Abstract
Background/Aims
Peripheral circulating endothelial cells (CEC) have been proposed as a prognostic marker in cardiovascular diseases. Cirrhosis and portal hypertension are associated with vascular injury yet little is known about CEC count in these conditions. Therefore, we evaluated CEC count in patients with cirrhosis, and correlated it with markers of portal hypertension/disease severity.
Patients/Methods
Fifteen patients with cirrhosis/portal hypertension and fifteen matched controls were prospectively recruited for study participation. An automated rare cell analysis system was used to enumerate CEC from peripheral blood and correlated with clinical features.
Results
Median CEC levels were significantly higher in patients with cirrhosis as compared to controls (median [interquartile range (IQR)]; cirrhosis: 73.7 cells/4ml [53.7-140.3]; controls: 28.7 cells/4ml [21-58.7]; p=0.021). Ratio of CEC to platelet count (CEC/PC) also distinguished patients with cirrhosis from controls (IQR; cirrhosis: 0.723[0.396-1.672]; controls: 0.126[0.103-0.333]; p<0.001). Receiver operator characteristic (ROC) analysis revealed that CEC cut-off of 42 cells/4 mL showed sensitivity of 87% and specificity of 74% for differentiating cirrhosis from controls (AUC:0.74), while CEC/PC ratio at 0.21 showed sensitivity of 100% and specificity of 73% (AUC:0.89). Furthermore, CEC/PC index was significantly elevated in patients with hepatic decompensation as defined by Child B/C (p<0.05). The intra- and interobserver variability correlation coefficients for CEC measurement were 0.9989 and 0.9986, respectively.
Conclusion
Median CEC count and CEC/PC ratio are significantly elevated in patients with cirrhosis, with CEC/PC also increased in patients with decompensated cirrhosis. These data provide rationale for larger validation studies to assess if CEC may have prognostic utility in patients with cirrhosis and portal hypertension.
Keywords: Circulating endothelial cells, liver cirrhosis, portal hypertension
Introduction
Cirrhosis and portal hypertension are characterized by marked anatomical and functional abnormalities in the hepatic and systemic circulations including vascular endothelial injury (1). Prior studies to predict the presence of cirrhosis and portal hypertension using various noninvasive approaches including routine laboratory tests, serum markers of fibrosis and inflammation, quantitative assays of liver function, and radiologic imaging have revealed varying levels of discriminatory capacity (2-4). Therefore, at present there is no well-recognized surrogate biomarker that can accurately detect the presence of histologically confirmed cirrhosis (5, 6). Similarly, the non-invasive determination of portal hypertension and its severity in patients with known cirrhosis is also suboptimal (7, 8).
Circulating endothelial cells (CEC) are a specific cell population exceeding 10 μm in size, and characterized by the expression of at least two endothelial markers (ie; CD146 and UEA-1) with the absence of expression of leukocyte markers (ie; CD14 and CD45) (9). CEC are thought to be present in very low quantities among healthy subjects (10); however, these cells gain access to the peripheral circulation after sloughing from the vessel wall following pathological injury patterns including mechanical stress, change in adhesion molecule expression and matrix degradation (11, 12). Recent studies indicate that CEC are increased in the peripheral blood of patients with various forms of vascular injury and endothelial damage (11, 13-21). In fact, CEC have been identified as a specific, non-invasive surrogate biomarker of vascular damage in specific cardiovascular diseases (16, 21).
Since cirrhosis and portal hypertension are commonly viewed as a vascular injury syndrome, we hypothesized that CEC count may also be elevated in patients with cirrhosis versus healthy controls. Furthermore, we aimed to establish the clinical value of CEC count in patients with cirrhosis, as we believed that CEC may have a systematic relationship with hepatic disease severity. We tested these hypotheses by prospectively measuring the CEC level in patients with cirrhosis (both compensated and decompensated) and in healthy controls. From these studies, we have demonstrated that the CEC count is significantly elevated in patients with cirrhosis. In addition, the ratio of CEC to platelet count (CEC/PC ratio) may be even more effective at detecting advanced cirrhosis as well as assessing the risk for clinically significant portal hypertension, thereby providing a rationale for larger validation studies.
Patients and Methods
Patients
This cross-sectional study was approved by the Institutional Review Board of Mayo Clinic. Fifteen patients with liver cirrhosis and 15 normal healthy subjects were recruited for study enrollment, and informed consent was obtained from all subjects. Control subjects were frequency matched by age and sex to patients with cirrhosis. They were chosen from Mayo clinic staff and employees who did not report a specific history of hepatic or vascular disease and this was confirmed by chart-reviews.
