRP78/BiP is known to bind to and inhibit UPR stress sensors including PERK and IRE1 (47, 48). In retrospective studies, elevated GRP78/BiP levels in patient tumor blocks have been associated with reduced survival, and chemotherapy resistance and tumor relapse (49–52). As geldanamycins have shown clinical activity in breast and prostate cancers, our data in the present manuscript, and those of prior studies using these tumor cell types, together with those showing that GRP78/BiP expression predicts for reduced survival in breast and prostate cancer argues that geldanamycin + MEK1/2 inhibitor treatment could have profound effects on tumor cell chemo-sensitivity (27, 51–53). Studies to prove or refute this possibility are beyond the scope of the present manuscript (Figure S14).
In conclusion, the present findings demonstrate that RAS transformed tumor cells are highly susceptible to being killed by a combination of 17AAG+MEK1/2 inhibitor exposure. Killing by activated RAS proteins was facilitated by RAL-GDS signaling to promote ROS-dependent activation of JNK1/2 and ultimately CD95. As many GI cell types also express highly activated wild type RAS proteins, e.g. HEP3B, our findings argue that the 17AAG+MEK1/2 inhibitor drug combination could have utility in treating many GI malignancies regardless of RAS mutational status.
Supplementary Material
Acknowledgments
Support was provided by P01-CA104177, R01-CA108325, R01-DK52825; R01-CA63753, R01-CA77141, R01-CA097318; R01-CA098712; P01-NS031492. P.D. is The Universal Professor in Signal Transduction and P.B.F. holds the Thelma Newmeyer Corman Chair in Cancer Research and is a SWCRF Investigator.
Abbreviations
- ERK
extracellular regulated kinase
- MEK
mitogen activated extracellular regulated kinase
- EGF
epidermal growth factor
- PARP
poly ADP ribosyl polymerase
- PI3K
phosphatidyl inositol 3 kinase
- −/−
null/gene deleted
- ERK
extracellular regulated kinase
- MAPK
mitogen activated protein kinase
- MEK
mitogen activated extracellular regulated kinase
- R
receptor
- JNK
c-Jun NH2-terminal kinase
- dn
dominant negative
- P
phospho-
- ca
constitutively active
- WT
wild type
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 3.Dent P. MAP kinase pathways in the control of hepatocyte growth, metabolism and survival. In: Dufour JF, Clavien P-A, editors. Signaling Pathways in Liver Diseases. Chapter 19. Springer Press; 2005. pp. 223–238. [Google Scholar]
- 4.Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003;22:5885–96. doi: 10.1038/sj.onc.1206701. [DOI] [PubMed] [Google Scholar]
- 5.Valerie K, Yacoub A, Hagan MP, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789–801. doi: 10.1158/1535-7163.MCT-06-0596. [DOI] [PubMed] [Google Scholar]
- 6.Hamad NM, Elconin JH, Karnoub AE, et al. Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes Dev. 2002;16:2045–57. doi: 10.1101/gad.993902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz ME, McCormick F. Signal transduction from multiple Ras effectors. Curr Opin Genet Dev. 1997;7:75–9. doi: 10.1016/s0959-437x(97)80112-8. [DOI] [PubMed] [Google Scholar]
- 8.Mori M, Uchida M, Watanabe T, et al. Activation of extracellular signal-regulated kinases ERK1 and ERK2 induces Bcl-xL up-regulation via inhibition of caspase activities in erythropoietin signaling. J Cell Physiol. 2003;195:290–7. doi: 10.1002/jcp.10245. [DOI] [PubMed] [Google Scholar]
- 9.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278:18811–6. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 10.Wang YF, Jiang CC, Kiejda KA, Gillespie S, Zhang XD, Hersey P. Apoptosis induction in human melanoma cells by inhibition of MEK is caspase-independent and mediated by the Bcl-2 family members PUMA, Bim, and Mcl-1. Clin Cancer Res. 2007;13:4934–42. doi: 10.1158/1078-0432.CCR-07-0665. [DOI] [PubMed] [Google Scholar]
