Abstract
Alveolar echinococcosis is one of the most important lethal zoonotic helminth infections in the northern hemisphere. Currently, the threat to public health is increasing, as evidenced by the rising prevalence rate of alveolar echinococcosis, as well as the invasion of urban areas by infected wild foxes. This threat is further increased due to the involvement of pet dogs, and probably cats, as emerging sources of infection. These increased threats to public health also have associated economic risks; therefore, there is a need for effective and sustainable methods of control. In this paper, initiatives to control alveolar echinococcosis by targeting its definitive hosts through anthelmintic baiting campaigns initiated by local residents who used local resources for bait production, distribution and collection of fecal samples for diagnosis are described. Further, when such distribution programs are coupled with the use of GIS-based maps, the optimum distribution of bait was obtained. These programs have also included the use of intravital diagnostic analyses of infection rates, which have been overseen by the Forum on Environment and Animals (FEA), and also allowed a nationwide monitoring of echinococcosis in difinitive hosts. In addition, a government initiative requiring mandatory reporting of echinococcosis in dogs to health authorities was recently initiated in Japan. Overall, the results of this study have shown that use of collaborative control initiatives targeting zoonotic agents of alveolar echinococcosis can be an effective method for reducing the threat of lethal echinococcosis in the northern hemisphere.
Keywords: alveolar echinococcosis, anthelmintic baiting, endogenous development, northern hemisphere, zoonosis
Introduction
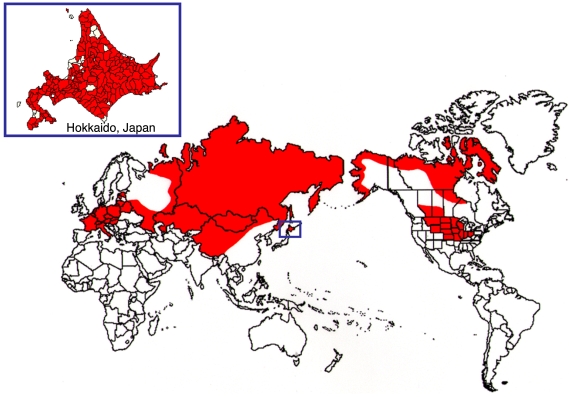
Alveolar echinococcosis is caused by Echinococcus multilocularis, which is a zoonotic tapeworm parasite of canids that is commonly distributed in the northern hemisphere (Fig. 1). The occurrence of the causative cestode in Japan is restricted to the northern island of Hokkaido, although sporadic cases of human infections have been reported on other islands [8]. Currently, this parasite is reported to be distributed throughout the island of Hokkaido. In addition, alveolar echinococcosis also occurs at low rates in central and eastern Europe [6]. However, recent studies of foxes in Europe have shown that E. multilocularis is more widespread than previously thought, being found as far south as Italy [25], as far north Lithuania and Estonia [27] and as far as east as Turkey [17]. The disease is also prevalent in Russia and the newly independent nations that were formerly part of the USSR, as well as China and Mongolia [38,44,45]. Echinococcus multilocularis has also been reported in North America, and is specifically found in the north central states and Alaska in the US as well as in parts of Canada [4,16,39].
Fig. 1.
The approximate geographic distribution of Echinococcus multilocularis in the northern hemisphere.
As of 2004, 482 patients in Hokkaido, Japan were confirmed to have been infected with alveolar echinococcosis, and one study found that the rate of occurrence during the endemic period was 48 cases per 100,000 residents every year [31]. In addition, more than 76 cases were reported from other islands [9], with most of these cases occurring in the northern part of the mainland of Japan. In addition to the cases reported in Japan, data from the European Echinococcosis Registry (EurEchinoReg: 1982-2000) [23] indicate that autochthonous cases of alveolar echinococcosis occurred in Austria (53 cases), Belgium (3 cases), France (235 cases), Germany (126 cases), Greece (1 case), and Switzerland (112 cases), and that 15 non-autochthonous cases were recorded from several European countries, imported especially from central Asia. In addition, 14 cases were reported in Poland, which was previously not considered to be endemic for alveolar echinococcosis [44], but were noted with increasing frequency since 1994 [29].
Because there is a long period between the time at which infection with alveolar echinococcosis occurs and the development of clinical disease, the recent increase in prevalence rates of infection in vectors may forecast higher prevalence rates in humans. A retrospective study of the disease in Switzerland that covered 50 years concluded that the incidence of human alveolar echinococcosis in that region appears to be increasing, and that this increase was preceded 10 years earlier by a parallel increase in the infection and urbanization of the fox population [37]. Humans become infected after ingestion of the echinococcus eggs from sources contaminated with feces from infected definitive hosts, such as foxes, dogs, and occasionally cats. However, general precautionary measures, such as avoidance of drinking water from springs or rivers, washing of hands and avoidance of contact with foxes has had no significant effect in suppressing alveolar echinococcosis in humans.