Patients were recruited from outpatient hepatobiliary clinics, liver transplant clinics and hospital services at, Mayo Clinic, Rochester, Minnesota. Inclusion criteria included age greater than 18; diagnosis of cirrhosis by histologic and/or accepted imaging, laboratory, and clinical criteria; and the presence of complications of cirrhosis/portal hypertension by one or more of the following clinical features: splenomegaly, esophageal varices, ascites, hepatic encephalopathy, and/or hepatocellular carcinoma. Patients with compensated and decompensated cirrhosis were included. Exclusion criteria included concomitant systemic or localized diseases associated with vascular endothelial injury (coronary artery disease, rheumatologic disorder, primary pulmonary hypertension, or solid organ transplantation); history of systemic hypercoagulable disorders; and the current use of HMG-CoA reductase inhibitors, warfarin, NSAIDs or, immunosuppressive/immunomodulatory agents within 3 months of recruitment.
Patients underwent routine clinical care that included serum liver function test, laboratory parameters required for MELD score, platelet count, coagulation parameters, and abdominal ultrasound. Child-Turcotte- Pugh and MELD scores were calculated for each patient, and the presence of splenomegaly (longitudinal diameter of the spleen > 9cm) and ascites was determined by ultrasound examination. The presence of esophageal varices was determined by diagnostic esophagogastroduodenoscopy.
CEC assay
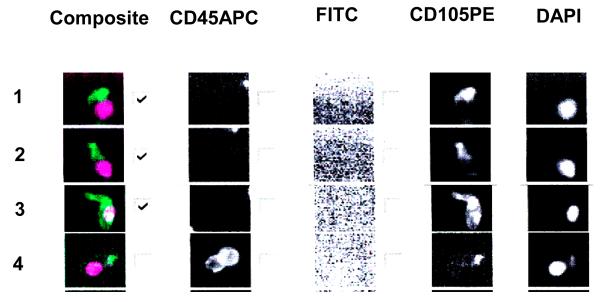
A four mL sample of peripheral blood was obtained by non-traumatic venipuncture from both patients and controls. Samples were collected in CellSave Preservation Tubes purchased from Veridex (Raritan, NJ) and transported to the Mayo Clinic Molecular Cytology and Imaging Laboratory at temperatures of 15-30°C (59-86°F). Analysis of the samples was done within 72 hours after collection. Four mL of blood was then added to 10 mL of dilution buffer in a 15 mL conical tube, mixed by inversion, sedimented at 800 × g for 10 minutes, and processed within 1 hour of collection using the CellSearch™ Circulating Endothelial Cell Kit purchased from Veridex (Raritan, NJ). The CellSearch™ Circulating Endothelial Cell Kit consists of 1) a ferrofluid reagent comprising nano-particles conjugated with CD146+ which allows for magnetic capture of CEC and 2) immunofluorescent reagents including anti-CD105-PE, anti-CD45-APC, and DAPI. Anti-CD105 is specific for the protein endoglin and expressed by endothelial cells, monocytes, stromal cells, and pre-B cells. The expression of anti-CD45 is restricted to leukocytes, and DAPI stains cell nuclei. Individual specimens consisting of CD146+ enriched cells were then placed on the CellTraks® Analyzer II (Veridex, Raritan, NJ). The analyzer produced a gallery of images from individual cells with CD105-PE and DAPI positivity (see Figure 1 for examples). The number of CEC was enumerated by quantifying cells with a phenotype of CD146+, CD105+, DAPI + and CD45−. CEC count was expressed as cells/4ml. Initial validation experiments in which human umbilical vein endothelial cells were spiked into normal volunteer samples were used to confirm that cell recovery exceeded 90% with excellent preservation of cell morphology (data not shown). Each gallery was reviewed by two independent operators (MBC and BRK) with expertise in laboratory fluorescence assays who were blinded to the origin of the samples. One operator (MBC) also analyzed each specimen a second time in blinded fashion to determine intra-observer variability.
Figure 1.
This figure is representative of thumbnails of circulating endothelial cell candidates from a blood sample. From right to left the columns show the DAPI, CD105 PE, FITC, CD45 APC staining and a composite of DAPI (purple) and CD105 (green) staining. In order to be considered an endothelial cell the image should be CD105+, DAPI+, CD45−, FITC−. Rows 1, 2, 3, show endothelial cell staining with DAPI and CD105 but lacking CD45. Row number 4 shows leucocyte staining with DAPI, CD105 and CD45. The checks in the boxes indicate endothelial cell type and are tabulated by the software.
