Abstract
Neuropilin is an essential cell surface receptor that functions in both semaphorin dependent axon guidance and vascular endothelial growth factor (VEGF) dependent angiogenesis. The interplay between these two seemingly distinct pathways is a source of considerable interest. Indeed, several semaphorin family members have been shown to have potent anti-angiogenic activity in vivo. However, reports conflict as to whether semaphorin and VEGF competitively bind to neuropilin. Previous work has demonstrated that all known ligands and inhibitors of neuropilin interact with the b1 domain of neuropilin via a C-terminal arginine. No semaphorin family member possesses a C-terminal arginine, leading to uncertainty as to the physical mechanism of interaction between the C-terminal domain of semaphorin and the b1 domain of neuropilin. Semaphorin 3F (Sema3F) possesses an RXRR furin recognition site in its C-terminus and we demonstrate that it is proteolytically processed. This processing is found to be essential for the interaction of the C-terminus of Sema3F with the b1 domain of neuropilin. We further demonstrate that furin activation of the C-terminus of Sema3F produces a species that potently inhibits the binding of VEGF to neuropilin. These studies provide a mechanistic basis for understanding the anti-angiogenic activity of semaphorin as well as the physical interaction and competition between neuropilin ligands.
Vertebrates employ a wide array of secreted growth factors and cell surface receptors to regulate the growth and guidance of axons. The semaphorins represent one of the largest families of cytokines that directly guide axon growth (1, 2). There are five recognized families of semaphorins in vertebrates, including the class III semaphorin family, all six members of which are secreted and able to diffuse through tissues (3). Neuropilin directly binds to most class III semaphorins and is essential for axonal guidance (4, 5).
Neuropilin interacts with members of the semaphorin family of ligands and functions together with plexin family receptors in semaphorin mediated axon guidance (6, 7). Neuropilin also interacts with the VEGF family of ligands and functions together with VEGF-R family receptors in VEGF mediated angiogenesis (8, 9). Higher eukaryotes possess two neuropilin family members, neuropilin-1 and neuropilin-2, which share 44% amino-acid sequence identity (10). They both function in semaphorin and VEGF signaling but differ in their substrate specificity among ligands and receptors, as well as specific control of protein expression and recycling (11). In vivo, Sema3F functions via neuropilin-2 to control axon guidance both in the CNS and peripheral nervous system (12).
The coagulation factor domains of neuropilin, b1 and b2, contain the high-affinity binding site for both VEGF and the C-terminal domain of semaphorin (13, 14). Because semaphorin and VEGF share an overlapping binding site within the b1 domain of neuropilin, the role of neuropilin in mediating interplay between the two seemingly distinct pathways of VEGF dependent angiogenesis and semaphorin dependent axon guidance is the source of considerable interest. However, there are conflicting reports as to the role and extent of ligand competition for neuropilin binding.
A number of researchers have observed direct competition between VEGF and semaphorin (15-17). This is consistent with both VEGF and semaphorin families possessing a highly basic C-terminal domain that interacts with the b1 domain of Nrp. Additionally, multiple class III semaphorin family members have been shown to have potent anti-angiogenic activity in vivo (18-20).
Surprisingly, a number of other researchers have recently reported that there is no competition between VEGF and semaphorin (21, 22). Previous studies defined the critical importance of a C-terminal arginine residue in the binding of both VEGF and inhibitory peptides to neuropilin (23-26). The observed lack of ligand competition for neuropilin is consistent with the fact that no class III semaphorin family members possess a C-terminal arginine, and it has been suggested that two distinct surfaces in the b1 domain of neuropilin may be employed for ligand binding.
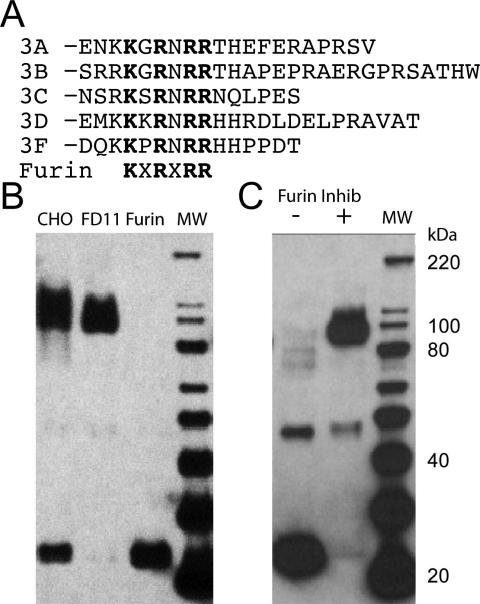
These conflicting reports suggest that a critical mechanistic feature of neuropilin ligand binding is not understood. This has motivated studies to determine the physical mechanism for the interaction of neuropilin and semaphorin and the basis for semaphorin's observed anti-angiogenic activity. The immediate C-terminus of class III semaphorins is not well conserved and does not contain a C-terminal arginine residue (Fig. 1A). However, a region just upstream of the C-termini is, in fact, highly conserved and has been shown to be a cleavage site for the furin family of pro-protein convertases. Proteolytic processing in semaphorin's C-terminal domain has been shown to regulate the anti-angiogenic potency of several semaphorins, which has been suggested to involve neuropilin binding (18, 20, 27). We hypothesized that proteolytic activation of the C-terminus of semaphorin may be critical for regulating interaction with neuropilin. We demonstrate that Sema3F is proteolytically processed at its C-terminus. This processing is essential for the production of a mature C-terminal region of Sema3F that can physically interact with neuropilin. Further, the mature form of semaphorin potently blocks VEGF binding to neuropilin. These data demonstrate that mature semaphorin and VEGF ligands do compete for binding to the overlapping binding site in the b1 domain of neuropilin, but that furin processing of semaphorin is essential for its physical interaction and anti-angiogenic potency. These findings resolve conflicting results in the literature by providing a physical basis for understanding the regulation of semaphorin interaction with neuropilin. Further, these results open new avenues to understand the cross-talk between neuronal and vascular guidance through ligand competition for a shared co-receptor.
Figure 1.