Human alveolar echinococcosis, although relatively rare and generally considered an accidental spill-over from wildlife, is one of the most difficult invasive helminthic infections to diagnose, effectively treat and effectively evaluate during the post-treatment period [3]. The disease is characterized by hepatic and sometimes cerebral disorders caused by the larval form (metacestode) of the tapeworm. The metacestode cells of E. multilocularis proliferate in a fashion similar to tumor cells, and by the time clinical signs are manifested, it is very difficult to treat, however, if no treatment is provided the disease is lethal. In addition, the disease can only be completely cured if confirmatory diagnosis is conducted during the early stages of the disease and is followed by complete resection of all of the lesions caused by the disease.
In addition to its adverse effects on human health, an epidemic of this disease could adversely affect the local economy of Japan due to its potential impact on agricultural and tourist industries [24]. Therefore, this disease warrants immediate attention and decisive action for its effective and sustainable control. To accomplish this, it has been suggested that public health authorities establish a coordinated system of continuous surveillance and risk assessment, and that these measures be combined with measures to reduce illness and death that occurs as a result of alveolar echinococcosis in the human population [10]. To date, the most effective control program encountered has been one that was introduced by the OIE Reference laboratory for Echinococcosis in Japan, which involves elimination of E. multilocularis in its definitive hosts through deworming [19]. The success of this program in Japan indicates that it could also be successful in other endemic countries.
Sources of infection
Wild red foxes
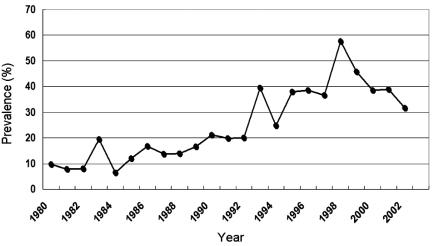
The prevalence rate of E. multilocularis in wild foxes has dramatically increased over the past few decades [30] (Fig. 2). In 1985, less than 10% of foxes were reportedly infected, however, by 1998 this figure had risen to 58.4%, and it has continued to increase over the past few years. Due to the high prevalence rate of echinococcosis in wild red foxes, as well as their increasing population, they are considered to be the major definitive hosts of the parasite in Japan [32]. Although most of the parasite biomass occurs in foxes, other definitive hosts may also serve as sources of infection [35], however, a mathematical model of egg excretion dynamics has suggested that foxes have a higher mean biotic potential than any other known definitive hosts [22].
Fig. 2.
Prevalence rates of Echinococcus multilocularis infection in wild foxes in Hokkaido island from 1980 to 2002 (necropsy survey data from the Hokkaido Government).
In addition to the high prevalence rate of echinococcosis in wild red foxes, the invasion of red foxes into urban areas has also been of concern because it indicates that an increasing infection pressure for densely populated areas is inevitable. For these reasons, it is expected that an urban cycle of E. multilocularis will eventually occur in the most populous city of Hokkaido, Sapporo, and most surveys conducted within the city, either by necropsy or coproantigen detection, have already registered the presence of Echinococcus-infected foxes in parks and woodlands [43]. Further, large amounts of E. multilocularis coproantigen positive fox feces have also been reported in urban areas adjacent to recreational parks [43]. Other suitable intermediate hosts have also been trapped in recreational parks, however none of these were found to be infected [43]. This phenomenon has also been documented in other endemic countries in which an increasing number of infected foxes have been found foraging in cities and villages [7]. For example, in Europe, prevalence rates in foxes have risen in many agricultural dominated areas in France, The Netherlands, Germany, Austria, Slovakia and Poland, however, the life cycle has also been established in many urban areas in which foxes are present in high population densities, which presents an increased risk of infection for large human populations [4,35].
In Japan, raccoon dogs have also been found to be infected with E. multilocularis [48]. Based on experimental infection studies they were suggested to be capable of playing a significant role in the epidemiology of alveolar echinococcosis [41]. Although their low population in Hokkaido indicates that they may have a lesser role in transmission of the disease, raccoon dogs have a greater reproduction potential than foxes, and their impact may increase with the effects of global warming [41].
Domestic pets
Prevalence studies of echinococcosis in dogs in Japan have been very limited; however, a 30 year survey by necropsy examinations of 9,930 dogs from 1966 conducted by the Hokkaido government revealed 98 infected dogs. Recently, annual examinations of less than 10 dogs conducted by the Hokkaido government registered zero incidence rate. However, the Forum on Environment and Animals (FEA), from April 2004 through August 2005, examined a total of 1,460 fecal samples obtained from domestic dogs nationwide by animal clinics found 4 (0.27%) dogs that were positive for echinococcosis based on coproantigen and PCR assays [18]. It was estimated that nearly ten thousand pet dogs are transported between Honshu and Hokkaido by plane and ferry every year, and that this includes up to 30 E. multilocularis-infected animals [8]. Further, it has been reported that 2 out of 69 dogs that were moved from Hokkaido to Honshu were found to be positive for E. multilocularis based on coproantigen examination [21]. This has raised concerns that echinococcosis might spread to Honshu as a result of pet dog translocation. In addition, a dog in Saitama prefecture on the main island (adjacent to Tokyo) was found to have E. multilocularis eggs in its feces [47].