Power and Sample Size Calculation
Power and sample size calculations were not performed, as the study was done to generate hypotheses aimed to determine whether CEC levels may be increased in patients with cirrhosis.
Statistical analysis
Statistical analysis was performed using SPSS version 9 for PC. Continuous data were summarized using median values with interquartile range [IQR]. Ordinal and categorical data was summarized by using ratios or proportions. Group differences were assessed using the Mann-Whitney’s U test. Correlations between CEC and clinical data were performed using the Spearman’s R test. The optimal diagnostic threshold value of CEC and CEC/PC in differentiating cirrhosis from controls was assessed using ROC curve methodology. Interobserver and intraobserver agreement were assessed by the intraclass correlation. A p value of < 0.05 was considered statistically significant.
Results
Patient demographics
The study included 15 patients with cirrhosis and 15 healthy volunteers frequency matched by age and sex. Demographic features are shown in Table 1. The patients included 5 females and 10 males with median age of 58 years (IQR 52-68). The control group included 6 females and 9 males with a median age of 56 years (IQR 50-64). Ten patients had splenomegaly, 9 had esophageal varices and 12 had ascites. Three patients had history of cancer (cholangiocarcinoma-1, esophageal carcinoma-1, and breast cancer- 1) and one patient had coexisting hepatocellular carcinoma. Three patients were Child A, 9 were Child B, and 3 were Child C. The median MELD score was 16 (IQR 10-22).
Table 1.
Patient demographic, clinical and biochemical features
| Demographics | Median (range) or Number (%) (n=15) |
|---|---|
| Age (yr) | 58 (52-68) |
|
| |
| Females | 5/15 (33%) |
|
| |
| Compensated cirrhosis (Child A) | 3 /15 (20%) |
|
| |
| Decompensated cirrhosis (Child B&C) | 12/15 (80%) |
|
| |
| Etiology of liver disease | |
| NASH | 6 (40%) |
| PSC | 3 (20%) |
| Alcoholic | 2 (13.2%) |
| HCV/HBV | 1 (6.7%) |
| PBC | 1 (6.7%) |
| Autoimmune hepatitis | 1 (6.7%) |
| Drugs | 1 (6.7%) |
|
| |
| Ascites | 12/15 (80%) |
|
| |
| Splenomegaly | 10/15 (66.6%) |
|
| |
| Esophageal varices | 9/15 (60%) |
|
| |
| Coexisting cancer | 4/15 (26%) |
|
| |
| Platelet count × 106/L | 80 (45-204) |
|
| |
| Total bilirubin (mg/dl) | 4.3 (1.4-5) |
|
| |
| Albumin (g/dl) | 3.4 (3.1- 3.7) |
|
| |
| Alanine aminotransferase (U/L) | 58 (26-81) |
|
| |
| Aspartate aminotransferase (U/L) | 61.5 (36.5-93.5) |
|
| |
| Alkaline phosphatase (U/L) | 160 (88-293) |
|
| |
| INR | 1.3 (1.2-1.3) |
|
| |
| Liver biopsy | 7 (47%) |
|
| |
| US abdomen | 13 (87%) |
|
| |
| CT abdomen | 12 (80%) |
|
| |
| EGD | 11 (73%) |
|
| |
| Beta-blocker | 4 (27%) |
CEC count and CEC/PC in patients and controls
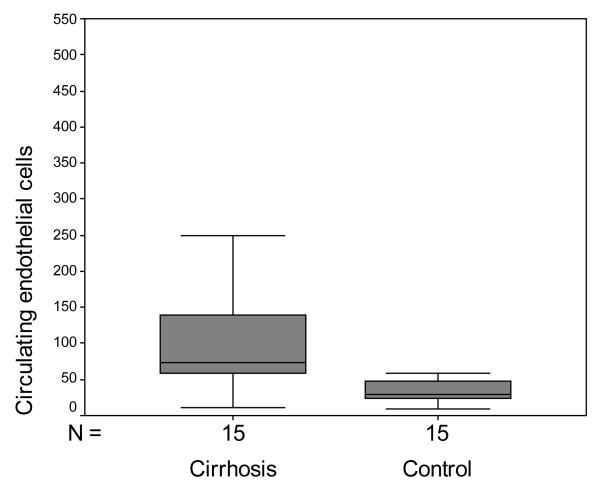
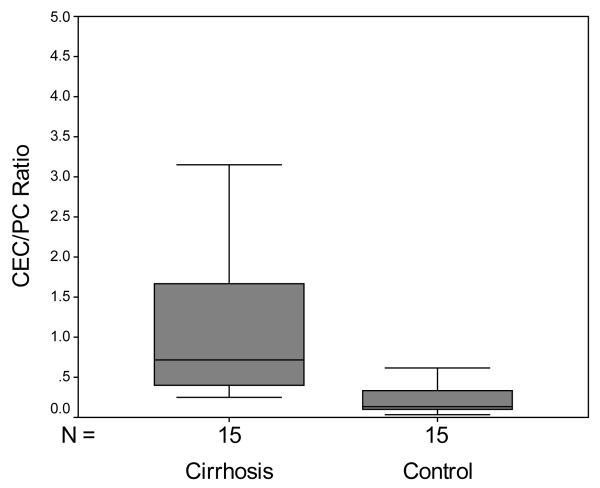
The median CEC count in cirrhosis was 73.7 cells/4ml [53.7-140.3] versus 28.7 cells/4ml [21-58.7] for the control group (Figure 2 A; p=0.021; Mann-Whitney). Since platelet count has also been proposed to be helpful in predicting cirrhosis, we also examined the CEC/PC ratio which was 0.723 [0.396-1.672] in patients with cirrhosis versus 0.126 [0.103-0.333] in control patients (Figure 2 B; p<0.001; Mann-Whitney).
