Abstract
Background
Obesity has been associated with an increased risk of developing several psychiatric illnesses including major depression and post-traumatic stress disorder. Likewise, these stress-related disturbances are associated with a higher rate of obesity, yet, the neurobiological mechanisms linking obesity and stress remain incompletely understood.
Methods
Following exposure to chronic social defeat stress (CSDS), mice were given free access to either regular chow or a Western-style diet high in triglycerides and cholesterol. Comprehensive metabolic and behavioral testing was then conducted.
Results
Mice subjected to CSDS and then fed a high-fat diet for 30 days display severe behavioral deficits accompanied by redistribution of body fat. Stressed mice have decreased adipose tissue as well as decreased serum leptin levels compared to control mice. Pharmacological inhibition of β3-adrenergic signaling during CSDS normalizes these metabolic abnormalities but worsens behavioral symptoms. Furthermore, mice subjected to CSDS displayed central leptin resistance including reduced expression of pro-opiomelanocortin in hypothalamus. Administration of a central melanocortin agonist worsens stress-induced behavioral deficits, while mice lacking the melanocortin 4-receptor display attenuated symptoms.
Conclusions
These results indicate that chronic signaling through β3-adrenergic receptors during social stress is an adaptive response that improves behavioral function. However, these responses come at the expense of central leptin resistance and melanocortin signaling alterations that contribute to significant and long-lasting metabolic abnormalities.
Keywords: Feeding, Metabolism, Leptin, Hypothalamus, Anxiety, Depression
Introduction
Metabolic disorders are thought to result from a complex interaction between genetic susceptibility and environmental influences. A growing literature indicates that obesity is one of the most important environmental risk factors for developing mental disorders. A longitudinal study of 1037 children in New Zealand found that major depression in late adolescent girls imparted a 2.3 fold increased risk of obesity in adulthood (1). A review of 221 male veterans with PTSD in the U.S. reported a significantly increased rate of obesity (2). Several recent epidemiological studies have confirmed a relationship between depressive symptoms and metabolic disorders. Two studies using data from the National Health and Nutritional Examination Survey (NHANES) reported that the presence of obesity positively correlated with depressive symptoms in women with the strongest risk found between morbid obesity and depressive symptoms (3, 4). In a prospective study of 429 women without metabolic syndrome at baseline, it was found that a history of major depression significantly increased the risk of developing metabolic syndrome at follow-up (5). Finally, using data from the Multi-Ethic Study of Atherosclerosis, Golden and colleagues conducted a bi-directional analysis of depressive symptoms and diabetes mellitus type II (DM II). In 5201 patients without DM II at baseline, the presence of elevated depressive symptoms was associated with an increased risk of developing DM II. Conversely, in an analysis of 4847 patients without depressive symptoms at baseline, the presence of treated DM II significantly elevated the risk of developing depressive symptoms at follow-up (6). Despite the clear bi-directional association between mental illness and metabolic disorders, relatively little is known about the underlying molecular and neuroanatomic mechanisms that mediate this risk.
Rodent models of chronic stress have provided some insight into the link between stress and the development of metabolic disturbances. Lu and colleagues examined the role of leptin and melanocortin signaling in rodent models of stress and antidepressant efficacy. They reported that leptin levels are diminished after chronic unpredictable stress, a rodent model associated with decreased sucrose preference—a purported sign of anhedonia, and that acute administration of leptin reverses the deficits observed in sucrose preference (7). Furthermore, they reported that leptin administration into the hippocampus, a key site for mood regulation, produces an antidepressant-like effect in the forced swim test. In a second study, this group demonstrated robust activation of the melanocortin system following restraint or forced swim stress, which contributed to the anorexic and anxiogenic effects of these acute stresses (8).
Recently, we demonstrated a novel role for the stomach-derived pro-appetite hormone ghrelin in mood regulation (9) following exposure to chronic social defeat stress (CSDS). CSDS is a preclinical model of chronic stress which shares features with many psychiatric disorders including major depressive disorder with anxiety, post-traumatic stress disorder, and social anxiety (10-12). In this model, mice subjected to repeated social aggression develop behavioral deficits including social avoidance and decreased preference for sucrose and other natural rewards. Here, we report a detailed analysis of changes in metabolism, body weight regulation, and behavioral symptoms in mice subjected to CSDS receiving regular chow or a Western-style diet high in fat and cholesterol.
Methods
Animals and housing
8-10 week old male c57BL6/J mice (Jackson Laboratories) and CD1 retired breeder mice (Charles River) were housed in the UTSW vivarium in a temperature-controlled environment (Lights ON: 0400-1600) with ad lib access to water and standard chow (4% fat diet #7001, Harlan-Teklad, Madison, WI) or high fat diet (42% kcal from fat, TD.88137, Harlan-Teklad) as noted. MC4R null mice (23) were back-crossed onto c57BL6/J seven generations. All animal procedures were carried out in accordance with the UTSW IACUC guidelines.