Additionally, a recent survey of dogs transported through ferry ports in Hokkaido found 2 dogs (2/183) that were positive for E. multilocularis coproantigen [28], and one of these dogs was a non-resident of Hokkaido that had been permitted to roam freely for only a few hours during its 5 day stay. Taken together, these findings suggest that there has been a rise in the infection rate of domestic dogs in Hokkaido, and that these dogs have the potential to spread the disease throughout Japan. It should be noted, however, that infection in domestic dogs can only be spread by way of highly contaminated rodent intermediate hosts, which are closely associated with the high infection prevalence rates observed in wild foxes [12]. Nevertheless, it has been demonstrated that dogs have a high biotic potential and contribution to the transmission cycle of echinococcosis [22], and the lifetime incidence in dogs being infected with E. multilocularis at least once can reach about 10% even in a population that has only a low prevalence rate of infection [7].
Echinococcosis infection in cats has also been reported, however, in all cases the tapeworms collected were immature in form [46], therefore, it has been suggested that cats only play a minor role in the maintenance of E. multilocularis in endemic areas [41]. In spite of this, in Japan a cat was recently shown to be excreting taeniid eggs that were confirmed to be E. multilocularis by PCR. This recent finding is similar to observations in Europe, which indicate that cats are potential sources of infective eggs.
Control strategies against echinococcosis
Anthelmintic baiting
Rausch et al. [33] conducted the first study on anthelmintic treatment in a 10-year trial involving monthly deworming of dogs with praziquantel in a village in St. Lawrence, Alaska a hyperendemic area to E. multilocularis. Although discontinued recently because of the cost of the drug, the replacement of sled-dogs by machines decreased remarkably the incidence of alveolar echinococcosis in the island. However, with the presence of other definitive hosts, i.e. arctic foxes, different dimensions of the problem are expected to surface.
It has since been asserted that there is no reliable, cost-effective method for the sustainable control or eradication of E. multilocularis in the sylvatic cycle [10]. In spite of this assertion, our reference laboratory has been deworming red foxes in Koshimizu (200 km2), Hokkaido, Japan since 1997. To accomplish this, a survey was initially conducted to locate fox dens, and then fox feces were collected from the vicinity of the dens and examined for the presence of taeniid eggs and Echinococcus coproantigen. The following year, anthelmintic-fortified bait, which consisted of commercial fish sausages (1.5 cm long) embedded with one half of a 25 mg praziquantel tablet (Droncit; Bayer, Germany), was distributed manually in approximately half of the total area around the fox dens on a monthly basis on foot. The baiting trial showed that there was a decrease in the taeniid egg infection rate in foxes in the baited area after one month, and that this suppressive effect persisted in the following years, despite a decrease in the number of times the bait was distributed. The trial also showed that intermediate host rodents born following the bait distribution had a significantly lower prevalence of infection than the overwintered older rodents [42].
In a follow-up study conducted in April 2001, praziquantel-fortified bait was distributed throughout the entire area of Koshimizu alongside roads, at intersections and at wind-shield forests by local residents using cars to allow for faster mobility. The bait was made from fish-waste products, using the same procedure that is used for manufacturing "kamaboko" (Fig. 3) fortified with praziquantel (50 mg/ piece of bait). Based on a comparison of feces collected from foxes within the treatment area to feces collected from foxes outside the treatment area, which were used as a control, taeniid egg infection rates and coproantigen infection rates were significantly decreased in foxes inside the treatment area. This significant reduction of taeniid egg infection rates, however, was not observed until six months after the start of bait distribution, and the lower coproantigen positive rates were not observed for almost a year. A recent study found that, after continuous annual distribution of bait manufactured from fish-waste products the prevalence rate of coproantigen positive feces in 2006 was reduced to zero. In this study, local residents used cars to distribute bait annually alongside roads, at intersections between roads, and in wind-shield forests, and this proved to be a rapid method that did not require a large number of personnel and was highly effective at suppressing the infection rate of E. multilocularis in wild red foxes [19].
Fig. 3.
Manufacture of baits using local resources such as the fish-waste products. Baits are fortified with praziquantel.
A control strategy initiated by local residents was also conducted in Kutchan, Hokkaido, Japan, which is another echinococcosis endemic area. A baseline study, in which fox feces were collected from a 100 km2 study area, was conducted in July, September and November of 2005, prior to the distribution of bait. The prevalence rates of taeniid egg and E. multilocularis coproantigen positive feces were 7% (19/268) and 21% (55/268), respectively. Between May and November of 2006, a monthly distribution of approximately 1,500 pieces of bait was conducted throughout the study area by volunteers comprised of local residents of Kutchan. The bait was distributed with the use of GIS- based maps to identify the foraging habitat of wild foxes. Remarkably, the prevalence rates of taeniid egg and coproantigen positive feces dropped to 0% and 2% after less than a year of baiting. The results obtained when this strategy was used indicate that distribution of praziquantel-fortified bait using GIS-based maps could allow bait distribution to be restricted to only areas commonly visited by foxes, thereby cutting costs and time.