Furin processing of Sema3F A) Class III semaphorins contain a conserved furin recognition sequence in their C-terminus. B) A mixture of unprocessed (100 kDa) and processed (24 kDa) forms of Sema3F-Hgh fusion are observed when overexpressed in CHO cells. Furin deficient (FD11) cells produce only the unprocessed form, whereas furin overexpressing cells produce only the processed form. C) Sema3F-Hgh expressed in COS-7 cells is nearly completely processed, whereas addition of furin inhibitors produces almost complete reversal to the unprocessed form. Protein molecular weights were estimated using the Magic Mark XP molecular weight standard (MW) (Invitrogen, Carlsbad, CA).
EXPERIMENTAL PROCEDURES
Protein production
The C-terminal neuropilin binding region of human Sema3F (residues 605-785 (16)) was produced as a C-terminal or an N-terminal Human Growth Hormone (Hgh) fusion from the pLexM vector (28, 29). Protein was produced from CHO, furin deficient FD11, and furin overexpressing cells (30). Protein was also produced in COS-7 cells in the absence and presence of Dec-RVKR-CMK and D-poly-Arg-NH2 furin inhibitors (Calbiochem, San Diego, CA). Cells were maintained in α-MEM supplemented with 5% FBS. For protein expression, cells were transfered to Hybridoma-SFM media (Invitrogen, Carlsbad, CA) when they reached 80% confluence and transfected with PEI-MAX (Polysciences Inc., Warrington, PA) using 1 μg DNA/mL media and a 3:1 PEI:DNA ratio.
The core ligand binding regions (b1b2) of human neuropilin-2 and neuropilin-1 were expressed and purified as previously described (24).
Western blot
Western blots were performed using an anti-Hgh polyclonal primary antibody (1:10,000 dilution, RDI-HGHabrX1 Fitzgerald Industries, Acton, MA), anti-rabbit-HRP secondary antibody (1:20,000 dilution, sc-2301 Santa Cruz Biotechnology, Santa Cruz, CA), and developed using SuperSignal West Pico (Pierce Biotechnology, Rockford, IL).
Peptide synthesis
Peptides were synthesized using solid phase synthesis and purified to >95% purity. The well characterized neuropilin inhibitory peptide ATWLPPR was used as a positive control (Sigma-Genosys, St. Louis, MO). Two dimeric disulfide linked peptides of the C-terminal region of Sema3F were synthesized, oxidized to produce the natural intramolecular disulfide, and purified (Genscript, Piscataway, NJ). One peptide, C-Sema, corresponds to the final 46 residues of Sema3F (GLIHQYCQGYWRHVPPSPREAPGAPRSPEPDQKKPRNRRHHPPDT) while the second peptide, C-furSema, is 40 residues and corresponds to the furin cleaved species (GLIHQYCQGYWRHVPPSPREAPGAPRSPEPQDQKKPRNRR). Each peptide contains a single tryptophan residue and peptide concentrations were determined using absorbance at 280 nm.
Affinity pull-down
Neuropilin-2 was coupled to AffiGel (Bio-Rad, Hercules, CA) according to manufacturer's recommendation at 5 mg protein/mL resin. N-terminally tagged Sema3F Ig-basic was expressed in Cos-7 cells with and without furin inhibitors. 200μL of conditioned media was diluted to 1 mL with Buffer A (20 mM Tris, pH=7.5, 100 mM NaCl) and incubated with 100 μL of neuropilin-2 affinity resin for thirty minutes. Resin was washed three times with Buffer A, and then eluted using 1 M NaCl. Eluted protein was resolved using SDS-PAGE and visualized by western blot.
Analytical Ultracentrifugation
Sedimentation velocity experiments were conducted with a Beckman XL-A analytical ultracentrifuge (Beckman-Coulter, Fullerton, CA). Experiments were conducted at 4°C in 10mM Tris pH 7.5, 150 mM NaCl, 1mM sodium azide at a speed of 40,000 rpm. Neuropilin-2 concentration was held constant at 8μM in all samples. Samples with Sema3F derived peptides contained peptides in 2.5X molar excess. Absorption measurements were made at 280 nm. Measurements of sedimentation coefficient distributions between 0.1 and 6s were resolved by fitting data to numerical solutions of the Lamm equation implemented in the program Sedfit (31). Parameters used, including buffer density, viscosity, and partial specific volumes of the proteins, were calculated with SEDNTERP (http://www.rasmb.bbri.org/) using standard methods (32).
Fluorescence Aniosotropy
C-furSema was synthesized with an N-terminal Fluorescein isothiocyanate (FITC) (Genscript). The peptide was resuspended and buffer exchanged into Buffer A. 1.8 μM FITC C-furSema was combined with increasing concentrations of neuropilin-2 and neuropilin-1. Fluorescence aniosotropy was measured at 23°C using a SpectraMax M5 (Molecular Devices, Sunnyvale, CA) with excitation at 485 nm, emission at 525 nm, and an emission filter at 515 nm). Anisotropy was calculated from the average of three independent samples at each point using the experimentally determined G-factor of 1.113. Dissociation constants (Kd) were calculated by fitting the data with Kaleida-Graph (Synergy Software, Reading, PA) using a single site model:
where ro is the initial aniosotropy and ra is the difference in aniosotropy between bound and free species.
Plate-based inhibition assay
A quantitative method to determine peptide inhibitory potency was developed. The core ligand binding domains (b1b2) of neuropilin-1 were physically coupled to high protein binding 96-well polystyrene plates (Corning, 9018). Neuropilin-1 was coupled, using a one hour incubation in pH=10.4 carbonate, followed by blocking with BSA. Using this method, 500 ng of purified neuropilin-1 could be efficiently coupled to each well. Inhibitory potency was measured by displacement of alkaline phosphatase (AP) tagged VEGF-A(164). AP was used because it allows rapid, sensitive, quantitative competition assays to be performed. Protein was produced with an N-terminal AP tag (pAPtag-5, GenHunter, Nashville, TN) in CHO-S cells (Invitrogen, Carlsbad, CA). Conditioned media was concentrated and buffer exchanged into 20 mM Tris pH 7.5, 50 mM NaCl.