Behavioral Testing
All behavioral testing was conducted as previously described (24). Social avoidance is presented as an interaction ratio: time spent in the interaction zone in the presence vs. absence of a social target mouse(12). See detailed descriptions of testing in supplemental material.
Injections
The β3-AR antagonist SR59230A (Sigma Aldrich, St Louis, MO), CL316243 (Tocris, Ellisville, MO), MT II (Bachem, Torrance, CA), and recombinant mouse leptin (National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance CA) were injected i.p. See detailed descriptions in supplemental material.
Hormone levels
Serum was collected from trunk blood after a three hour fast. Leptin levels were determined from serum using ELISA kits as per the manufacturer’s instructions (#90030 Crystal Chem, Downers Grove, IL).
Quantitative PCR analysis
All genes were standardized to cyclophilin and gene expression was determined for all mRNA data by fold change relative to control using the Ct method as reported (24). Primer sequences and mRNA preparation are included in supplemental material.
Western Blotting
Mice subjected to CSDS received a single injection of leptin (1 mg/kg i.p.) on Day 12 at 13:00. One hour later, the hypothalamus was collected by gross dissection and stored at −80°C until processing for SDS-PAGE. Blots were probed for STAT3 (#06-596, Upstate Biochem, Lake Placid, NY) and phospho-STAT3 (#9131S, Upstate Biochem, Lake Placid, NY). Images were quantified using ImageJ software (NIH, Bethesda, MD). Data are presented as fold difference of phospho-STAT3/STAT between control and CSDS mice treated with leptin. No phospho-STAT3 signal was visualized in saline-treated mice.
Body composition
Experiments were performed by the UTSW Metabolic Phenotyping Core. Body composition was determined by an mq10 series Bruker Minispec (Bruker Optics, The Woodlands, TX) 28 days after CSDS.
Statistical analyses
Data are reported as mean±SEM. Statistical analyses were performed using Student’s t-test, repeated measures analysis of variance (RMANOVA) or two way ANOVA followed by Bonferroni post hoc tests. All statistical analyses were performed using Prism (v 5.0) software. Statistical significance was defined as p < 0.05.
Results
Metabolic Effects of Chronic Social Defeat Stress
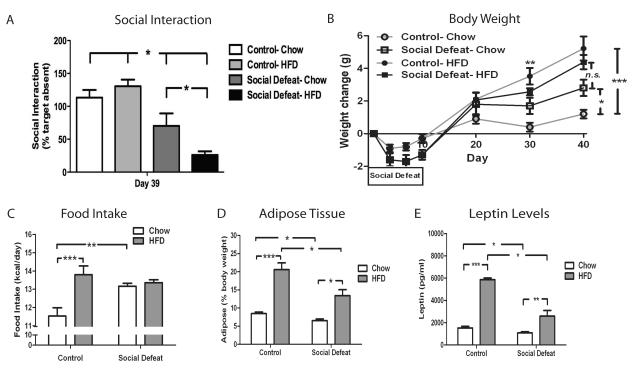
Following ten days of CSDS, wild-type c57BL/6 male mice were tested for social interaction and randomized into two groups with equivalent interaction scores and then received either regular chow or a Western-style diet that is high in fat and cholesterol (high fat diet, HFD). In the first set of experiments, we characterized the effect CSDS on food intake and body weight regulation in chow fed animals. Mice initially lose weight during the first days of the CSDS paradigm, however, beginning at day seven, they increase their food intake and rapidly regain the lost weight (Fig. 1B and 1C). Mice exposed to CSDS maintained on chow continue to gain more body weight than control littermates and by day 40 (30 days after the last episode of CSDS), defeated mice have a significantly increased weight change compared to control mice on chow (Fig. 1B). Because of the alteration in body weight regulation we observed, we next analyzed the body composition of animals subjected to CSDS. Despite having a significant increase in weight change during the experiment, mice exposed to CSDS actually demonstrated reduced adipose tissue (8.47% ± 0.41 vs. 6.51% ± 0.44, Fig. 1D) compared to control mice, suggesting that CSDS leads to a redistribution of energy stores with less relative storage of energy in adipose tissue. Because the satiety hormone leptin is synthesized in adipose tissue, we speculated that leptin levels would be lower in defeated mice. Consistent with this hypothesis, leptin levels in defeated mice on chow were significantly lower than in control mice (1521 pg/ml ± 159 vs. 1082 pg/ml ± 106, Fig. 1E).