The baiting system implemented in Japan varies from that of Germany, which started wild fox deworming in 1990. Although the vegetation, quantity of snowfall, the species of voles involved and their habitat in Hokkaido are different from those of Europe, the primary difference in baiting systems used is that the post-deworming prevalence rates of infection in Europe are evaluated by hunting the foxes. In a baiting campaign that utilized planes to distribute bait over a large area, stronger effects were observed in the 156 km2 core area than in the 6 to 10 km border area. It has been suggested that the border effect observed in this campaign may have occurred as a result of immigration of young, infected foxes [14,36]. Similarly, following control trials in northern Germany, the prevalence rate of infection recovered unexpectedly and rapidly, reaching pre-control levels 15 months after the end of the baiting campaign [13]. It is believed that this occurred as a result of young foxes being dispersed due to hunting pressure upon foxes in the border area, which resulted in there being vacant territories available for younger generations of infected foxes. While some studies have indicated that there were no significant age-dependent differences in the rate of E. multilocularis infection, other studies have found juvenile foxes to be more frequently infected than adults, and infection rates in young foxes have been found to be significantly greater under highly-endemic conditions than low-endemic conditions [11,40]. In addition, it has been reported that subadult foxes carry significantly higher worm burdens than adult foxes [15]. Taken together, these findings indicate that invasion of young infected foxes into the territories left by the hunted foxes could maintain or increase the prevalence rate of infection.
In Japan, however, the ecological niches of the foxes being treated were not disturbed because the efficacy of deworming was assessed using coproantigen detection in fox feces collected from the environment of baited areas instead of hunting. The differences observed in the efficacy of the Japanese treatment method and the German method indicate that the use of fox culling or hunting for evaluation of control efficacy is actually detrimental to the success of the baiting campaign [20]. Similarly, anthelmintic baiting of foxes against urban contamination with E. mutilocularis using intravital diagnosis for the assessment of efficacy was highly successful in Switzerland [14]. In the Swiss study, a pronounced reduction of E. multilocularis prevalence rates was observed in both the definitive and intermediate hosts when an approach combining anthelmintic baiting and coproantigen diagnosis was used.
Taken together, these results indicate that the use of intravital diagnosis, such as coproantigen [1] or copro-DNA [26] examination provides a superior means for assessing control interventions while preserving both the animals being treated and their environments.
The role of local residents in treatment initiatives
"Endogenous development" involves building on local resources, enhancing in situ development, maximizing local control of the development process, and recognizing the needs and the values of local residents [34]. As the Dag Hammarskjöld Report [5] puts it, such development relies on what a human group has: its natural environment, its cultural heritage, the creativity of the men and women who constitute it, becoming richer through exchange between them and with other groups and entails the autonomous definition of development styles and of life styles.
In all of the baiting campaigns reviewed in this paper that were conducted in Japan, the endogenous initiative of local residents, which was facilitated by NonProfit Organization (NPO), was highly instrumental. Zoonotic diseases are of concern not only to public health personnel but also to individual residents who are at risk of infection. Moreover, the use of local resources, including local residents for fecal collection and bait distribution, locally produced fish-waste products for bait manufacturing, and local fund-raising to support the deworming program was found to be imperative for the success of a sustainable program for the control and prevention of echinoccocosis.
In a follow-up campaign that has been ongoing in Koshimizu since 2002, bait distribution, fecal collection and monitoring of echinococcosis in wild foxes was conducted by Okhotsk Sanctuary, a local NPO. Endogenous control initiatives introduced by this NPO provided a necessary solution that allowed the prolongation of the baiting campaign, thereby enabling a sustainable approach to the suppression of the E. multilocularis infection in wild red foxes, as indicated by the most recent prevalence rate of coproantigen positive feces of wild foxes in Koshimizu, which was 0%.
Another control initiative introduced by another NPO such as WAO in Kutchan, Hokkaido [31] also produced favorable results. To finance a long term preventive measure, WAO organized an echinococcosis control sticker sale campaign, in which stickers containing information regarding the life cycle of E. multilocularis and the threat of echinococcosis to public health are sold at approximately US $4. The proceeds of the sticker sales are then used to finance the program, including the costs of the anthelmintic-fortified baits and fecal examinations. In addition, WAO, with the help of the OIE reference lab, spearheaded an information dissemination initiative that involved local residents, including university students and children in elementary school.
National government initiatives
The results of our research project, entitled "Prevention on the spread of areas that are endemic for zoonotic parasitic diseases" disclosed a strong possibility that dogs infected with Echinococcus could transmit the infection to their owners. Based on these findings, the Ministry of Health, Labor and Science, Japan directed the Hokkaido Prefectural Government to take measures to prevent the infection of pet owners from occurring, including campaigns to make the public aware of the potential threat [19].