Figure 2.
Box and whisker plot of CEC and CEC/PC ratio. A. CEC were significantly increased in cirrhotic patients compared to controls (p=0.021; Mann-Whitney). B. CEC/PC ratio was significantly increased in cirrhotic patients compared to controls (p<0.001; Mann-Whitney).
Sensitivity and specificity of CEC and CEC/PC for detecting cirrhosis
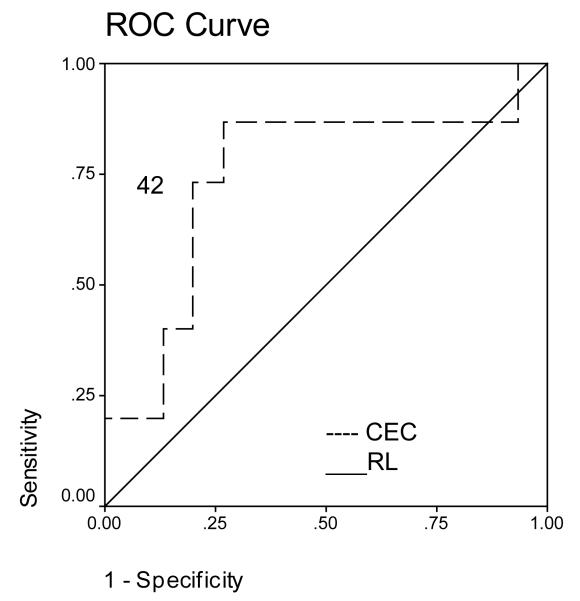
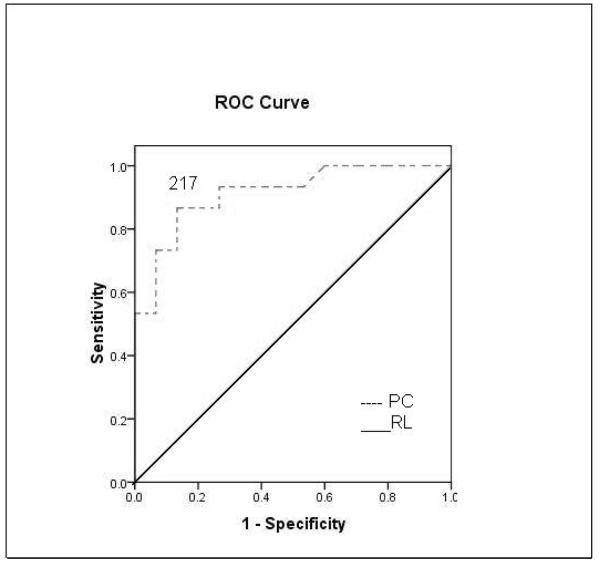
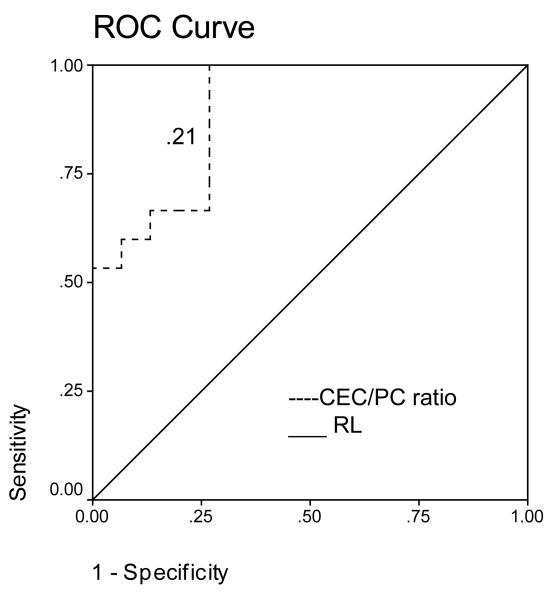
ROC analysis revealed that a CEC cut-off value of 42 cells/4 ml had an initial sensitivity of 87% and specificity of 74% for differentiating cirrhosis from controls (Figure 3A). ROC analysis revealed that a platelet count cut-off value of 217 cells/ ml had an initial sensitivity of 93% and specificity of 73% for differentiating cirrhosis from controls (Figure 3B). A CEC/PC cut-off value of 0.21 showed an initial sensitivity of 100% and specificity of 73% for differentiating cirrhosis from controls (Figure 3C). The area under the curve was 0.74 for CEC count, 0.91 for platelet count and 0.89 for CEC/PC.