AP-tagged VEGF-A and inhibitory peptides were premixed and incubated in the 96-well plate for one hour at 25°C. Wells were washed three times with PBS-T and incubated with PBS-T for another five minutes. Wash solution was removed and 100 μL of 1X AP Assay A Reagent was added (GenHunter). The reaction was stopped after eight minutes by addition of 100mL 0.5N NaOH. Evolution of p-Nitrophenol (p-NP) was quantitated at 405nm using a 96-well plate reader and converted to AP activity according to manufacturer's instructions. Displacement of AP-tagged VEGFs from the plate with increasing peptide concentration was fit, using a standard four parameter sigmoidal curve, yielding the IC50.
RESULTS
Sema3F is proteolytically processed in its C-terminal domain
Sema3F possesses an RXRR consensus furin-like protease recognition sequence at its C-terminus. This region is highly conserved in five of the class III semaphorin family members (Figure 1A). To test whether Sema3F is proteolytically processed in its C-terminus, we expressed the C-terminal neuropilin binding region of Sema3F (Ig-basic) with a C-terminal human growth hormone (Hgh) fusion. The construct was expressed in CHO cells, FD11 CHO cells lacking furin activity, and CHO cells overexpressing furin (30). Wild-type CHO cells expressed a mixture of processed and unprocessed forms of Sema3F (Figure 1B). FD11 cells produced solely the unprocessed form of Sema3F, whereas furin overexpressing cells produced only the processed form (Figure 1B). To further confirm that the observed proteolytic processing is the result of furin activity, the construct was expressed in Cos7 cells. The protein was found to be >95% processed (Figure 1C). The observed proteolytic processing is fully blocked by addition of furin inhibitors (Figure 1C). Thus, as has been previously observed with other class III semaphorin family members (18, 20), Sema3F is proteolytically processed in its C-terminal basic domain.
C-terminal processing of Sema3F regulates interaction with neuropilin
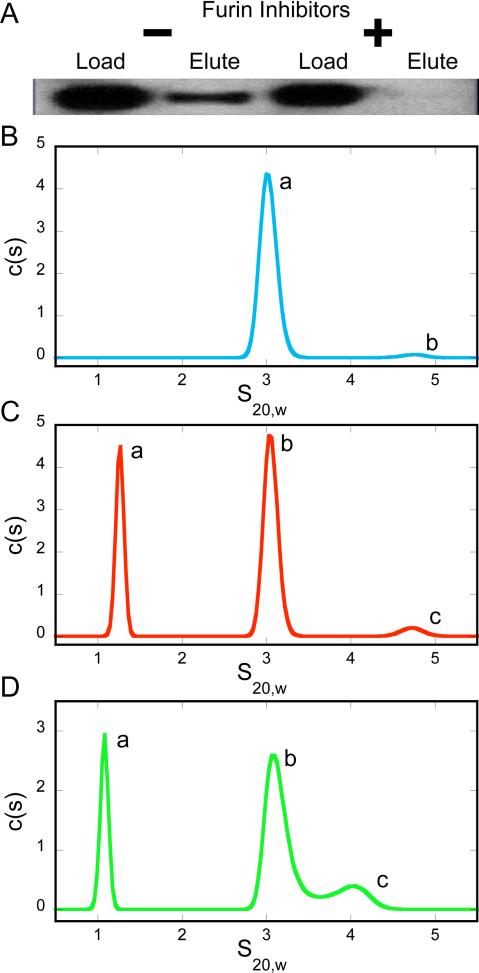
A specific interaction has been shown between the C-terminus of Sema3F and core ligand binding domains (b1b2) of neuropilin-2 (13). To determine the effect of the observed proteolytic processing, we tested the ability of the unprocessed and processed forms of Sema3F to interact with neuropilin, utilizing a neuropilin-2 affinity pull-down of the C-terminus of Sema3F. In order to test the effect of the C-terminal sequence, a construct was produced with a native C-terminal sequence and the Hgh attached to the N-terminus. Furin cleavage would remove only six residues at the C-terminus and so, as expected, no difference in apparent molecular weight is observed between N-terminally tagged protein expressed in Cos-7 cells in the absence or presence of furin inhibitors. However, a dramatic difference is observed in their ability to interact with neuropilin-2. The processed form of Sema3F, expressed from Cos-7 cells in the absence of furin inhibitors, shows a robust interaction with the neuropilin-2 affinity resin (Figure 2A). This result is consistent with previous reports describing the domain specific interaction between the C-terminal Ig-basic domain of Sema3F and neuorpilin-2 (13). In contrast, the unprocessed form, expressed from Cos-7 cells in the presence of furin inhibitors, shows little if any ability to interact with the neuropilin-2 affinity resin (Figure 2A). This result suggests that the mechanism underlying the profound physiological effect of furin processing of semaphorin may be direct regulation of the physical interaction between the C-terminus of semaphorin and the b1 domain of neuopilin.
Figure 2.
Interaction of the C-terminus of semaphorin with neuropilin-2. A) Affinity purification of the Hgh-Sema3F C-terminus using neuropilin-2 affinity resin demonstrates that only the protein with a furin processed C-terminus is efficiently pulled down. B) Sedimentation velocity experiments reveal that neuropilin-2 exists predominantly as a monomer, with a small fraction of dimer. Peak labels (a, b, c) correspond to the numerical peak data in Table 1. C) C-Sema shows no significant interaction with neuropilin-2. D) C-furSema interacts with neuropilin-2 in a stable 1:1 complex and a higher order species with an intermediate molecular weight, suggesting the species is in exchange between 1:1 and 2:1 neuropilin-2:C-furSema complexes.
Physical interaction between the C-terminus of Sema3F and neuropilin-2
To more fully characterize the interaction of the C-terminus of semaphorin with neuropilin, we produced peptides from the C-terminus of Sema3F that include the C-terminal intermolecular disulfide and basic domain. This allowed production of a pure, chemically defined species corresponding to the unprocessed (C-Sema, the final 46 residues of Sema3F) and processed (C-furSema, the same with the final six residues removed, thus possessing a C-terminal arginine) forms of Sema3F.