In addition, an amendment to the Infectious Disease Law in Japan was made that required inclusion of certain specific zoonotic diseases in the 4th category (diseases which must be reported). The diseases added to this category included echinococcosis in dogs as well as bacterial dysentery in primates and West Nile fever in birds. In addition, during their 20th session, the Infectious Disease Evaluation Committee of the Ministry of Health, Labor, and Science passed a resolution that made it mandatory for veterinarians to report cases of echinococcosis in dogs to the health authorities. Thus, a national reporting system for dogs infected with E. multilocularis has been used by veterinarians since October 2004 [19].
The Ministry of Health, Labor, and Science, with the assistance of our laboratory, has also published guidelines regarding standard procedures and diagnostic measures to be taken when reports are submitted by veterinarians, and these guidelines have been distributed to local health offices, as well as to practicing veterinarians throughout Japan. The following three criteria for diagnosis are stipulated in the national reporting system, and a positive result in any of these should be reported to health authorities [19,30]:
locating a parasite body that can be morphologically identified
detecting parasite DNA in eggs or a part of a parasite body
detecting parasite coproantigen, which should become negative after deworming.
Research laboratory initiatives
It is believed that research institutions have an important role to play in extending practical assistance to the public as the knowledge and understanding of the disease advances, especially with regards to its control and prevention. Thus, according to Zinsstag [49] "Although there is no doubt that progress in animal health research must continue, it must also respond to societal needs and lead to solutions that can be delivered quickly." Therefore, in 1999, this OIE reference laboratory organized a scheme called the FEA, Japan. This scheme is able to link important organizations including government offices, academic institutions, international agencies (e.g. the OIE), veterinary associations and non-governmental organizations, such as NPOs comprised of local residents, that all have the primary goal of controlling echinococcosis in Hokkaido.
The FEA is presently serving as a hub for private veterinarians involved in small animal practice throughout Japan for the confirmatory diagnosis of echinococcosis. Veterinarians who suspect Echinococcus infection in dogs, cats, or other susceptible definitive hosts send fecal samples to the FEA, which then conducts laboratory examinations. Further, the FEA has assisted with the endogenous initiative of NPOs by providing them with technical expertise, laboratory examinations of fox feces, anthelmintic- fortified baits and necessary materials such as the "eki-bin" (echi-bottle), which are containers used for the collection of fecal samples safely. In addition, the FEA provides intravital diagnosis using ELISA (EmA9) to determine the prevalence rate of echinococcosis in foxes during pre- and post-baiting campaigns. Overall, the FEA has enhanced the connection between laboratory findings and field applications through accurate diagnosis and proper monitoring of echinococcosis in Japan.
Recently, "Full-Echinococcus", a database of full-length cDNAs obtained from a human parasite, E. multilocularis, was produced in cooperation with this reference laboratory. The full-length cDNA library was produced using the Vector-trapper method on hydatid cysts developed in cotton rats that were infected with E.multilocularis. A total of 10,966 5'end-one-pass sequences were compared with the non-redundant database, DDBJ/Genbank/EMBL, using the BLAST and TBLASTX programs. Two-thirds of the sequences were considered to be derived from Echinococcus, while the remaining one-third represented host genes. Many of the former clones represent full-length cDNAs that are expressed in the larval stage, and these clones are available for further analysis and experiments.
Conclusions
The increasing prevalence rates of red foxes infected with the parasite, E. multilocularis, in the northern hemisphere represent a public health threat. In addition, it is feared that the invasion of infected foxes into urban areas and the proportional increase in infection pressure upon pet dogs may cause an epidemic in these endemic areas and disperse the infection to neighboring non-endemic areas. Ultimately, echinococcosis, which is endemic in the northern island of Japan, may spread into the mainland.
Because there is still no vaccine available for echinococcosis, the best means for control at present is deworming the definitive hosts, especially foxes, which produce the greatest biomass of the zoonotic agent. Baiting campaigns conducted in Hokkaido have been found to be very effective at reducing the prevalence rates of infection in wild foxes, and praziquantel-fortified bait distribution using cars to deliver the bait to strategic locations identified by GIS-based maps was found to be a valuable method for reducing costs and saving time. Complementing this, the OIE Reference Laboratory and FEA strongly advocate the use of intravital techniques for assessment of the efficacy of deworming trials to avoid the recurrence of high prevalence rates of infection due to immigration of young infected foxes into territories left by culled foxes, as well as to preserve environmental animals and their ecology.
These control programs may also be applied in other endemic areas in the northern hemisphere to avoid dispersion of zoonotic agents. In addition, collaborative efforts initiated by local residents herein referred as "endogenous development" may be a significant and sustainable approach in the control of other vector borne zoonotic diseases such as found in the rest of the world.
The initiative of local NPOs, coupled with the aid of this reference laboratory was successful at facilitating control of echinococcosis. The establishment of the FEA, which is currently helping to protect the public health and regional economy of the northern island of Japan from alveolar echinococcosis, strengthened this collaborative inititiatives. In addition, the national initiative put forth by the government requiring mandatory reporting of echinococcosis in dogs has also strengthened public health safety protection and welfare. It is recommended, however, that the Ministry of Environment and the Ministry of Agriculture take part in the collaborative efforts to help ensure successful control of alveolar echinococcosis in Japan.