Figure 3.
A. ROC curve for CEC for diagnosis of cirrhosis. A CEC cut-off value of 42 cells/4ml showed a sensitivity of 87% and specificity of 74% for differentiating cirrhosis from controls. Area under the curve was 0.74. B. ROC curve for platelet count for diagnosis of cirrhosis. ROC analysis revealed that a platelet count cut-off value of 217 cells/ ml had an initial sensitivity of 93% and specificity of 73% for differentiating cirrhosis from controls. Area under the curve was 0.91. C. ROC curve for CEC/platelet count (CEC/PC) ratio for diagnosis of cirrhosis. A CEC/PC ratio at cut-off value of 0.21 showed a sensitivity of 100% and specificity of 73%. Area under the curve was 0.89. RL- Reference Line
Correlation between CEC and CEC/PC with hepatic disease severity scores
Although the CEC count generally tracked with Child-Pugh scores, the results were not statistically significant (Table 2). However as seen in Table 2, CEC/PC was significantly elevated in patients with splenomegaly (p value 0.037) as well as those with portal hypertensive decompensation proxied by Child Class B and C status (p<0.05). MELD score also generally tracked with CEC count however it was not statistically significant (R 0.097, p value 0.732).
Table 2.
Median CEC count and CEC/PC in patient cohorts
| CEC count Cells/4ml |
p * | Platelet count Cells×106/L |
p * | CEC/PC | p * | |
|---|---|---|---|---|---|---|
| Coexistent cancer | ||||||
| Yes (n=4) | 139 [127.6-221.6] | 0.13 | 142[79.25-206.25] | 0.296 | 1.2 [0.62-2.82] | 0.361 |
| No (n= 11) | 70 [49-138] | 67[42-191] | 0.72 [0.31-1.62] | |||
|
| ||||||
| Splenomegaly | ||||||
| Yes (n= 10) | 105 [41-254] | 0.86 | 65.50[40.75-91.50] | 0.005 | 1.32 [0.6-2.4] | 0.037 |
| No (n= 5) | 73.7 [61.8-131.1] | 207[176-228.50] | 0.43 [0.28-0.66] | |||
|
| ||||||
| Varices | ||||||
| Yes (n= 9) | 131 [80-170] | 0.556 | 67[43.50-182.50] | 0.279 | 0.74 [0.39-2.39] | 0.637 |
| No (n= 6) | 70 [51-194] | 158.50[69.25- 215.25] |
0.66[0.31-1.82] | |||
|
| ||||||
| Child score | ||||||
| Child A (n= 3) | 73.7[53.7- 124.3] | 0.73 | 217[212-228.50] | 0.009 | 0.31 [0.28-0.45] | 0.043 |
| Child B&C (n=12) | 105 [53.6-221.8] | 73[42.75-152.25] | 0.88 [0.49-2.03] | |||
Mann-Whitney’s test
Inter-and intra-observer variability of CEC count
Correlation coefficient of intra- and interobserver variability for CEC measurement using the automated rare cell analysis system showed an intraclass correlation coefficient of 0.9989 and 0.9986, respectively. The intraclass correlation coefficient for the interobserver and intraobserver variation is shown in Table 3.
Table 3.