Figure 1. Metabolic effects of CSDS.
C57BL/6 mice were subjected to CSDS (n=25) or non-aggressive encounters (control, n = 34) for ten days and then randomized into groups receiving either regular chow or HFD for 30 days. CSDS increases (A) social avoidance at day 39 [significant defeat effect (F1,55 = 41.76, p<0.0001, and (B) body weight [significant group × dayRM interaction (F18, 391 = 2.36, p=0.0015)]. A sub-group of animals (n = 6/group) were further analyzed for (C) food intake [significant defeat effect (F1, 20 = 8.81, p=0.0076)], (D) body composition [significant defeat × diet interaction effect (F1, 20 = 5.81, p=0.0257), and (E) leptin [significant defeat × diet interaction (F1, 20 = 26.11, p<0.0001)]. Data are presented as mean±SEM, with * indicating significant differences (*p<0.05, **p<0.01, ***p<0.001). RM (repeated measure).
Because access to a HFD is known to increase food intake and body weight, we next determined the effect of HFD on mice subjected to CSDS. As expected, control mice given free access to HFD consumed more kilocalories and gained significantly more weight than control mice on chow (Fig 1B and 1C). Interestingly, this same increase in weight change was not observed in the CSDS groups. Mice exposed to CSDS showed no difference in number of kilocalories consumed or weight gained regardless of diet (Fig 1B and 1C). As predicted, body composition analysis confirmed that control mice on HFD significantly increase their adipose stores (Fig. 1D). However, mice fed HFD after CSDS do not have as much adipose tissue (20.58% ± 1.85 vs. 13.41% ± 1.65, Fig. 1D) or leptin (5865 pg/ml ± 135 vs. 2591 pg/ml ± 503, Fig 1E) as control mice on HFD despite no difference in body weight change. This finding demonstrates that mice on HFD are resistant to storing excess energy in adipose tissue following exposure to CSDS.
Finally, because of the association between obesity and depressive symptoms, we tested the effect of exposure to HFD on CSDS-induced behavioral deficits. After CSDS, the mice were randomized into two equal groups based upon Interaction scores (data not shown) and then re-tested for social interaction after four weeks on the specified diet. Interestingly, mice on HFD demonstrated significantly greater social avoidance than mice receiving regular chow (Fig 1A). This finding indicates that access to highly palatable, calorically dense food can worsen recovery from stress-induced behavioral deficits. Because CSDS induces persisting changes in social interaction as well as food intake and body weight regulation, this model allows for a detailed analysis of the molecular adaptations that mediate this effect. Therefore, we conducted a detailed examination to identify signaling pathways that contribute to the exacerbation of depressive symptoms by HFD.
Role of β3-adrenergic Signaling in CSDS-Induced Metabolic and Behavioral Abnormalities
Our initial data presented a discrepancy. Leptin is a well-known regulator of food intake and has previously been reported to improve measures of depression-related behavior (7). The observed decrease in leptin after CSDS is consistent with the increased food intake observed in chow fed mice (Fig. 1C). However, elevation of leptin levels following exposure to HFD was associated with worsening of behavioral deficits, not an improvement. Therefore, we investigated the regulation of leptin levels after CSDS as well as the role of leptin signaling in the development of social avoidance. To determine how CSDS induces long-lasting decreases in adipose tissue and leptin levels, we first examined the role of the sympathetic nervous system. Increased sympathetic activity has been demonstrated following CSDS as part of the ‘fight or flight’ response (12-14). Importantly, sympathetic tone stimulates lipolysis and suppresses leptin levels via signaling through the β3-adrenergic receptor (β3-AR) in white adipose tissue (15). These findings led us to speculate that increased sympathetic activity through the β3-AR induces the decreased adipose tissue and leptin levels observed during CSDS.
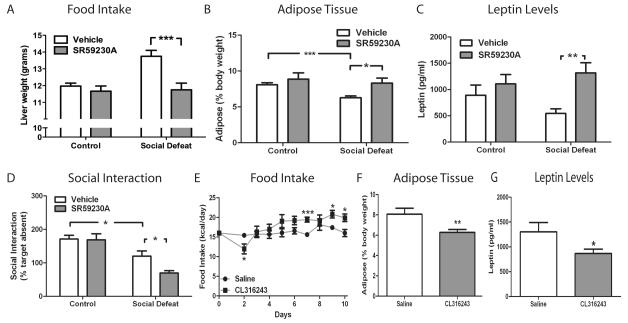
To test this hypothesis, we administered the β3-AR antagonist SR59230A during CSDS. While β3-AR blockade had no effect upon body weight at day 10 (data not shown), the subsequent CSDS-induced hyperphagic response was prevented (Fig. 2A) and adipose tissue levels were normalized (Fig. 2B) in chow fed mice receiving SR59230A. Consistent with the observed adipose tissue levels, administration of SR59230A during 10 days of CSDS blocked defeat-induced decreases in circulating leptin levels, but had no effect on leptin levels in control mice (Fig. 2C). Next, we determined the effect of SR59230A administration on behavior in the social interaction test. In control mice, SR59230A had no effect on social interaction when compared to vehicle treated mice (Fig 2D). However, mice that received the compound during CSDS demonstrated a significant worsening of social avoidance compared to vehicle treated animals despite the elevation in leptin levels. This finding suggested that suppression of leptin signaling is associated with resistance to behavioral deficits induced by CSDS and may be an important compensatory response that develops during CSDS.