- 11.Qiao L, Han SI, Fang Y, et al. Bile acid regulation of C/EBP beta, CREB, and c-Jun function, via the extracellular signal-regulated kinase and c-Jun NH2-terminal kinase pathways, modulates the apoptotic response of hepatocytes. Mol Cell Biol. 2003;23:3052–66. doi: 10.1128/MCB.23.9.3052-3066.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li N, Batt D, Warmuth M. B-Raf kinase inhibitors for cancer treatment. Curr Opin Investig Drugs. 2007;8:452–6. [PubMed] [Google Scholar]
- 13.Davies BR, Logie A, McKay JS, et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther. 2007;6:2209–19. doi: 10.1158/1535-7163.MCT-07-0231. [DOI] [PubMed] [Google Scholar]
- 14.Bagatell R, Whitesell L. Altered Hsp90 function in cancer: a unique therapeutic opportunity. Mol Cancer Ther. 2004;3:1021–30. [PubMed] [Google Scholar]
- 15.Neckers L, Neckers K. Heat-shock protein 90 inhibitors as novel cancer chemotherapeutic agents. Expert Opin Emerg Drugs. 2002;7:277–88. doi: 10.1517/14728214.7.2.277. [DOI] [PubMed] [Google Scholar]
- 16.DeBoer C, Meulman PA, Wnuk RJ, Peterson DH. Geldanamycin, a new antibiotic. J Antibiot (Tokyo) 1970;23:442–7. doi: 10.7164/antibiotics.23.442. [DOI] [PubMed] [Google Scholar]
- 17.Isaacs JS, Xu W, Neckers L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell. 2003;3:213–7. doi: 10.1016/s1535-6108(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 18.Nimmanapalli R, O’Bryan E, Kuhn D, Yamaguchi H, Wang HG, Bhalla KN. Regulation of 17-AAG-induced apoptosis: role of Bcl-2, Bcl-XL, and Bax downstream of 17-AAG-mediated down-regulation of Akt, Raf-1, and Src kinases. Blood. 2003;102:269–75. doi: 10.1182/blood-2002-12-3718. [DOI] [PubMed] [Google Scholar]
- 19.Stancato LF, Silverstein AM, Owens-Grillo JK, Chow YH, Jove R, Pratt WB. The hsp90-binding antibiotic geldanamycin decreases Raf levels and epidermal growth factor signaling without disrupting formation of signaling complexes or reducing the specific enzymatic activity of Raf kinase. J Biol Chem. 1997;272:4013–20. doi: 10.1074/jbc.272.7.4013. [DOI] [PubMed] [Google Scholar]
- 20.Nimmanapalli R, O’Bryan E, Bhalla K. Geldanamycin and its analogue 17-allylamino-17-demethoxygeldanamycin lowers Bcr-Abl levels and induces apoptosis and differentiation of Bcr-Abl-positive human leukemic blasts. Cancer Res. 2001;61:1799–804. [PubMed] [Google Scholar]
- 21.Ramanathan RK, Trump DL, Eiseman JL, et al. Phase I pharmacokinetic-pharmacodynamic study of 17-(allylamino)-17-demethoxygeldanamycin (17AAG, NSC 330507), a novel inhibitor of heat shock protein 90, in patients with refractory advanced cancers. Clin Cancer Res. 2005;11:3385–91. doi: 10.1158/1078-0432.CCR-04-2322. [DOI] [PubMed] [Google Scholar]
- 22.Lane D, Robert V, Grondin R, Rancourt C, Piche A. Malignant ascites protect against TRAIL-induced apoptosis by activating the PI3K/Akt pathway in human ovarian carcinoma cells. Int J Cancer. 2007;121:1227–37. doi: 10.1002/ijc.22840. [DOI] [PubMed] [Google Scholar]
- 23.Georgakis GV, Li Y, Rassidakis GZ, Martinez-Valdez H, Medeiros LJ, Younes A. Inhibition of heat shock protein 90 function by 17-allylamino-17-demethoxy-geldanamycin in Hodgkin’s lymphoma cells down-regulates Akt kinase, dephosphorylates extracellular signal-regulated kinase, and induces cell cycle arrest and cell death. Clin Cancer Res. 2006;12:584–90. doi: 10.1158/1078-0432.CCR-05-1194. [DOI] [PubMed] [Google Scholar]