Correlation coefficient of intra- and inter-observer agreement of CEC
| R2* | 95% lower limit | 95% upper limit | |
|---|---|---|---|
| Inter-observer | 0.9989 | 0.9976 | 0.9995 |
| Intra-observer | 0.9986 | 0.9970 | 0.9993 |
Intra-class correlation coefficient
Discussion
This initial study is the first to assess CEC levels in chronic liver disease, and the results suggests that CEC levels are significantly higher in patients with cirrhosis as compared to healthy controls. Furthermore, the ratio of CEC/PC, which incorporates platelet count (22), also appears to initially discriminate between patients with cirrhosis and normal controls, as well as those with compensated versus decompensated liver disease. However, caution should be exercised in interpreting these findings given the small sample size of the current study. Prior studies to predict the presence of cirrhosis, portal hypertension, and likelihood of hepatic decompensation using various non-invasive approaches including routine laboratory tests, serum markers of fibrosis/inflammation, quantitative assays of liver function, and radiologic imaging, have revealed varying levels of discriminatory capacity (2-4). In this study, CEC levels were assayed using a commercially available automated analytical system from a routine peripheral blood collection (23, 24). The high interobserver and intraobserver agreement of this technique depicted by our group and others (14, 23), suggest that this diagnostic test may have methodologic advances compared to some currently available techniques such as ultrasonography, which are limited by high inter- and intraobserver variability (25). Initial estimates of diagnostic accuracy (sensitivity, specificity, AUC) of CEC count and CEC/PC ratio for distinguishing patients with cirrhosis from healthy controls also compares favorably to metrics reported for other non-invasive tests such as aspartate aminotransferase-to-platelet ratio index (APRI) (26) and ultrasonography (27). However, prospective studies with larger numbers of subjects encompassing a broader spectrum of liver disease are necessary for verifying the precision of these initial estimates.
In this pilot study, where a big spread in mean platelet count values was seen between normals and patients with decompensated cirrhosis, we were not surprised to see that platelet count performed reasonably well to distinguish normals from cirrhotics. Interestingly, we found that 6 out of 15 cirrhotic patients had a normal or near normal platelet count but a relatively high CEC count. Indeed, the sensitivity of cut-off value of CEC/PC to differentiate cirrhosis patients from controls was 100 % as compared to 93 % for platelet count alone. This suggests that CEC in combination with PC may have some potential value for prognosis compared to platelet count alone. This needs validation in larger future studies.
Elevated CEC levels has been observed in a variety of pathological conditions associated with vascular disease (18, 28), and considered by some to be a biomarker of disease severity in vascular conditions (24). Indeed, the vascular endothelium is intimately linked with a variety of conditions including cardiovascular, autoimmune, infectious, and neoplastic diseases (11, 13-21). Similarly, cirrhosis and portal hypertension are characterized by prominent vascular changes involving the endothelium, including angiogenesis, sinusoidal vascular distortion, hyperdynamic systemic circulation, and intrahepatic endothelial dysfunction (1). Furthermore, the prominent cytokine derangements in molecules such as nitric oxide and tumor necrosis factor may also contribute to disturbances in the endothelial component of the vessel wall and ensuing increases in peripheral CEC levels (29-31).
The precise origin of CEC in patients with cirrhosis and portal hypertension remains to be determined. Theoretically, CEC may derive from mature endothelial cells sloughed from the vessel wall in response to vascular injury, or from endothelial progenitor cells (EPC) which are bone-marrow derived and postulated to contribute to vascular repair (32-34). A recent study by Poon et al., (35) reported higher circulating levels of EPC in patients with advanced unresectable hepatoma as compared to patients with resectable disease, cirrhosis, or normal controls. Interestingly, patients with cirrhosis and cancer were also observed to have some of the highest CEC levels in our study (see Table 2).
This observation may reflect the relationship between malignancy, angiogenesis, and recruitment of bone marrow derived endothelial cells through the blood stream (35). However, it is important to note that the study by Poon focused on EPC, which are selected by CD133, VEGFR2, and CD34, while our study gated on a more mature population of cells which are positive for CD146, CD105, and are in fact negative for CD34. This may reflect a bone marrow origin of EPC in the Poon study compared to the more mature CEC in our study derived from sloughing of endothelium from injured vasculature.
A number of questions were raised by our study. While our initial results are quite promising, future studies of diagnostic accuracy will need to include patients with a broader spectrum of liver disease severity. Accounting for clinical stage of cirrhosis (36) would be a useful analysis in this regard. In addition, portal venous pressure measurements will also need to be performed to identify significant quantitative correlations with CEC count and CEC/PC ratios.