Figure 2. β3-AR signaling in CSDS.
C57BL/6 mice (n=10/group) were given twice daily injections of the β3-AR antagonist SR59230A (5 mg/kg i.p.) or vehicle during CSDS or control encounters. (A) Food intake [significant defeat effect (F1, 36 =4.17, p=0.0484) and significant treatment effect (F1, 36 =6.59, p=0.0146)] and (B) body composition [significant treatment effect (F1, 36 =5.92, p=0.0201)]. (C) Leptin levels (n=7/group) [significant treatment effect (F1, 24 =5.14, p=0.0075)]. (D) SR59230A worsens social avoidance behavior on Day 11 [significant defeat effect (F1, 36=30.22, p<0.0001)]. C57BL/6 mice were given injections of the β3-AR agonist CL316243 (1 mg/kg i.p., n=5) or saline (n=6) once daily for ten days. (E) CL316243 effect on food intake [significant treatment × dayRM interaction (F8, 81 = 2.54, p=0.0163)]. (F) Body composition [p=0.005 by Student’s t-test], and (G) leptin levels [p=0.0493 by Student’s t-test]. Data are presented as mean±SEM, with * indicating significant differences (*p<0.05, **p<0.01, ***p<0.001). RM (repeated measure).
To complement our β3-AR blockade data, we treated mice with the β3-AR agonist CL316243 to determine if activation of β3-AR alone could replicate certain aspects of the observed CSDS phenotype. Control (non-stressed) mice receiving CL316243 once daily initially responded with decreased food intake, however, following repeated treatments the mice developed a hyperphagic response on days seven, nine, and ten (Fig. 2E) similar to that observed during CSDS. Consistent with the known ability of CL316243 to stimulate the β3-AR, adipose tissue decreased (Fig. 2F) along with circulating leptin levels (Fig. 2G) similar to the changes observed after 10 days of CSDS. Together, these data demonstrate that activating β3-AR signaling is both necessary and sufficient to recapitulate several metabolic changes observed after CSDS. We next wanted to determine if CL316243 administration during CSDS could affect development of social avoidance. Unfortunately, the initial suppression in food intake induced by this compound (Fig. 2E) exacerbated the CSDS-induced weight loss (Fig. 1B) and prohibiting completion of the CSDS protocol.
Leptin Responses in Chronic Social Defeat Stress
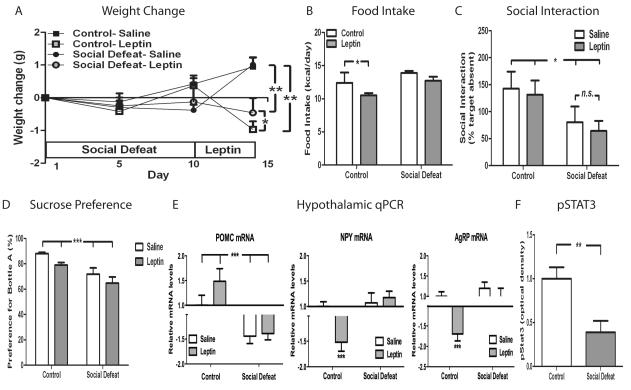
To further elucidate the role of leptin signaling in CSDS-induced changes in behavior and metabolism, we administered exogenous leptin following 10 days of CSDS and then determined its effect on food intake, body weight, and social interaction. As expected, twice-daily leptin treatment (1 mg/kg i.p.) produced significant weight loss and hypophagia in control (non-stressed) mice (Fig. 3A and 3B). On the first day of leptin treatment (day 10), mice exposed to CSDS started at a lower baseline weight than control mice. Interestingly, defeated mice were resistant to the anorexic effect of leptin (Fig 3B) and did not lose as much weight as control mice receiving leptin (Fig 3A). Even though mice exposed to CSDS started at a lower body weight, after four days of leptin administration they actually weighed significantly more than control mice, which further demonstrates their resistance to the effects of leptin administration.