- 24.Powers MV, Clarke PA, Workman P. Dual targeting of HSC70 and HSP72 inhibits HSP90 function and induces tumor-specific apoptosis. Cancer Cell. 2008;14:250–62. doi: 10.1016/j.ccr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Grem JL, Morrison G, Guo XD, et al. Phase I and pharmacologic study of 17-(allylamino)-17-demethoxygeldanamycin in adult patients with solid tumors. J Clin Oncol. 2005;23:1885–93. doi: 10.1200/JCO.2005.12.085. [DOI] [PubMed] [Google Scholar]
- 26.Banerji U, O’Donnell A, Scurr M, et al. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol. 2005;23:4152–61. doi: 10.1200/JCO.2005.00.612. [DOI] [PubMed] [Google Scholar]
- 27.Park MA, Zhang G, Mitchell C, et al. Mitogen-activated protein kinase kinase 1/2 inhibitors and 17-allylamino-17-demethoxygeldanamycin synergize to kill human gastrointestinal tumor cells in vitro via suppression of c-FLIP-s levels and activation of CD95. Mol Cancer Ther. 2008;7:2633–48. doi: 10.1158/1535-7163.MCT-08-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelman JA, Chen L, Tan X, et al. Effective use of PI3K and MEK inhibitors to treat mutant K ras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang G, Park M, Mitchell C, et al. Vorinostat and sorafenib synergistically kill tumor cells via FLIP suppression and CD95 activation. Clin Cancer Res. 2008;14:5385–99. doi: 10.1158/1078-0432.CCR-08-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park MA, Zhang G, Martin AP, et al. Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol & Ther. 2008;7:135–149. doi: 10.4161/cbt.7.10.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park MA, Yacoub A, Rahmani M, et al. OSU-03012 stimulates PERK-dependent increases in HSP70 expression, attenuating its lethal actions in transformed cells. Mol Pharm. 2008;73:1168–84. doi: 10.1124/mol.107.042697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell C, Park MA, Zhang G, et al. 17-Allylamino-17-demethoxygeldanamycin enhances the lethality of deoxycholic acid in primary rodent hepatocytes and established cell lines. Mol Cancer Ther. 2007;6:618–32. doi: 10.1158/1535-7163.MCT-06-0532. [DOI] [PubMed] [Google Scholar]
- 33.White-Gilbertson S, Mullen T, Senkal C, Lu P, Ogretmen B, Obeid L, Voelkel-Johnson C. Ceramide synthase 6 modulates TRAIL sensitivity and nuclear translocation of active caspase-3 in colon cancer cells. Oncogene. 2009 Jan 12; doi: 10.1038/onc.2008.468. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin AP, Miller A, Emad L, et al. Lapatinib resistance in HCT116 cells is mediated by elevated MCL-1 expression and decreased BAK activation and not by ERBB receptor kinase mutation. Mol Pharmacol. 2008;74:807–22. doi: 10.1124/mol.108.047365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ihle N, Lemos R, Wipf P, et al. Activating PI-3-kinase mutation confers sensitivity, while oncogenic Ras mutation confers resistance to PI-3-kinase inhibition. Cancer Res. 2008;69:143–50. doi: 10.1158/0008-5472.CAN-07-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carpinteiro A, Dumitru C, Schenck M, Gulbins E. Ceramide-induced cell death in malignant cells. Cancer Lett. 2008;264:1–10. doi: 10.1016/j.canlet.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 37.Fang Y, Han SI, Mitchell C, et al. Bile acids induce mitochondrial ROS, which promote activation of receptor tyrosine kinases and signaling pathways in rat hepatocytes. Hepatology. 2004;40:961–71. doi: 10.1002/hep.20385. [DOI] [PubMed] [Google Scholar]