In summary, this pilot study suggests that CEC levels are elevated in patients with cirrhosis, and a ratio of CEC/PC is not only elevated in cirrhosis but also correlates with hepatic decompensation. These interesting yet preliminary results, should they be verified by larger studies, not only provide important pathophysiologic clues pertaining to vascular injury in cirrhosis, but also justify the conduct of prospective studies to ascertain the clinical utility of this novel diagnostic methodology.
Acknowledgments
Grant Support: This work was funded through Mayo Clinic and NIH (DK 59615 and HL 86990 to VS).
Abbreviations
- APRI
(aminotransferase-to-platelet ratio index)
- CEC
(circulating endothelial cells)
- EPC
(endothelial progenitor cells)
- IQR
(interquartile range)
Footnotes
Disclosures: All authors disclose that they have no potential conflicts that are relevant to the manuscript.
References
- 1.Bosch J. Vascular deterioration in cirrhosis: the big picture. J Clin Gastroenterol. 2007;41(Suppl 3):S247–253. doi: 10.1097/MCG.0b013e3181572357. [DOI] [PubMed] [Google Scholar]
- 2.George J. Biochemical markers of hepatic fibrogenesis: single measurements are not reliable enough to replace liver biopsy. J Gastroenterol Hepatol. 2000;15:819–821. doi: 10.1046/j.1440-1746.2000.02175.x. [DOI] [PubMed] [Google Scholar]
- 3.McHutchison JG, Blatt LM, de Medina M, et al. Measurement of serum hyaluronic acid in patients with chronic hepatitis C and its relationship to liver histology. Consensus Interferon Study Group. J Gastroenterol Hepatol. 2000;15:945–951. doi: 10.1046/j.1440-1746.2000.02233.x. [DOI] [PubMed] [Google Scholar]
- 4.Imbert-Bismut F, Ratziu V, Pieroni L, et al. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–1075. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 5.Oberti F, Valsesia E, Pilette C, et al. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology. 1997;113:1609–1616. doi: 10.1053/gast.1997.v113.pm9352863. [DOI] [PubMed] [Google Scholar]
- 6.Lok AS, Ghany MG, Goodman ZD, et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology. 2005;42:282–292. doi: 10.1002/hep.20772. [DOI] [PubMed] [Google Scholar]
- 7.de Franchis R. Noninvasive diagnosis of esophageal varices: is it feasible? Am J Gastroenterol. 2006;101:2520–2522. doi: 10.1111/j.1572-0241.2006.00880.x. [DOI] [PubMed] [Google Scholar]
- 8.Thabut D, Moreau R, Lebrec D. Screening for esophageal varices: Endoscopy, other tools, or endoscopy and other tools? Hepatology. 2008;47:1434–1436. doi: 10.1002/hep.22315. [DOI] [PubMed] [Google Scholar]
- 9.Woywodt A, Blann AD, Kirsch T, et al. Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: proposal of a definition and a consensus protocol. J Thromb Haemost. 2006;4:671–677. doi: 10.1111/j.1538-7836.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- 10.Widemann A, Sabatier F, Arnaud L, et al. CD146-based immunomagnetic enrichment followed by multiparameter flow cytometry: a new approach to counting circulating endothelial cells. J Thromb Haemost. 2008;6:869–876. doi: 10.1111/j.1538-7836.2008.02931.x. [DOI] [PubMed] [Google Scholar]
- 11.Goon PK, Boos CJ, Lip GY. Circulating endothelial cells: markers of vascular dysfunction. Clin Lab. 2005;51:531–538. [PubMed] [Google Scholar]
- 12.Ruegg C, Yilmaz A, Bieler G, et al. Evidence for the involvement of endothelial cell integrin alphaVbeta3 in the disruption of the tumor vasculature induced by TNF and IFN-gamma. Nat Med. 1998;4:408–414. doi: 10.1038/nm0498-408. [DOI] [PubMed] [Google Scholar]
- 13.Bonello L, Basire A, Sabatier F, et al. Endothelial injury induced by coronary angioplasty triggers mobilization of endothelial progenitor cells in patients with stable coronary artery disease. J Thromb Haemost. 2006;4:979–981. doi: 10.1111/j.1538-7836.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 14.Bull TM, Golpon H, Hebbel RP, et al. Circulating endothelial cells in pulmonary hypertension. Thromb Haemost. 2003;90:698–703. doi: 10.1160/TH03-04-0251. [DOI] [PubMed] [Google Scholar]
- 15.Quilici J, Banzet N, Paule P, et al. Circulating endothelial cell count as a diagnostic marker for non-ST-elevation acute coronary syndromes. Circulation. 2004;110:1586–1591. doi: 10.1161/01.CIR.0000142295.85740.98. [DOI] [PubMed] [Google Scholar]