Sedimentation velocity analysis was performed to determine the interaction and stoichiometry of Sema3F in complex with neuropilin-2. On its own, neuropilin-2 exists primarily as a monomer (s20,w=3.03, c(M)=37.9 kDa ± 1.9 kDa, expected 38.4 kDa) with a small fraction (3%) of dimer (s20,w=4.73) (Figure 2B, Table 1). When a 2.5 fold molar excess of C-Sema is added, neuropilin-2 monomer (s20,w=3.04) and dimer (s20,w=4.65) are observed, along with free C-Sema (s20,w=1.26) (Figure 2C, Table 1).
Table 1.
Sedimentation velocity data of neuropilin-2 alone and in complex with C-Sema and C-furSema
| s20,w (S=10-13sec) : % | c(M) (kDa) | Expected MW | |
|---|---|---|---|
| Neuropilin-2: | |||
| Peak a | 3.03 : 97% | 37.9 ± 1.9 kDa | 38.4 kDa (monomer) |
| Peak b | 4.73 : 3% | 74.3 ± 3.0 kDa | 76.8 kDa (dimer) |
| Neuropilin-2 + C-Sema: | |||
| Peak a | 1.26 : 32% | 11.1 ± 0.6 kDa | 10.7 kDa (C-Sema) |
| Peak b | 3.04 : 58% | 41.7 ± 1.8 kDa | 38.4 kDa (monomer) |
| Peak c | 4.65 : 4% | 80.2 ± 3.0 kDa | 76.8 kDa (dimer) |
| Neuropilin-2 + C-furSema: | |||
| Peak a | 1.09 : 24% | 9.4 ± 0.6 kDa | 9.4 kDa (C-furSema) |
| Peak b | 3.15 : 62% | 46.7 ± 3.4 kDa | 47.7 kDa (1:1 complex) |
| Peak c | 4.02 : 12% | 66.7± 4.9 kDa | 86.2 kDa (2:1 complex) |
When C-furSema is added, significant differences are observed. The major species shifts, with molecular weight consistent with a 1:1 complex (s20,w=3.15, c(M)=46.7 ± 3.4 kDa, expected weight of a 1:1 complex, 47.7 kDa). Interestingly, the 1:1 complex appears to predominate even though C-furSema is a disulfide linked dimer. A second unique species is formed (s20,w=4.02, c(M)=66.7± 4.9 kDa, expected weight of a 2:1 complex, 86.2 kDa). This species sediments at an intermediate mass between that expected for the 1:1 and 2:1 neuropilin-2:C-furSema complex, likely representing a species in exchange between the two forms of the complex. Free C-furSema is also observed, as expected since it is in molar excess (s20,w=1.09) (Figure 2D, Table 1).
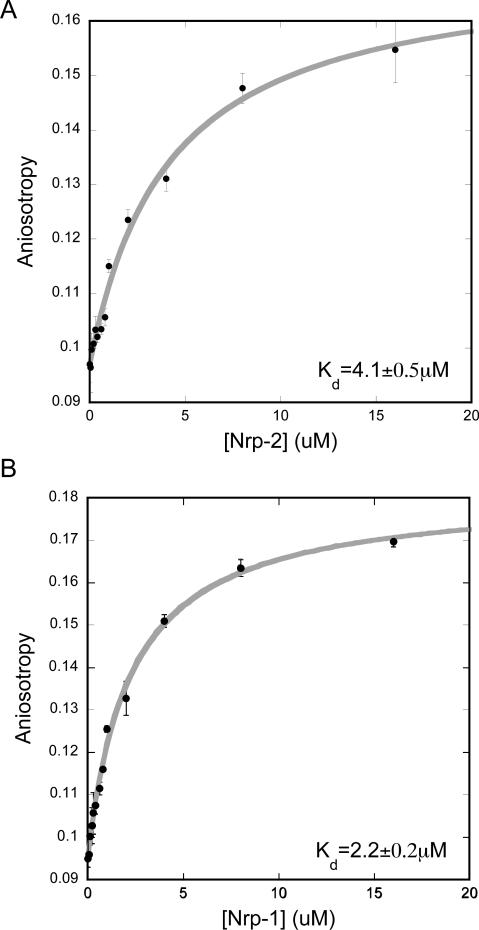
To quantitatively characterize the interaction of the two proteins, the binding of FITC labeled C-furSema to neuropilin-2 was measured using fluorescence anisotropy. Incubation with neuropilin-2 resulted in a significant increase in anisotropy consistent with a decrease in the rotational diffusion of the bound complex. The observed binding was well fit with a single site binding model (R2=0.99) and allowed determination of the dissociation constant Kd=4.1±0.5 μM (Figure 3A).
Figure 3.
Binding of C-furSema to neuropilin. A) FITC C-furSema shows a significant increase in anisotropy when bound to neuropilin-2, which is fit well with a single site binding curve. B) Neuropilin-1 also binds to FITC C-furSema with slightly higher affinity.
Based on these data, we conclude that furin mediated activation of Sema3F is critical for the physical interaction of the C-terminus of Sema3F with neuropilin-2.
Binding of C-furSema to neuropilin-1
Sema3F functions with neuropilin-2 in axon guidance, but functionally blocks VEGF-A binding to neuropilin-1 to block angiogenesis. To determine the basis for Sema3F anti-angiogenic activity, we first tested if C-furSema was also able to bind to neuropilin-1. The binding of FITC C-furSema to neuropilin-1 was determined using fluorescence anisotropy as with neuropilin-2. Binding was again well fit with a single site binding model (R2=0.99) and a dissociation constant Kd=2.2±0.2 μM (Figure 3B). Thus, C-furSema can bind both to neuropilin-2 and neuropilin-1, the latter with slightly higher affinity.