Figure 3. Leptin resistance after CSDS.
C57BL/6 mice subjected to CSDS (n=10/group) or control encounters (n=10/group) were given leptin (1 mg/kg i.p.) twice daily for four days. (A) Body weight [significant effect of leptin × dayRM (F9, 144 = 4.77, p<0.0001). (B) Food intake [significant effect for leptin (F1, 36 = 10.19, p=0.0029) and defeat (F1, 36 = 14.86, p=0.0005)]. (C) Social interaction scores on day 15 [significant effect of defeat (F1, 36 = 5.81, p=0.0211)]. (D) Sucrose preference after leptin treatment [significant effect of defeat (F1,35 = 16.94, p=0.0002)]. (E) Hypothalamic expression of POMC, NPY, and AgRP (n=7/group) determined by qPCR on day 15 [POMC: significant effect of defeat (F1, 24 = 196.82, p<0.0001), NPY: significant effect of leptin × defeat (F1, 24 = 72.17, p<0.0001), AgRP: significant effect of leptin × defeat (F1, 24 = 59.60, p<0.0001). In a separate experiment, mice were subjected to CSDS (n=9/group) or control encounters (n=9/group) and given a single injection of leptin (1 mg/kg). (F) Levels of phosphorylated STAT3 [p=0.004 by Student’s t-test]. Data are presented as mean±SEM, with * indicating significant differences (*p<0.05, **p<0.01, **p<0.001). RM (repeated measure).
We next tested whether leptin administration influences CSDS-induced social avoidance. Leptin treatment had no effect on social interaction scores in either control or defeated mice (Fig. 3C). We also examined the effect of leptin administration on sucrose preference, a purported measure of anhedonia, in control and defeated mice. Consistent with our social interaction findings, leptin had no effect on this behavioral measure compared to saline-treated animals (Fig. 3D).
As several of the aforementioned findings in mice subjected to CSDS are suggestive of a state of leptin resistance, we directly measured leptin responses in control and defeated animals. We first analyzed leptin’s effects on hypothalamic gene expression. Consistent with leptin’s known actions, peripheral leptin administration significantly increased POMC mRNA levels and decreased agouti-related peptide (AgRP) and neuropeptide Y (NPY) mRNA levels in the hypothalamus of control mice (Fig. 3E). However, in mice subjected to 10 days of CSDS, leptin administration showed no difference in POMC, AgRP, or NPY mRNA expression compared to saline-treated mice. These findings indicate that central leptin resistance occurs after CSDS. Because leptin receptor activation is coupled to intracellular signaling by the janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway, we measured phosphorylation of the STAT3 protein in hypothalamus after leptin treatment as a measure of leptin action. Following leptin treatment, mice subjected to CSDS exhibit a significant reduction in the amount of phospo-STAT3 (Fig. 3F), the active form of the protein, compared to control mice. This observation indicates that CSDS-induced leptin resistance occurs in the hypothalamus at the level of leptin receptor signaling.
Role of Central Melanocortin Signaling in Chronic Social Defeat Stress
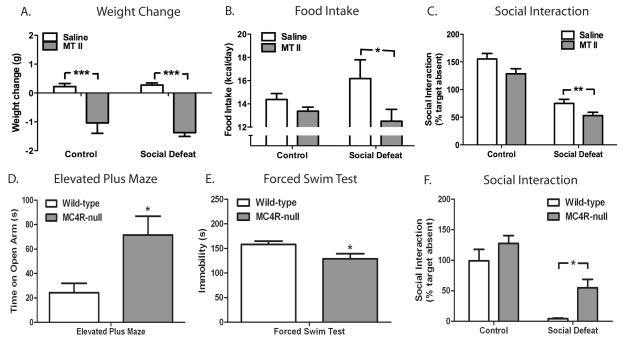
The central melanocortin system is a critical regulator of the actions of leptin in regulating food intake and metabolism (16, 17). This system consists of an endogenous agonist, α-melanocyte-stimulating hormone ( α-MSH, also known as melanocortin, processed from the precursor POMC), an endogenous antagonist (AgRP) as well as two receptors, melanocortin 3 receptor (MC3R) and melanocortin 4 receptor (MC4R). Of these two receptors, MC4R has been shown to have a major role in feeding and metabolism because absence of MC4R induces morbid obesity in mice and humans (16). The combination of decreased circulating leptin levels and central leptin resistance induced by CSDS converge within the hypothalamus to decrease expression of POMC (Fig. 3E). Because melanocortin signaling is a potent inhibitor of food intake, we hypothesized that a selective decrease in melanocortin signaling contributes to the hyperphagia observed in defeated mice. We tested this hypothesis by administering melanotan-II (MT II), an agonist at the melanocortin 3 and 4 receptors, to mice after 10 days of CSDS. In contrast to the lack of effect of leptin in mice exposed to CSDS, defeated mice were sensitive to the anorectic effect of MT II, which significantly decreased both food intake and body weight (Fig 4A and 4B).