Overall, this collaborative initiative revealed the dynamic and essential roles of local residents, the national government and our research laboratory in seeking out potential and optimum means of controlling diseases that are of public health and veterinary importance. This model is also applicable for developed and developing countries that desire a safer society and a cleaner environment.
Acknowledgments
I especially thank Dr. Yuzaburo Oku, Center of Excellence (COE), Hokkaido University, for providing the bulk of the data for this paper and Dr. Nariaki Nonaka, Hokkaido University, for his data on pet dogs, Dr. Jose Trinipil Lagapa, Central Mindanao University, Philippines, for the helpful assistance, and Dr. Sumiya Ganzorig and Mr. Fumio Kobayashi of the FEA for their invaluable support to this control program. I also wish to convey my deep gratitude to the international researchers who have cooperated with us in conceptualizing this control program: Dr. Bruno Gottstein of The University of Bern, Switzerland; Dr. Robert L. Rausch of The University of Washington, USA; Dr. Dominique Vuitton of The University of Franche-Comte, France; Dr. Jun Watanabe of the University of Tokyo, Japan; and Dr. Hee-Jeong Youn of Seoul National University, Korea. Special recognition to the NPO's of Koshimizu and Kutchan towns of Hokkaido, Japan.
References
- 1.Allan JC, Craig PS. Coproantigens in taeniasis and echinococcosis. Parasitol Int. 2006;55:S75–S80. doi: 10.1016/j.parint.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Bružinskaitė R, Marcinkutė A, Strupas K, Sokolovas V, Deplazes P, Mathis A, Eddi C, Šarkūnas M. Alveolar echinoccoccosis, Lithuania. Emerg Infect Dis. 2007;13:1618–1619. doi: 10.3201/eid1310.061161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craig PS. Epidemiology of echinococcosis in western China. In: Torgerson PR, Shaikenov BS, editors. Echinococcosis in Central Asia: Problems and Solution. Almaty: Publishing House Dauir; 2004. pp. 43–58. [Google Scholar]
- 4.Craig PS. Echinococcus multilocularis. Curr Opin Infect Dis. 2003;16:437–444. doi: 10.1097/00001432-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Dag Hammarskjöld Foundation. The 1975 Dag Hammarskjöld Report on Development and International Cooperations. Uppsala, Sweden: Dag Hammarskjöld Foundation; 1982. p. 28. [Google Scholar]
- 6.Deplazes P. Ecology and epidemiology of Echinococcus multilocularis in Europe. Parassitologia. 2006;48:37–39. [PubMed] [Google Scholar]
- 7.Deplazes P, Hegglin D, Gloor S, Romig T. Wilderness in the city: the urbanization of Echinococcus multilocularis. Trends Parasitol. 2004;20:77–84. doi: 10.1016/j.pt.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Doi R, Matsuda H, Uchida A, Kanda E, Kamiya H, Konno K, Tamashiro H, Nonaka N, Oku Y, Kamiya M. Possibility of invasion of Echinococcus into Honshu with pet dogs from Hokkaido and overseas. Nippon Koshu Eisei Zasshi. 2003;50:639–649. [PubMed] [Google Scholar]
- 9.Doi R, Nakao M, Nihei N, Kutsumi H. Epidemiology of alveolar hydatid disease (AHD) and estimation of infected period of AHD on Rebun Island, Hokkaido. Nippon Koshu Eisei Zasshi. 2000;47:145–152. [PubMed] [Google Scholar]
- 10.Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ewald D, Eckert J, Gottstein B, Straub M, Nigg H. Parasitological and serological studies on the prevalence of Echinococcus multilocularis Leuckart, 1863 in red foxes (Vulpes vulpes Linnaeus, 1758) in Switzerland. Rev Sci Tech. 1992;11:1057–1061. doi: 10.20506/rst.11.4.640. [DOI] [PubMed] [Google Scholar]
- 12.Gottstein B, Saucy F, Deplazes P, Reichen J, Demierre G, Busato A, Zuercher C, Pugin P. Is high prevalence of Echinococcus multilocularis in wild and domestic animals associated with disease incidence in humans? Emerg Infect Dis. 2001;7:408–412. doi: 10.3201/eid0703.010307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen F, Tackmann K, Jeltsch F, Wissel C, Thulke HH. Controlling Echinococcus multilocularis-ecological implications of field trials. Prev Vet Med. 2003;60:91–105. doi: 10.1016/s0167-5877(03)00084-9. [DOI] [PubMed] [Google Scholar]