Inhibition of VEGF binding
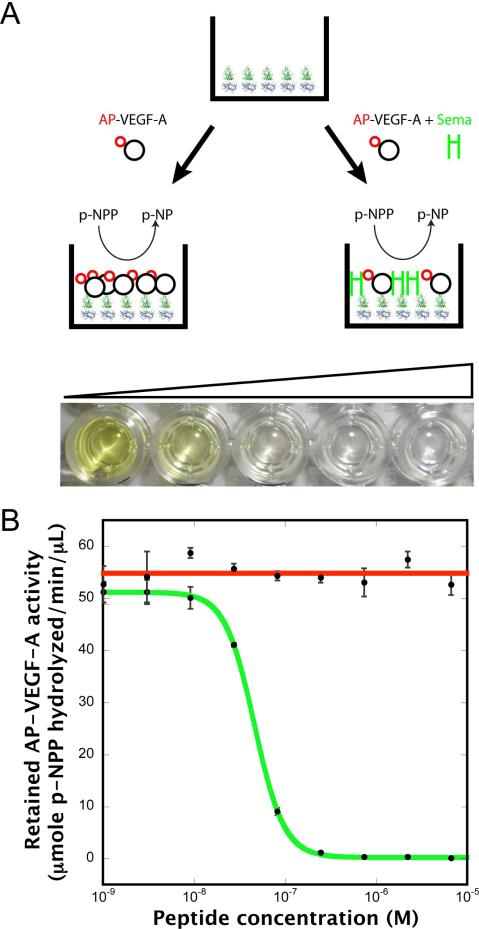
To test whether the observed anti-angiogenic activity of Sema3F is due to direct competition with VEGF-A for binding to neuropilin-1, we developed a novel inhibitory assay. This assay measures the ability of Sema3F to compete with VEGF-A for binding to the core ligand binding domains (b1b2) of neuropilin-1. Neuropilin-1 was adsorbed to 96-well plates to which AP-tagged VEGF-A binds specifically. VEGF-A binding could be competitively blocked using anti-angiogenic inhibitory peptides or other blocking reagents. Bound AP-VEGF-A was quantitatively determined using a colorometric p-NPP based assay (Figure 4A).
Figure 4.
C-furSema potently inhibits VEGF-A binding to neuropilin. A) Design of a novel plate based inhibition assay measuring the displacement of AP-tagged VEGF-A from neuropilin-1 b1b2 coated plates with increasing concentration of peptide. B) C-furSema potently inhibits the binding of AP-VEGF-A to neuropilin-1 with an IC50=45nM (green). C-Sema shows no inhibitor potency even at high concentrations (red). Each point is the average of three independent samples with error bars representing +/- one standard deviation.
To validate the assay, we used the well characterized neuropilin inhibitory hepta-peptide ATWLPPR. Increasing concentrations of ATWLPPR were able to block AP-VEGF-A binding. The inhibitory potency of the peptide determined using this novel assay was IC50=10.5 ± 2 μM (Supplementary Figure 1). This corresponds well to its previously reported inhibitory potency IC50=19 μM, determined by measuring displacement of biotinylated VEGF-A from the neuropilin ectodomain (33).
C-furSema, representing the processed form of Sema3F, was able to fully inhibit the binding of AP-VEGF-A to neuropilin. C-furSema was found to be a very potent inhibitor with an IC50=46 ± 3 nM (R2=0.9999) (Figure 4B, green). This demonstrates that, in fact, Sema3F and VEGF-A do directly compete for binding to the core ligand binding domains of neuropilin, explaining the anti-angiogenic potency of Sema3F in vivo.
To determine the effect of proteolytic activation of Sema3F on anti-angiogenic potency, we tested the ability of C-Sema to competitively block VEGF-A binding to neuropilin. C-Sema, representing the unprocessed form of Sema3F, showed no inhibition of VEGF-A binding even at high concentrations (Figure 4B, red).
These results underline the essential importance of furin processing of semaphorin, and provide a mechanism for the observed anti-angiogenic potency of Sema3F. In summary, we demonstrate that furin processing produces a form of Sema3F that binds to the core ligand binding domains of neuropilin and directly competes with VEGF-A for receptor binding.
DISCUSSION
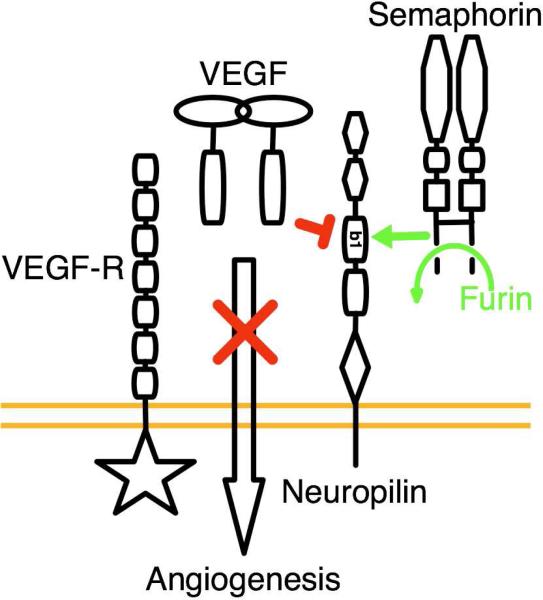
We demonstrate that Sema3F is proteolytically processed at its C-terminus. This processing is essential for the interaction of Sema3F with the core ligand binding domains of neuropilin. Our data provides a physical explanation for this, since furin processing liberates a C-terminal arginine. Possession of a C-terminal arginine has been demonstrated to be critical for the interaction of both VEGF and peptide inhibitors with neuropilin (24, 25). Further, the b1 domain of neuropilin is utilized for binding both VEGF and semaphorin families of neuropilin ligands, yet the nature of the different ligand interactions with and competition for neuropilin has been unclear. We demonstrate that the two classes of ligands directly compete for neuropilin binding, but only when semaphorin is processed. A C-terminal peptide representing the proteolytically processed form of Sema3F potently blocks the binding of VEGF to neuropilin, explaining the anti-angiogenic activity of Sema3F (Figure 5).
Figure 5.
Model for the mechanism of anti-angiogenic activity of semaphorin. Our data demonstrates that furin dependent activation of the C-terminus of Sema3F is essential for direct interaction with neuropilin and anti-angiogenic activity via competition with VEGF-A for binding to the b1 domain of neuropilin.