Figure 4. Melanocortin signaling in anxiety and depression.
C57BL/6 mice subjected to CSDS (n=10/group) or control encounters (n=10/group) were given MT II (50 μg i.p.) four times daily for two days. (A) Body weight change [significant effect of treatment (F1, 36 = 52.59, p<0.0001)] and (B) food intake [significant effect of treatment (F1, 36 = 5.41, p=0.0257)] on Days 11 and 12. (C) Social interaction scores on day 13 (n=18 control-saline, n=16 control-MTII, n=19 social defeat-saline, n=18 social defeat-MTII) [significant effect for defeat (F1, 67 = 89.59, p<0.0001) and MT II (F1, 67 = 8.80, p=0.0042). A cohort of wild-type (n=7) and MC4R-null (n=9) littermates were tested in rodent models of depression and anxiety including (D) time spent on open arm of elevated plus maze (p=0.027 by Student’s t-test), (E) immobility in the forced swim test (p=0.0478), and (F) social avoidance after CSDS [significant defeat effect (F1, 26 =38.45, p<0.0001) and significant genotype effect (F1, 26 = 8.47, p=0.0073)]. Data are presented as mean±SEM, * indicating significant differences (*p<0.05, **p<0.01, ***p<0.001).
We next determined if melanocortin signaling could also regulate behavioral symptoms induced by CSDS. Defeated mice treated with MT II displayed increased social avoidance compared to saline-treated mice (Fig. 4C), implicating melanocortin signaling not only in the control of food intake, but also in the regulation of social avoidance. To complement our pharmacologic data with a genetic approach, we analyzed mice deficient in MC4R. A battery of behavioral tests was used to evaluate various aspects of mood and anxiety in MC4R-null and wild-type littermate mice. MC4R-null mice spent significantly more time exploring the open arm of the elevated plus maze compared to controls, a finding interpreted as decreased anxiety (Fig. 4D). Additionally, MC4R-null mice spent less time immobile in the forced swim test, an antidepressant-like response (Fig. 4E). Finally, in the CSDS paradigm, MC4R-null mice spent significantly more time interacting with a social target than wild-type controls (Fig. 4F). Of note, the wild-type littermates used as control mice in this experiment exhibited significantly greater levels of avoidance than similar wild-type C57BL6 mice used in earlier experiments (Fig 1A, 2D, 3C, 4C). This finding is likely a result of the social isolation required to pair-feed mice in order to maintain similar body compositions (see Methods for details). We have previously observed greater social avoidance in mice housed individually prior to CSDS (data not shown). Collectively, these findings indicate that melanocortin signaling through the MC4R strongly regulates behavioral responses to chronic stress in rodents.
Discussion
Determining the effects of chronic stress on the regulation of feeding and body weight is of critical importance because of the well known association between psychiatric disorders and metabolic diseases, as outlined in the Introduction. The current findings provide a molecular and neurobiologic model that explains, in part, the link between altered mood and metabolic status. This model, and the extensive characterization provided here, can now be used in future studies to further investigate the underlying molecular and cellular mechanisms involved.
Sympathetic Nervous System
Our data suggest that CSDS induces long lasting changes in the functioning of peripheral organs such as white adipose tissue via sympathetic nervous system activation and β3-AR signaling that persists long after termination of the stress. For example, adipose tissue levels and leptin production remain suppressed, while body weight is increased, up to 4 weeks after the last episode of defeat. Along with the suppression of leptin signaling by chronic stress demonstrated in the present study, there are likely to be many other organs affected by chronic stress. For instance, we reported recently that CSDS enhances production of ghrelin by the stomach: circulating levels of active ghrelin are increased four weeks after the last defeat (9). Elevated ghrelin levels likely work in concert with low leptin to induce a potent feeding response that increases available energy stores.
Our behavioral data suggest that activation of the sympathetic nervous system and β3-AR signaling is a positive coping mechanism that opposes the deleterious behavioral effects of CSDS. Previous work has demonstrated antidepressant-like and anxiolytic-like effects induced by the β3-AR agonist SR58611A in rodent models (18). The β3-AR antagonist used in this study, SR59023A, has never been shown to have pro-depressant properties and has no effect on control mice in the social interaction test (Fig 2E). However, we cannot rule out the possibility that SR59023A exacerbates social avoidance after CSDS through a central mechanism independent of its effect on leptin levels and POMC expression. Determining the relative contribution of peripheral vs. central β3-AR blockade will be of great interest in future studies.