- 14.Hegglin D, Ward PI, Deplazes P. Anthelmintic baiting of foxes against urban contamination with Echinococcus multilocularis. Emerg Infect Dis. 2003;9:1266–1272. doi: 10.3201/eid0910.030138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofer S, Gloor S, Muller U, Mathis A, Hegglin D, Deplazes P. High prevalence of Echinococcus multilocularis in urban red foxes (Vulpes vulpes) and voles (Arvicola terrestris) in the city of Zurich, Switzerland. Parasitology. 2000;120:135–142. doi: 10.1017/s0031182099005351. [DOI] [PubMed] [Google Scholar]
- 16.Holt DW, Hanns C, O'Hara T, Burek K, Frantz R. New distribution records of Echinococcus multilocularis in the brown lemming from Barrow, Alaska, USA. J Wildl Dis. 2005;41:257–259. doi: 10.7589/0090-3558-41.1.257. [DOI] [PubMed] [Google Scholar]
- 17.Inceboz T, Korkmaz M, Tokat Y, Uner A. The first report of Echinococcus multilocularis strain isolation from human in Turkey. Turkiye Parazitol Derg. 2005;29:31–33. [PubMed] [Google Scholar]
- 18.Kamiya M, Lagapa JT, Ganzorig S, Kobayashi F, Nonaka N, Oku Y. Echinococcosis risk among domestic definitive hosts, Japan. Emerg Infect Dis. 2007;13:346–347. doi: 10.3201/eid1302.051377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamiya M, Lagapa JT, Nonaka N, Ganzorig S, Oku Y, Kamiya H. Current control strategies targeting sources of echinococcosis in Japan. Rev Sci Tech. 2006;25:1055–1065. [PubMed] [Google Scholar]
- 20.Kamiya M, Lagapa JT, Oku Y. Research on targeting sources of alveolar echinococcosis in Japan. Comp Immunol Microbiol Infect Dis. 2007;30:427–448. doi: 10.1016/j.cimid.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Kamiya M, Nonaka N, Ganzorig S, Oku Y. Effective countermeasures against alveolar echinococcosis in the red fox population of Hokkaido, Japan. In: Torgerson PR, Shaikenov BS, editors. Echinococcosis in Central Asia: Problems and Solutions. Almaty: Publishing House Dauir; 2004. pp. 273–282. [Google Scholar]
- 22.Kapel CM, Torgerson PR, Thompson RC, Deplazes P. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. Int J Parasitol. 2006;36:79–86. doi: 10.1016/j.ijpara.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Kern P, Bardonnet K, Renner E, Auer H, Pawlowski Z, Ammann RW, Vuitton DA, Kern P. European Echinococcosis Registry. European echinococcosis registry: human alveolar echinococcosis, Europe, 1982-2000. Emerg Infect Dis. 2003;9:343–349. doi: 10.3201/eid0903.020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konno K, Oku Y, Tamashiro H. Prevention of alveolar echinococcosis-ecosystem and risk management perspectives in Japan. Acta Trop. 2003;89:33–40. doi: 10.1016/j.actatropica.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Manfredi MT, Casulli A, La Rosa G, Di Cerbo AR, Trevisio K, Genchi C, Pozio E. Echinococcus multilocularis in north Italy. Parassitologia. 2006;48:43–46. [PubMed] [Google Scholar]
- 26.Mathis A, Deplazes P. Copro-DNA tests for diagnosis of animal taeniid cestodes. Parasitol Int. 2006;55:S87–S90. doi: 10.1016/j.parint.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Moks E, Saarma U, Valdmann H. Echinococcus multilocularis in Estonia. Emerg Infect Dis. 2005;11:1973–1974. doi: 10.3201/eid1112.050339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morishima Y, Sugiyama H, Arakawa K, Kawanaka M. Echinococcus multilocularis in dogs, Japan. Emerg Infect Dis. 2006;12:1292–1294. doi: 10.3201/eid1208.051241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myjak P, Nahorski W, Pietkiewicz H, von Nickisch-Rosenegk M, Stolarczyk J, Kacprzak E, Felczak-Korzybska I, Szostakowska B, Lucius R. Molecular confirmation of human alveolar echinococcosis in Poland. Clin Infect Dis. 2003;37:e121–e125. doi: 10.1086/378296. [DOI] [PubMed] [Google Scholar]
- 30.Nonaka N, Kamiya M, Oku Y. Towards the control of Echinococcus multilocularis in the definitive host in Japan. Parasitol Int. 2006;55:S263–S266. doi: 10.1016/j.parint.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 31.Oku Y. Expansion of the distribution of Echinococcus multilocularis which propagates and spreads in the human body. J Modern Vet Med. 2000;48:5–17. [Google Scholar]
- 32.Oku Y, Kamiya M. Biology of Echinococcus. In: Otsuru M, Kamegai S, Hayashi S, editors. Progress of Medical Parasitology in Japan. Vol. 8. Tokyo: Meguro Parasitological Museum; 2003. pp. 293–318. [Google Scholar]