Understanding the mechanistic basis for the interaction of the C-terminus of semaphorin with neuropilin also provides a simple yet elegant explanation for the divergent literature reports regarding the competition of VEGF and semaphorin ligands for neuropilin binding. C-terminal fusions, such as Fc or AP, are often used in the expression and purification of semaphorin family members. This includes commercially available semaphorins, which are expressed and purified using a C-terminal Fc-fusion (R & D Systems). These proteins represent solely the unprocessed form of semaphorin and, as expected from our studies, are unable or have dramatically decreased ability to compete with VEGF for binding to neuropilin. When using a C-terminal tag, such as AP, for quantitation but not purification, the protein produced will likely be a mixture of the processed and unprocessed forms. When using an N-terminal tag, care should be taken since proteolytic processing of the C-terminus does not produce an appreciable shift in molecular weight and thus a mixture of processed forms will be produced unless furin activity is specifically inhibited or enhanced. It is interesting to note that Sema3F produced in wild-type CHO cells has a larger percentage of unprocessed protein, whereas that produced in COS-7 cells is almost completely processed. In vitro, the ratio of processed to unprocessed semaphorin will be highly dependent on the cell type used to express the protein. In vivo, cell and tissue-specific proteolytic processing of semaphorin family members may well represent an important mechanism controlling the production of anti-angiogenic semaphorins.
The role of proteolytic processing of the C-terminus of Sema3F is the simplest case for class III semaphorins, since it possesses a single furin consensus site in its C-terminus. Other family members have additional furin-like consensus sites. For instance, it has been demonstrated experimentally that semaphorin 3A can be processed at three different sites in its final forty-five residues (18). It will be interesting to determine if proteolytic processing at these different sites produces proteins with differing anti-angiogenic potency.
Paradoxically, it has been shown that furin processing of semaphorin 3B can inactivate its anti-angiogenic potency (34). However, the observed cleavage is at a known site in the middle of the semaphorin gene, upstream of the Ig domain, and removes the entire Ig-basic region, including the cysteine that forms the critical intermolecular disulfide essential for semaphorin function (3). Thus, proteolytic processing of semaphorin can either activate or inactivate its anti-angiogenic potency, depending on the site of proteolysis.
While it is clear that only the processed form of semaphorin is able to function as a VEGF pathway inhibitor, the role of C-terminal processing of semaphorin in axon guidance is an intriguing area that remains to be explored. The C-terminal domain of semaphorin is necessary, but not sufficient, for its function in axon guidance. Semaphorin additionally requires an interaction between its sema domain and the a1 domain of neuropilin (14, 16, 35). It is interesting to note that the C-terminal Fc fusion of semaphorin, which represents the unprocessed form of semaphorin, is able to cause axon repulsion in situ (21, 36). Thus, both processed and unprocessed forms of semaphorin are able to function in situ in axon guidance. In vivo, furin processing may be solely utilized as a mechanism regulating the anti-angiogenic activity of semaphorin or it may alter the potency and range of activity of semaphorin in axon guidance. Alternatively, VEGF has well characterized neurotrophic and neuroprotective effects and it is possible that furin processing of semaphorin could affect the ability of VEGF to compete for neuropilin binding on the surface of neuronal and glial cells.
Neuropilin ligand mimicry
Semaphorin-like proteins are produced by a variety of viruses that utilize molecular mimicry. Various poxviruses encode SemaV family members, which are sema domain proteins homologous to the N-terminus of semaphorin (37). SemaV family members have been shown to induce changes in host cytoskeletal dynamics, thereby altering the adherence and spreading of infected cells (38). It has recently been shown that neuropilin is essential for HTLV-1 viral entry (39). The HTLV-1 coat protein is a heparin binding protein that directly interacts with neuropilin (40). Further, the interaction and infectivity of HTLV-1 can be attenuated by both VEGF-A and peptide inhibitors of neuropilin (40). Intriguingly, the HTLV coat protein that interacts with neuropilin requires furin processing for maturation and infectivity (41). From our studies, we suggest that HTLV-1 utilizes molecular mimicry of the mature furin processed form of semaphorin to target the shared semaphorin/VEGF binding site in the b1 domain of neuropilin. This insight provides a novel avenue for potential therapeutic intervention in HTLV-1 infected individuals.
Novel inhibitors of angiogenesis
The difference between the observed direct binding affinity of C-furSema to neuropilin and its inhibitory potency in blocking VEGF binding to neuropilin is of interest. The observed binding affinity of C-furSema for neuropilin (2.2 μM) is comparable to those reported for VEGF binding to neuropilin-1 (2 μM) by surface plasmon resonance with low neuropilin density (42). Our analytical ultracentrifugation results suggest a 1:1 stoichiometry of binding in dilute solution conditions, suggesting that this affinity respresents the monomeric binding of C-furSema to neuropilin. However, VEGF binding to neuropilin has been shown to be highly dependent on neuropilin density, with four fold higher density of neuropilin leading to a twenty-fold increase in apparent affinity (42). The higher potency of inhibition observed in our plate-based inhibitory assay is consistent with this result, since maximal amounts of neuropilin are coupled to the plate. C-furSema's inhibitory potency in the plate based assay is thus likely due to avidity affects of the dimeric ligand binding to two neuropilin molecules, and accurately reproduces the inhibitory potency of previously reported peptides measured both in vitro and in situ (Supplementary Figure 1).
Two major classes of peptide-based neuropilin inhibitors have been described (23, 43). Both are relatively small monomeric peptides (5-7 residues) with modest inhibitory potency (mid-μM). It has been unclear if this modest potency is due to specific features of the peptides or if it represents a general problem with this mode of inhibiting angiogenesis. Our results reveal that C-furSema is able to inhibit binding of VEGF-A to neuropilin with an increase in potency of two to three orders of magnitude relative to previous inhibitors. It will be interesting to determine the physical basis for this enhanced potency. It is notable that while dimeric VEGF was found to directly antagonize semaphorin mediated growth cone collapse, a monomeric peptide inhibitor derived from the C-terminus of VEGF reversed this effect (36). C-furSema contains the strictly conserved intermolecular disulfide, and the multimeric state of the peptide may well contribute to its enhanced potency. Additionally, the C-terminal region of all known anti-angiogenic semaphorins shows conservation beyond the terminal 5-7 residues. This suggests that additional binding pockets on neuropilin may be employed which are not exploited by current generation peptides. Together, these results strongly suggest that potent peptide inhibitors of neuropilin can be produced, opening exciting avenues to design novel inhibitors based on Sema3F and other endogenous angiogenesis inhibitors.