- 38.Reinehr R, Sommerfeld A, Häussinger D. CD95 ligand is a proliferative and anti-apoptotic signal in quiescent hepatic stellate cells. Gastroenterology. 2008;134:1494–506. doi: 10.1053/j.gastro.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 39.Reinehr R, Becker S, Eberle A, Grether-Beck S, Häussinger D. Involvement of NADPH oxidase isoforms and Src family kinases in CD95-dependent hepatocyte apoptosis. J Biol Chem. 2005;280:27179–94. doi: 10.1074/jbc.M414361200. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Viciana P, McCormick F. RalGDS comes of age. Cancer Cell. 2005;7:205–6. doi: 10.1016/j.ccr.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Heigold S, Sers C, Bechtel W, Ivanovas B, Schäfer R, Bauer G. Nitric oxide mediates apoptosis induction selectively in transformed fibroblasts compared to nontransformed fibroblasts. Carcinogenesis. 2002;23:929–41. doi: 10.1093/carcin/23.6.929. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Singh R, Massey AC, et al. Loss of macroautophagy promotes or prevents fibroblast apoptosis depending on the death stimulus. J Biol Chem. 2008;283:4766–77. doi: 10.1074/jbc.M706666200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han J, Hou W, Goldstein LA, et al. Involvement of protective autophagy in TRAIL resistance of apoptosis-defective tumor cells. J Biol Chem. 2008;283:19665–77. doi: 10.1074/jbc.M710169200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin J, Hou Q, Mullen TD, et al. Ceramide generated by sphingomyelin hydrolysis and the salvage pathway is involved in hypoxia/reoxygenation-induced Bax redistribution to mitochondria in NT-2 cells. J Biol Chem. 2008;283:26509–17. doi: 10.1074/jbc.M801597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novgorodov SA, Gudz TI, Obeid LM. Long-chain ceramide is a potent inhibitor of the mitochondrial permeability transition pore. J Biol Chem. 2008;283:24707–17. doi: 10.1074/jbc.M801810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan Y, Wei CL, Zhang WR, Cheng HP, Liu J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol Sin. 2006;27:821–6. doi: 10.1111/j.1745-7254.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 47.Rao RV, Hermel E, Castro-Obregon S, del Rio G, Ellerby LM, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J Biol Chem. 2001;276:33869–74. doi: 10.1074/jbc.M102225200. [DOI] [PubMed] [Google Scholar]
- 48.Fu Y, Li J, Lee AS. GRP78/BiP inhibits endoplasmic reticulum BIK and protects human breast cancer cells against estrogen starvation-induced apoptosis. Cancer Res. 2007;67:3734–40. doi: 10.1158/0008-5472.CAN-06-4594. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez PM, Tabbara SO, Jacobs LK, Manning FC, Tsangaris TN, Schwartz AM, Kennedy KA, Patierno SR. Overexpression of the glucose-regulated stress gene GRP78 in malignant but not benign human breast lesions. Breast Cancer Res Treat. 2000;59:15–26. doi: 10.1023/a:1006332011207. [DOI] [PubMed] [Google Scholar]
- 50.Lee E, Nichols P, Spicer D, Groshen S, Yu MC, Lee AS. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 2006;66:7849–53. doi: 10.1158/0008-5472.CAN-06-1660. [DOI] [PubMed] [Google Scholar]
- 51.Pootrakul L, Datar RH, Shi SR, Cai J, Hawes D, Groshen SG, Lee AS, Cote RJ. Expression of stress response protein Grp78 is associated with the development of castration-resistant prostate cancer. Clin Cancer Res. 2006;12:5987–93. doi: 10.1158/1078-0432.CCR-06-0133. [DOI] [PubMed] [Google Scholar]
- 52.Daneshmand S, Quek ML, Lin E, Lee C, Cote RJ, Hawes D, Cai J, Groshen S, Lieskovsky G, Skinner DG, Lee AS, Pinski J. Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum Pathol. 2007;38:1547–52. doi: 10.1016/j.humpath.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 53.Powers MV, Workman P. Targeting of multiple signalling pathways by heat shock protein 90 molecular chaperone inhibitors. Endocr Relat Cancer. 2006 Dec;13:S1, S125–35. doi: 10.1677/erc.1.01324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.