- 16.Lee KW, Lip GY, Tayebjee M, et al. Circulating endothelial cells, von Willebrand factor, interleukin-6, and prognosis in patients with acute coronary syndromes. Blood. 2005;105:526–532. doi: 10.1182/blood-2004-03-1106. [DOI] [PubMed] [Google Scholar]
- 17.Percivalle E, Revello MG, Vago L, et al. Circulating endothelial giant cells permissive for human cytomegalovirus (HCMV) are detected in disseminated HCMV infections with organ involvement. J Clin Invest. 1993;92:663–670. doi: 10.1172/JCI116635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clancy R, Marder G, Martin V, et al. Circulating activated endothelial cells in systemic lupus erythematosus: further evidence for diffuse vasculopathy. Arthritis Rheum. 2001;44:1203–1208. doi: 10.1002/1529-0131(200105)44:5<1203::AID-ANR204>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 19.Woywodt A, Schroeder M, Gwinner W, et al. Elevated numbers of circulating endothelial cells in renal transplant recipients. Transplantation. 2003;76:1–4. doi: 10.1097/01.TP.0000074569.65127.26. [DOI] [PubMed] [Google Scholar]
- 20.Bertolini F, Shaked Y, Mancuso P, et al. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 21.Furstenberger G, von Moos R, Lucas R, et al. Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy of primary breast cancer. Br J Cancer. 2006;94:524–531. doi: 10.1038/sj.bjc.6602952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu SN, Wang JH, Liu SL, et al. Thrombocytopenia as a surrogate for cirrhosis and a marker for the identification of patients at high-risk for hepatocellular carcinoma. Cancer. 2006;107:2212–2222. doi: 10.1002/cncr.22242. [DOI] [PubMed] [Google Scholar]
- 23.Rowand JL, Martin G, Doyle GV, et al. Endothelial cells in peripheral blood of healthy subjects and patients with metastatic carcinomas. Cytometry A. 2007;71:105–113. doi: 10.1002/cyto.a.20364. [DOI] [PubMed] [Google Scholar]
- 24.Erdbruegger U, Haubitz M, Woywodt A. Circulating endothelial cells: a novel marker of endothelial damage. Clin Chim Acta. 2006;373:17–26. doi: 10.1016/j.cca.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Taniguchi N, Itoh F, et al. Ultrasonographic evaluation using the internal echo in normal and cirrhotic livers: Comparison of accuracy of gray-scale and binary black-and-white images and their intraobserver reproducibility and interobserver agreement. Journal of Medical Ultrasonics. 2006;30:21–29. doi: 10.1007/BF02485166. [DOI] [PubMed] [Google Scholar]
- 26.Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007;46:912–921. doi: 10.1002/hep.21835. [DOI] [PubMed] [Google Scholar]
- 27.Zheng RQ, Wang QH, Lu MD, et al. Liver fibrosis in chronic viral hepatitis: an ultrasonographic study. World J Gastroenterol. 2003;9:2484–2489. doi: 10.3748/wjg.v9.i11.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 29.Llorent L, Richaud-Patin Y, Alcocer-Castillejos N, et al. Cytokine gene expression in cirrhotic and non-cirrhotic human liver. J Hepatol. 1996;24:555–563. doi: 10.1016/s0168-8278(96)80140-1. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 31.Ozuyaman B, Ebner P, Niesler U, et al. Nitric oxide differentially regulates proliferation and mobilization of endothelial progenitor cells but not of hematopoietic stem cells. Thromb Haemost. 2005;94:770–772. doi: 10.1160/TH05-01-0038. [DOI] [PubMed] [Google Scholar]
- 32.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 33.Kim HK, Song KS, Kim HO, et al. Circulating numbers of endothelial progenitor cells in patients with gastric and breast cancer. Cancer Lett. 2003;198:83–88. doi: 10.1016/s0304-3835(03)00268-4. [DOI] [PubMed] [Google Scholar]
- 34.Strijbos MH, Gratama JW, Kraan J, et al. Circulating endothelial cells in oncology: pitfalls and promises. Br J Cancer. 2008;98:1731–1735. doi: 10.1038/sj.bjc.6604383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho JW, Pang RW, Lau C, et al. Significance of circulating endothelial progenitor cells in hepatocellular carcinoma. Hepatology. 2006;44:836–843. doi: 10.1002/hep.21353. [DOI] [PubMed] [Google Scholar]
- 36.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]