Supplementary Material
ACKNOWLEDGMENT
We thank Drs. Daniel Leahy, David Ginty, Alex Kolodkin, David Rodgers and Mr. Hou-Fu Guo for valuable advice and discussions, and Dr. Steven Leppla for providing the CHO C16, FD11, and furin overexpressing cell lines.
Abbreviations
- Sema3F
Semaphorin 3F
- VEGF
vascular endothelial growth factor
- AP
alkaline phosphatase
- Hgh
Human Growth Hormone
- FITC
Fluorescein isothiocyanate
Footnotes
This work was supported by NIH grants P20RR020171 (C.W.V.K), GM-070662 (M.G.F.) and NSF REU grant DBI-0648233 (M.W.P.) and by the Kentucky Lung Cancer Research Program.
SUPPORTING INFORMATION AVAILABLE
Inhibitory potency of the peptide ATWLPPR. Supplemental materials may be accessed free of charge online at http://pubs.acs.org.
REFERENCES
- 1.Koncina E, Roth L, Gonthier B, Bagnard D. Role of semaphorins during axon growth and guidance. Adv Exp Med Biol. 2007;621:50–64. doi: 10.1007/978-0-387-76715-4_4. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y, Gunput RA, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci. 2008;33:161–170. doi: 10.1016/j.tibs.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- 4.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura F, Tanaka M, Takahashi T, Kalb RG, Strittmatter SM. Neuropilin-1 extracellular domains mediate semaphorin D/III-induced growth cone collapse. Neuron. 1998;21:1093–1100. doi: 10.1016/s0896-6273(00)80626-1. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- 7.Fujisawa H. Discovery of semaphorin receptors, neuropilin and plexin, and their functions in neural development. J Neurobiol. 2004;59:24–33. doi: 10.1002/neu.10337. [DOI] [PubMed] [Google Scholar]
- 8.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 9.Klagsbrun M, Takashima S, Mamluk R. The role of neuropilin in vascular and tumor biology. Adv Exp Med Biol. 2002;515:33–48. doi: 10.1007/978-1-4615-0119-0_3. [DOI] [PubMed] [Google Scholar]
- 10.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron. 1997;19:547–559. doi: 10.1016/s0896-6273(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 12.Sahay A, Molliver ME, Ginty DD, Kolodkin AL. Semaphorin 3F is critical for development of limbic system circuitry and is required in neurons for selective CNS axon guidance events. J Neurosci. 2003;23:6671–6680. doi: 10.1523/JNEUROSCI.23-17-06671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giger RJ, Urquhart ER, Gillespie SK, Levengood DV, Ginty DD, Kolodkin AL. Neuropilin-2 is a receptor for semaphorin IV: insight into the structural basis of receptor function and specificity. Neuron. 1998;21:1079–1092. doi: 10.1016/s0896-6273(00)80625-x. [DOI] [PubMed] [Google Scholar]
- 14.Mamluk R, Gechtman Z, Kutcher ME, Gasiunas N, Gallagher J, Klagsbrun M. Neuropilin-1 binds vascular endothelial growth factor 165, placenta growth factor-2, and heparin via its b1b2 domain. J Biol Chem. 2002;277:24818–24825. doi: 10.1074/jbc.M200730200. [DOI] [PubMed] [Google Scholar]
- 15.Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu C, Limberg BJ, Whitaker GB, Perman B, Leahy DJ, Rosenbaum JS, Ginty DD, Kolodkin AL. Characterization of neuropilin-1 structural features that confer binding to semaphorin 3A and vascular endothelial growth factor 165. J Biol Chem. 2002;277:18069–18076. doi: 10.1074/jbc.M201681200. [DOI] [PubMed] [Google Scholar]
- 17.Narazaki M, Tosato G. Ligand-induced internalization selects use of common receptor neuropilin-1 by VEGF165 and semaphorin3A. Blood. 2006;107:3892–3901. doi: 10.1182/blood-2005-10-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams RH, Lohrum M, Klostermann A, Betz H, Puschel AW. The chemorepulsive activity of secreted semaphorins is regulated by furin-dependent proteolytic processing. EMBO J. 1997;16:6077–6086. doi: 10.1093/emboj/16.20.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kessler O, Shraga-Heled N, Lange T, Gutmann-Raviv N, Sabo E, Baruch L, Machluf M, Neufeld G. Semaphorin-3F is an inhibitor of tumor angiogenesis. Cancer Res. 2004;64:1008–1015. doi: 10.1158/0008-5472.can-03-3090. [DOI] [PubMed] [Google Scholar]
- 20.Christensen C, Ambartsumian N, Gilestro G, Thomsen B, Comoglio P, Tamagnone L, Guldberg P, Lukanidin E. Proteolytic processing converts the repelling signal Sema3E into an inducer of invasive growth and lung metastasis. Cancer Res. 2005;65:6167–6177. doi: 10.1158/0008-5472.CAN-04-4309. [DOI] [PubMed] [Google Scholar]
- 21.Appleton BA, Wu P, Maloney J, Yin J, Liang WC, Stawicki S, Mortara K, Bowman KK, Elliott JM, Desmarais W, Bazan JF, Bagri A, Tessier-Lavigne M, Koch AW, Wu Y, Watts RJ, Wiesmann C. Structural studies of neuropilin/antibody complexes provide insights into semaphorin and VEGF binding. Embo J. 2007;26:4902–4912. doi: 10.1038/sj.emboj.7601906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vieira JM, Schwarz Q, Ruhrberg C. Selective requirements for NRP1 ligands during neurovascular patterning. Development. 2007;134:1833–1843. doi: 10.1242/dev.002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Wronski MA, Raju N, Pillai R, Bogdan NJ, Marinelli ER, Nanjappan P, Ramalingam K, Arunachalam T, Eaton S, Linder KE, Yan F, Pochon S, Tweedle MF, Nunn AD. Tuftsin binds neuropilin-1 through a sequence similar to that encoded by exon 8 of vascular endothelial growth factor. J Biol Chem. 2006;281:5702–5710. doi: 10.1074/jbc.M511941200. [DOI] [PubMed] [Google Scholar]