- 33.Rausch RL, Wilson JF, Schantz PM. A programme to reduce the risk of infection by Echinococcus multilocularis: the use of praziquantel to control the cestode in a village in the hyperendemic region of Alaska. Ann Trop Med Parasitol. 1990;84:239–250. doi: 10.1080/00034983.1990.11812463. [DOI] [PubMed] [Google Scholar]
- 34.Rist A. Endogenous development as a social learning process. Compas Magazine. 2004;7:26–29. [Google Scholar]
- 35.Romig T, Dinkel A, Mackenstedt U. The present situation of echinococcosis in Europe. Parasitol Int. 2006;55:S187–S191. doi: 10.1016/j.parint.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 36.Schelling U, Frank W, Will R, Romig T, Lucius R. Chemotherapy with praziquantel has the potential to reduce the prevalence of Echinococcus multilocularis in wild foxes (Vulpes vulpes) Ann Trop Med Parasitol. 1997;91:179–186. doi: 10.1080/00034983.1997.11813128. [DOI] [PubMed] [Google Scholar]
- 37.Schweiger A, Ammann RW, Candinas D, Clavien PA, Eckert J, Gottstein B, Halkic N, Muellhaupt B, Prinz BM, Reichen J, Tarr PE, Torgerson PR, Deplazes P. Human alveolar echinococcosis after fox population increase, Switzerland. Emerg Infect Dis. 2007;13:878–882. doi: 10.3201/eid1306.061074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaikenov BS. Distribution and ecology of Echinococcus multilocularis in Central Asia. Parasitol Int. 2006;55:S213–S219. doi: 10.1016/j.parint.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 39.Storandt ST, Virchow DR, Dryden MW, Hygnstrom SE, Kazacos KR. Distribution and prevalence of Echinococcus multilocularis in wild predators in Nebraska, Kansas, and Wyoming. J Parasitol. 2002;88:420–422. doi: 10.1645/0022-3395(2002)088[0420:DAPOEM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 40.Tackmann K, Loschner U, Mix H, Staubach C, Thulke HH, Ziller M, Conraths FJ. A field study to control Echinococcus multilocularis-infections of the red fox (Vulpes vulpes) in an endemic focus. Epidemiol Infect. 2001;127:577–587. doi: 10.1017/s0950268801006112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson RC, Kapel CM, Hobbs RP, Deplazes P. Comparative development of Echinococcus multilocularis in its definitive hosts. Parasitology. 2006;132:709–716. doi: 10.1017/S0031182005009625. [DOI] [PubMed] [Google Scholar]
- 42.Tsukada H, Hamazaki K, Ganzorig S, Iwaki T, Konno K, Lagapa JT, Matsuo K, Ono A, Shimizu M, Sakai H, Morishima Y, Nonaka N, Oku Y, Kamiya M. Potential remedy against Echinococcus multilocularis in wild red foxes using baits with anthelmintic distributed around fox breeding dens in Hokkaido, Japan. Parasitology. 2002;125:119–129. doi: 10.1017/s0031182002001968. [DOI] [PubMed] [Google Scholar]
- 43.Tsukada H, Morishima Y, Nonaka N, Oku Y, Kamiya M. Preliminary study of the role of red foxes in Echinococcus multilocularis transmission in the urban area of Sapporo, Japan. Parasitology. 2000;120:423–428. doi: 10.1017/s0031182099005582. [DOI] [PubMed] [Google Scholar]
- 44.Vuitton DA, Zhou H, Bresson-Hadni S, Wang Q, Piarroux M, Raoul F, Giraudoux P. Epidemiology of alveolar echinococcosis with particular reference to China and Europe. Parasitology. 2003;127:S87–S107. [PubMed] [Google Scholar]
- 45.Wang Q, Vuitton DA, Qiu J, Giraudoux P, Xiao Y, Schantz PM, Raoul F, Li T, Yang W, Craig PS. Fenced pasture: a possible risk factor for human alveolar echinococcosis in Tibetan pastoralist communities of Sichuan, China. Acta Trop. 2004;90:285–293. doi: 10.1016/j.actatropica.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Yagi K, Takahashi K, Hattori K. A case of immature Echinococcus multilocularis in a domestic cat in Nemuro, eastern Hokkaido, Japan. Rep Hokkaido Inst Pub Health. 1984;34:68–69. [Google Scholar]
- 47.Yamamoto N, Morishima Y, Kon M, Yamaguchi M, Tanno S, Koyama M, Maeno N, Azuma H, Mizusawa H, Kimura H, Sugiyama H, Arakawa K, Kawanaka M. The first reported case of a dog infected with Echinococcus multilocularis in Saitama prefecture, Japan. Jpn J Infect Dis. 2006;59:351–352. [PubMed] [Google Scholar]
- 48.Yimam AE, Nonaka N, Oku Y, Kamiya M. Prevalence and intensity of Echinococcus multilocularis in red foxes (Vulpes vulpes schrencki) and raccoon dogs (Nyctereutes procyonoides albus) in Otaru City, Hokkaido, Japan. Jpn J Vet Res. 2002;49:287–296. [PubMed] [Google Scholar]
- 49.Zinsstag J. Animal health research. Science. 2007;315:1193. doi: 10.1126/science.1141278. [DOI] [PubMed] [Google Scholar]