Melanocortin Signaling
The combination of decreased leptin along with central leptin resistance results in a robust decrease in the expression of POMC mRNA in the hypothalamus. The subsequent decrease in melanocortin signaling not only helps to increase food intake, as would be expected, but also seems to improve mood symptoms consistent with previous reports. Notably, we found that administration of a melanocortin agonist worsens the development of depressive-like behavior. Pharmacological studies have supported a role for MC4R in animal models of depression and anxiety. Administration of α-MSH increases immobility in the forced swim test, a pro-depression-like effect, and blocks the antidepressant-like action of NPY in this test (19). Additionally, the synthetic MC4R antagonist, MCL0129, induces anxiolytic-like responses in the light/dark and marble burying tests, as well as producing antidepressant-like responses in the learned helplessness and forced swim tests (20). Recently, the melanocortin system has been implicated in acute responses to stress. Both restraint and forced swim stress induce rapid activation of POMC neurons in the arcuate nucleus of hypothalamus as inferred from c-Fos staining. This activation is associated with anxiety-like behavior, and stress-induced anorexia and can be prevented by pretreatment with the melanocortin receptor antagonist SHU9119 (8). Activation of melanocortin signaling by acute stress can be viewed as a beneficial adaptation as both behaviors (anorexia and anxiety) enhance survival in the short term. However, as energy stores are depleted, mice must respond with new strategies to promote survival. Decreased melanocortin signaling may have this effect in part by increasing food intake. Additionally, decreased melanocortin signaling may decrease anxiety and depressive symptoms thereby increasing the ability to scavenge for food. An important prediction of this theory is that chronic stress promotes increased feeding and perhaps obesity via reduced melanocortin signaling, while obesity likewise increases the vulnerability to stressful life events via enhanced melanocortin signaling. Put another way, obese individuals have larger stores of energy that may oppose a stress-induced decrease in leptin and melanocortin signaling, which serve to counteract the deleterious effects of chronic stress. Consistent with this hypothesis, we found that defeated mice with access to a calorically dense high fat diet show increased social avoidance 40 days after the last episode of defeat when compared to defeated mice on regular chow diet (Fig 1A).
Site of Behavioral Action
How the brain integrates numerous signals of peripheral energy balance to regulate mood and anxiety is an important unanswered question. Chronic unpredictable stress decreases leptin levels in rats, while leptin administration exerts antidepressant-like effects in certain tests (7). The hippocampus has been suggested to be the site of leptin’s antidepressant-like effect, because infusion of leptin directly into the hippocampus decreases depression-like behavior in the forced swim test. Our findings reveal more complex actions of leptin in control of mood. During repeated exposure to severe stress as in CSDS, leptin levels fall and leptin resistance increases, leading to a sustained decrease in melanocortin signaling. This fall in melanocortin tone then exerts an antidepressant-like effect. The site of melanocortin’s action on mood regulation is unknown. MC4R are expressed predominately in the brain, and enriched in several brain regions associated with mood control, including the nucleus accumbens and lateral hypothalamus (21, 22).
Collectively, our results indicate that the development of certain neurovegetative symptoms seen in many people with major depression or other stress-related conditions may be a concomitant of homeostatic, compensatory responses to manage the chronic stress. Specifically, enhanced β3-AR signaling, and consequent decreases in leptin and melanocortin signaling, may promote emotional coping with stress at the expense of metabolic derangements. Better understanding the association between feeding and mood regulation may assist in the development of improved psychiatric medications, as well as novel treatments for obesity that lack psychiatric side effects.
Supplementary Material
Acknowledgments
We thank the National Hormone and Peptide Program (Harbor-UCLA Medical Center, Torrance, CA) for supplying leptin. This work was supported by: K08 MH084058-1A1, UL1-DE019584-02, R01 MH51399, R37 DK53301; R01DK071320; RL1 DK081182, 1RL1 DK081185-01, 1PL1DK081182-01, K08 DK068069-01A2, P50 MH066172, NARSAD, Astra-Zeneca, The Physician Scientist Training Program, The Disease Oriented Clinical Scholars Program, the Klarman Family Foundation, and The Medical Scientist Training Program.