- 24.Vander Kooi CW, Jusino MA, Perman B, Neau DB, Bellamy HD, Leahy DJ. Structural basis for ligand and heparin binding to neuropilin B domains. Proc Natl Acad Sci U S A. 2007;104:6152–6157. doi: 10.1073/pnas.0700043104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starzec A, Ladam P, Vassy R, Badache S, Bouchemal N, Navaza A, du Penhoat CH, Perret GY. Structure-function analysis of the antiangiogenic ATWLPPR peptide inhibiting VEGF(165) binding to neuropilin-1 and molecular dynamics simulations of the ATWLPPR/neuropilin-1 complex. Peptides. 2007;28:2397–2402. doi: 10.1016/j.peptides.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Teesalu T, Sugahara KN, Kotamraju VR, Ruoslahti E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc Natl Acad Sci U S A. 2009;106:16157–16162. doi: 10.1073/pnas.0908201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potiron VA, Roche J, Drabkin HA. Semaphorins and their receptors in lung cancer. Cancer Lett. 2009;273:1–14. doi: 10.1016/j.canlet.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leahy DJ, Dann CE, 3rd, Longo P, Perman B, Ramyar KX. A mammalian expression vector for expression and purification of secreted proteins for structural studies. Protein Expr Purif. 2000;20:500–506. doi: 10.1006/prep.2000.1331. [DOI] [PubMed] [Google Scholar]
- 29.Aricescu AR, Lu W, Jones EY. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr D Biol Crystallogr. 2006;62:1243–1250. doi: 10.1107/S0907444906029799. [DOI] [PubMed] [Google Scholar]
- 30.Gordon VM, Klimpel KR, Arora N, Henderson MA, Leppla SH. Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infect Immun. 1995;63:82–87. doi: 10.1128/iai.63.1.82-87.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuck P, Perugini MA, Gonzales NR, Howlett GJ, Schubert D. Size-distribution analysis of proteins by analytical ultracentrifugation: strategies and application to model systems. Biophys J. 2002;82:1096–1111. doi: 10.1016/S0006-3495(02)75469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laue TM, Shah BD, Ridgeway TM, Pelletier SL. Computer-Aided Interpretation of Analytical Sedimentation Data For Proteins. In: Harding SE, Rowe AJ, Harding JC, editors. Analytical Ultracentrifugation in Biochemistry and Polymer Science. The Royal Society of Chemistry; Cambridge, England: 1992. pp. 90–125. [Google Scholar]
- 33.Tirand L, Frochot C, Vanderesse R, Thomas N, Trinquet E, Pinel S, Viriot ML, Guillemin F, Barberi-Heyob M. A peptide competing with VEGF165 binding on neuropilin-1 mediates targeting of a chlorin-type photosensitizer and potentiates its photodynamic activity in human endothelial cells. J Control Release. 2006;111:153–164. doi: 10.1016/j.jconrel.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Varshavsky A, Kessler O, Abramovitch S, Kigel B, Zaffryar S, Akiri G, Neufeld G. Semaphorin-3B is an angiogenesis inhibitor that is inactivated by furin-like pro-protein convertases. Cancer Res. 2008;68:6922–6931. doi: 10.1158/0008-5472.CAN-07-5408. [DOI] [PubMed] [Google Scholar]
- 35.Klostermann A, Lohrum M, Adams RH, Puschel AW. The chemorepulsive activity of the axonal guidance signal semaphorin D requires dimerization. J Biol Chem. 1998;273:7326–7331. doi: 10.1074/jbc.273.13.7326. [DOI] [PubMed] [Google Scholar]
- 36.Cheng L, Jia H, Lohr M, Bagherzadeh A, Holmes DI, Selwood D, Zachary I. Anti-chemorepulsive effects of vascular endothelial growth factor and placental growth factor-2 in dorsal root ganglion neurons are mediated via neuropilin-1 and cyclooxygenase-derived prostanoid production. J Biol Chem. 2004;279:30654–30661. doi: 10.1074/jbc.M402488200. [DOI] [PubMed] [Google Scholar]
- 37.Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walzer T, Galibert L, Comeau MR, De Smedt T. Plexin C1 engagement on mouse dendritic cells by viral semaphorin A39R induces actin cytoskeleton rearrangement and inhibits integrin-mediated adhesion and chemokine-induced migration. J Immunol. 2005;174:51–59. doi: 10.4049/jimmunol.174.1.51. [DOI] [PubMed] [Google Scholar]
- 39.Ghez D, Lepelletier Y, Lambert S, Fourneau JM, Blot V, Janvier S, Arnulf B, van Endert PM, Heveker N, Pique C, Hermine O. Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry. J Virol. 2006;80:6844–6854. doi: 10.1128/JVI.02719-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lambert S, Bouttier M, Vassy R, Seigneuret M, Petrow-Sadowski C, Janvier S, Heveker N, Ruscetti FW, Perret G, Jones KS, Pique C. HTLV-1 uses HSPG and neuropilin-1 for entry by molecular mimicry of VEGF165. Blood. 2009;113:5176–5185. doi: 10.1182/blood-2008-04-150342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasegawa H, Tatsumi M, Ogawa-Goto K, Takahashi H, Kojima A, Iwasaki T, Kurata T, Sata T, Takeuchi T, Sheehy N, Sawa H, Nagashima K, Hall WW. Processing of the HTLV-II envelope precursor glycoprotein gp63 by furin is essential for cell fusion activity. AIDS Res Hum Retroviruses. 2002;18:1253–1260. doi: 10.1089/088922202320886299. [DOI] [PubMed] [Google Scholar]
- 42.Fuh G, Garcia KC, de Vos AM. The interaction of neuropilin-1 with vascular endothelial growth factor and its receptor flt-1. J Biol Chem. 2000;275:26690–26695. doi: 10.1074/jbc.M003955200. [DOI] [PubMed] [Google Scholar]
- 43.Starzec A, Vassy R, Martin A, Lecouvey M, Di Benedetto M, Crepin M, Perret GY. Antiangiogenic and antitumor activities of peptide inhibiting the vascular endothelial growth factor binding to neuropilin-1. Life Sci. 2006;79:2370–2381. doi: 10.1016/j.lfs.2006.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.