Footnotes
Financial disclosure: Dr. Nestler consults for Merck Research Laboratories and PsychoGenics, and has a research alliance with AstraZeneca. Dr. Elmquist has received research support from Sanofi-Aventis. The remaining authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Richardson LP, Davis R, Poulton R, McCauley E, Moffitt TE, Caspi A, et al. A longitudinal evaluation of adolescent depression and adult obesity. Archives of pediatrics & adolescent medicine. 2003;157:739–745. doi: 10.1001/archpedi.157.8.739. [DOI] [PubMed] [Google Scholar]
- 2.Vieweg WV, Julius DA, Benesek J, Satterwhite L, Fernandez A, Feuer SJ, et al. Posttraumatic stress disorder and body mass index in military veterans. Preliminary findings. Progress in neuro-psychopharmacology & biological psychiatry. 2006;30:1150–1154. doi: 10.1016/j.pnpbp.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Ma J, Xiao L. Obesity and Depression in US Women: Results From the 2005-2006 National Health and Nutritional Examination Survey. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.213. [DOI] [PubMed] [Google Scholar]
- 4.Beydoun MA, Wang Y. Pathways linking socioeconomic status to obesity through depression and lifestyle factors among young US adults. J Affect Disord. 2009 doi: 10.1016/j.jad.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldbacher EM, Bromberger J, Matthews KA. Lifetime history of major depression predicts the development of the metabolic syndrome in middle-aged women. Psychosom Med. 2009;71:266–272. doi: 10.1097/PSY.0b013e318197a4d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golden SH, Lazo M, Carnethon M, Bertoni AG, Schreiner PJ, Roux AV Diez, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299:2751–2759. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu XY, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc Natl Acad Sci U S A. 2006;103:1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Garza JC, Truong HV, Henschel J, Zhang W, Lu XY. The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology. 2007;148:5531–5540. doi: 10.1210/en.2007-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avgustinovich DF, Gorbach OV, Kudryavtseva NN. Comparative analysis of anxiety-like behavior in partition and plus-maze tests after agonistic interactions in mice. Physiol Behav. 1997;61:37–43. doi: 10.1016/s0031-9384(96)00303-4. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan V, Berton O, Nestler E. The use of animal models in psychiatric research and treatment. Am J Psychiatry. 2008;165:1109. doi: 10.1176/appi.ajp.2008.08071076. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Keeney AJ, Hogg S, Marsden CA. Alterations in core body temperature, locomotor activity, and corticosterone following acute and repeated social defeat of male NMRI mice. Physiol Behav. 2001;74:177–184. doi: 10.1016/s0031-9384(01)00541-8. [DOI] [PubMed] [Google Scholar]
- 14.Sgoifo A, Koolhaas J, De Boer S, Musso E, Stilli D, Buwalda B, et al. Social stress, autonomic neural activation, and cardiac activity in rats. Neurosci Biobehav Rev. 1999;23:915–923. doi: 10.1016/s0149-7634(99)00025-1. [DOI] [PubMed] [Google Scholar]
- 15.Mantzoros CS, Qu D, Frederich RC, Susulic VS, Lowell BB, Maratos-Flier E, et al. Activation of beta(3) adrenergic receptors suppresses leptin expression and mediates a leptin-independent inhibition of food intake in mice. Diabetes. 1996;45:909–914. doi: 10.2337/diab.45.7.909. [DOI] [PubMed] [Google Scholar]
- 16.Williams DL, Schwartz MW. The melanocortin system as a central integrator of direct and indirect controls of food intake. Am J Physiol Regul Integr Comp Physiol. 2005;289:R2–3. doi: 10.1152/ajpregu.00226.2005. [DOI] [PubMed] [Google Scholar]
- 17.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 18.Stemmelin J, Cohen C, Terranova JP, Lopez-Grancha M, Pichat P, Bergis O, et al. Stimulation of the beta3-Adrenoceptor as a novel treatment strategy for anxiety and depressive disorders. Neuropsychopharmacology. 2008;33:574–587. doi: 10.1038/sj.npp.1301424. [DOI] [PubMed] [Google Scholar]
- 19.Goyal SN, Kokare DM, Chopde CT, Subhedar NK. Alpha-melanocyte stimulating hormone antagonizes antidepressant-like effect of neuropeptide Y in Porsolt’s test in rats. Pharmacology, biochemistry, and behavior. 2006;85:369–377. doi: 10.1016/j.pbb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Chaki S, Hirota S, Funakoshi T, Suzuki Y, Suetake S, Okubo T, et al. Anxiolytic-like and antidepressant-like activities of MCL0129 (1-[(S)-2-(4-fluorophenyl)-2-(4-isopropylpiperadin-1-yl)ethyl]-4-[4-(2-met hoxynaphthalen-1-yl)butyl]piperazine), a novel and potent nonpeptide antagonist of the melanocortin-4 receptor. The Journal of pharmacology and experimental therapeutics. 2003;304:818–826. doi: 10.1124/jpet.102.044826. [DOI] [PubMed] [Google Scholar]
- 21.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, et al. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci. 2003;23:7143–7154. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 24.Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci. 2008;28:3071–3075. doi: 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.